Abstract
In a preliminary analysis, 21 Colletotrichum strains with large conidia preserved in the CBS culture collection clustered with a recently described species, C. gigasporum, forming a clade distinct from other currently known Colletotrichum species complexes. Multi-locus phylogenetic analyses (ITS, ACT, TUB2, CHS-1, GAPDH) as well as each of the single-locus analyses resolved seven distinct species, one of them being C. gigasporum. Colletotrichum gigasporum and its close allies thus constitute a previously unknown species complex with shared morphological features. Five of the seven species accepted in the C. gigasporum species complex are described here as novel species, namely C. arxii, C. magnisporum, C. pseudomajus, C. radicis and C. vietnamense. A species represented by a single sterile strain, namely CBS 159.50, was not described as novel species, and is treated as Colletotrichum sp. CBS 159.50. Furthermore, C. thailandicum is reduced to synonymy with C. gigasporum.
Keywords: Ascomycota, morphology, phylogeny, systematics
INTRODUCTION
Colletotrichum gigasporum was originally reported from healthy leaves of Centella asiatica in Madagascar and Stylosanthes guianensis in Mexico, as well as from Coffea arabica in Colombia (Rakotoniriana et al. 2013). It has an endophytic growth habit and could be isolated from various host plants occurring in geographically distant areas.
The most distinctive morphological feature of C. gigasporum is the long straight conidia (up to 32 μm long, av. length 26 μm). Rakotoniriana et al. (2013) discussed the morphological differences between C. gigasporum and other species that produce large conidia, e.g. C. crassipes, C. echinatum, C. macrosporum, C. taiwanense and C. vinosum. Based on phylogenetic analyses of ITS and TUB2 sequence data, they showed C. gigasporum to belong to a distinct clade, distant from other currently accepted Colletotrichum species.
Numerous Colletotrichum isolates detected in a blastn search on GenBank have similar ITS sequences to that of the ex-type strain of C. gigasporum, e.g. isolates from Coffea arabica in Vietnam (Nguyen et al. 2010), Hibiscus rosa-sinensis in Thailand (Noireung et al. 2012), Magnolia liliifera in Thailand (Promputtha et al. 2007), Taxus chinensis var. mairei in China (Wu et al. 2013) and Theobroma cacao, Trichilia tuberculata and Virola surinamensis in Panama (Rojas et al. 2010). In our preliminary ITS analysis, 21 isolates retrieved from the CBS collection clustered with C. gigasporum, but showed considerable genetic variability, suggesting further species belonging to a previously unreported species complex.
The objectives of this study are to clarify the genetic and taxonomic relationships of Colletotrichum strains from various hosts and geographic areas thought to be closely related to C. gigasporum, and to describe the new species from this complex.
MATERIALS AND METHODS
Isolates
Colletotrichum isolates with large conidia were obtained from the culture collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands (CBS). All descriptions are based on ex-type cultures. Features of other strains are added if deviant. Cultures of additional isolates used for morphological and phylogenetic analyses are maintained in the CBS culture collection (Table 1).
Table 1.
Strains of Colletotrichum studied in this paper with details about host/substrate and location, and GenBank accessions of the sequences generated. Strains studied in this paper are in bold.
| Species | Accession number1 | Host / Substrate | Locality | GenBank accessions |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ITS | ACT | Tub2 | CHS-1 | GAPDH | HIS32 | CAL2 | GS2 | ||||
| C. acutatum | CBS 112996, ATCC 56816* | Carica sp. | Australia | JQ005776 | JQ005839 | JQ005860 | JQ005797 | JQ948677 | |||
| C. anthrisci | CBS 125334* | Anthriscus sylvestris | Netherlands | GU227845 | GU227943 | GU228139 | GU228335 | GU228237 | |||
| CBS 125335 | Anthriscus sylvestris | Netherlands | GU227845 | GU227943 | GU228139 | GU228335 | GU228237 | ||||
| C. arxii | CBS 169.59, IMI 304050, IMI 309371 | Oncidium excavatum | Netherlands | KF687717 | KF687784 | KF687868 | KF687781 | KF687824 | KF687846 | – | KF687740 |
| CBS 132511, Paphi 2-1* | Paphiopedilum sp. | Germany | KF687716 | KF687802 | KF687881 | KF687780 | KF687843 | KF687858 | KF687819 | KF687756 | |
| C. boninense | CBS 123755, MAFF 305972* | Crinum asiaticum var. sinicum | Japan | JQ005153 | JQ005501 | JQ005588 | JQ005327 | JQ005240 | |||
| CBS 128526 | Dacrycarpus dacrydioides, leaf endophyte | New Zealand | JQ005162 | JQ005510 | JQ005596 | JQ005336 | JQ005249 | ||||
| C. brevisporum | BCC 38876* | Neoregalia sp. | Thailand | JN050238 | JN050216 | JN050244 | KF687760 | JN050227 | |||
| MFLUCC 100182, BTL 23 | Pandanus pygmaeus | Thailand | JN050239 | JN050217 | JN050245 | – | JN050228 | ||||
| C. chlorophyti | IMI 103806* | Chlorophytum sp. | India | GU227894 | GU227992 | GU228188 | GU228384 | GU228286 | |||
| C. circinans | CBS 111.21 | Allium cepa | USA | GU227854 | GU227952 | GU228148 | GU228344 | GU228246 | |||
| CBS 221.81* | Allium cepa | Serbia | GU227855 | GU227953 | GU228149 | GU228345 | GU228247 | ||||
| C. cliviae | CBS 125375, CSSK4* | Clivia miniata | China | GQ485607 | GQ856777 | GQ849440 | GQ856722 | GQ856756 | |||
| C. coccodes | CBS 164.49 | Solanum tuberosum | Netherlands | JQ005775 | JQ005838 | JQ005859 | JQ005796 | HM171672 | |||
| CBS 369.75* | Solanum tuberosum | Netherlands | HM171679 | HM171667 | JX546873 | JX546681 | HM171673 | ||||
| C. dracaenophilum | CBS 118199* | Dracaena sanderana | China | JX519222 | JX519238 | JX519247 | JX519230 | JX546707 | |||
| C. fructi | CBS 346.37* | Malus sylvestris | USA | GU227844 | GU227942 | GU228138 | GU228334 | GU228236 | |||
| C. gigasporum | MAFF 242697 | Diospyros kaki | Japan | 242697_ITS3 | 242697_ACT3 | 242697_Tub23 | – | 242697_GAPDH3 | |||
| CBS 101881 | Solanum betaceum | New Zealand | KF687736 | KF687797 | KF687886 | KF687777 | KF687841 | KF687861 | KF687808 | KF687745 | |
| CBS 109355, FMR 6728 | Homo sapiens | Brazil | KF687729 | KF687798 | KF687870 | KF687774 | KF687827 | KF687848 | KF687809 | KF687746 | |
| CBS 124947 | Theobromae cacao | Panama | KF687731 | KF687786 | KF687871 | KF687763 | KF687828 | KF687849 | KF687810 | KF687747 | |
| CBS 125385, E2452 | Virola surinamensis | Panama | KF687732 | KF687787 | KF687872 | KF687764 | KF687835 | KF687850 | KF687811 | KF687748 | |
| CBS 125387, 4801 | Theobroma cacao | Panama | KF687733 | KF687788 | KF687873 | KF687765 | KF687834 | KF687851 | KF687812 | KF687749 | |
| CBS 125475, LD30a(T4) | Coffea sp. | Vietnam | KF687723 | KF687789 | KF687874 | KF687766 | KF687836 | KF687852 | KF687813 | KF687750 | |
| CBS 125476, LD35b(B2) | Coffea sp. | Vietnam | KF687728 | KF687790 | KF687875 | KF687767 | KF687833 | KF687853 | KF687814 | KF687751 | |
| CBS 125730, 3386 | Theobroma cacao | Panama | KF687735 | KF687793 | KF687878 | KF687770 | KF687840 | KF687856 | KF687817 | KF687754 | |
| CBS 125731, E1249 | Trichilia tuberculata | Panama | KF687727 | KF687794 | KF687879 | KF687771 | KF687837 | KF687857 | KF687818 | KF687755 | |
| CBS 132881, CPC 12084 | Acacia auriculiformis | Thailand | KF687725 | KF687795 | KF687880 | KF687772 | KF687829 | KF687859 | KF687820 | KF687757 | |
| CBS 132884, CPC 16323 | Musa sp. | Mexico | KF687730 | KF687796 | – | KF687773 | KF687830 | KF687860 | – | KF687737 | |
| CBS 133266, MUCL 44947* | Centella asiatica | Madagascar | KF687715 | – | KF687866 | KF687761 | KF687822 | KF687844 | – | – | |
| CBS 159.75 | air and stored grains | India | KF687726 | KF687783 | KF687884 | KF687776 | KF687839 | KF687863 | KF687821 | KF687739 | |
| CBS 181.52 | Theobroma cacao | East Africa | KF687734 | KF687799 | KF687885 | KF687775 | KF687838 | KF687862 | KF687805 | KF687741 | |
| (syn. C. thailandicum) | BCC 38879, LC0596, HR01MFU | Hibiscus rosa-sinensis | Thailand | JN050242 | JN050220 | JN050248 | KF687758 | JN050231 | |||
| MFLUCC 100192, LC0958, CMSP34 | Alocasia sp. | Thailand | JN050243 | JN050221 | JN050249 | KF687759 | JN050232 | ||||
| C. gloeosporioides | CBS 953.97* | Citrus sinensis | Italy | GQ485605 | GQ856782 | GQ849434 | GQ856733 | GQ856762 | |||
| CORCG5 | Vanda sp. | China | HM034809 | HM034801 | HM034811 | HM034805 | HM034807 | ||||
| C. graminicola | CBS 130836, M 1.001* | Zea mays | USA | JQ005767 | JQ005830 | JQ005851 | JQ005788 | – | |||
| C. karstii | CBS 132134, | ||||||||||
| CGMCC 3.14194* | Vanda sp. | China | HM585409 | HM581995 | HM585428 | HM582023 | HM585391 | ||||
| C. lindemuthianum | CBS 523.97 | Phaseolus coccineus | Costa Rica | JX546815 | JX546623 | JX546861 | JX546669 | JX546719 | |||
| CBS 144.31* | Phaseolus vulgaris | Germany | JQ005779 | JQ005842 | JQ005863 | JQ005800 | JX546712 | ||||
| C. lineola | CBS 125339 | Apiaceae | Czech Republic | GU227830 | GU227928 | GU228124 | GU228320 | GU228222 | |||
| CBS 125337* | Apiaceae | Czech Republic | GU227829 | GU227927 | GU228123 | GU228319 | GU228221 | ||||
| C. liriopes | CBS 122747 | Liriope muscari | Mexico | GU227805 | GU227903 | GU228099 | GU228295 | GU228197 | |||
| CBS 119444* | Liriope muscari | Mexico | GU227804 | GU227902 | GU228098 | GU228294 | GU228196 | ||||
| C. magnisporum | CBS 398.84* | unknown | unknown | KF687718 | KF687803 | KF687882 | KF687782 | KF687842 | KF687865 | – | KF687742 |
| C. nigrum | CBS 128507 | Capsicum annuum | New Zealand | JX546843 | JX546651 | JX546890 | JX546698 | JX546747 | |||
| CBS 169.49* | Capsicum sp. | Argentina | JX546838 | JX546646 | JX546885 | JX546693 | JX546742 | ||||
| C. oncidii | CBS 129828* | Oncidium sp., leaf | Germany | JQ005169 | JQ005517 | JQ005603 | JQ005343 | JQ005256 | |||
| CBS 130242 | Oncidium sp., leaf | Germany | JQ005170 | JQ005518 | JQ005604 | JQ005344 | JQ005257 | ||||
| C. pseudomajus | CBS 571.88* | Camellia sinensis | Taiwan | KF687722 | KF687801 | KF687883 | KF687779 | KF687826 | KF687864 | KF687807 | KF687744 |
| C. radicis | CBS 529.93* | unknown | Costa Rica | KF687719 | KF687785 | KF687869 | KF687762 | KF687825 | KF687847 | KF687806 | KF687743 |
| C. rusci | CBS 119206* | Ruscus sp. | Italy | GU227818 | GU227916 | GU228112 | GU228308 | GU228210 | |||
| C. sansevieriae | MAFF 239721* | Sansevieria trifasciata | Japan | AB212991 | 239721_ACT3 | 239721_Tub23 | – | 239721_GAPDH3 | |||
| MAFF 239175 | Sansevieria trifasciata | Japan | 239175_ITS3 | 239175_ACT3 | 239175_Tub23 | – | 239175_GAPDH3 | ||||
| C. simmondsii | CBS 130421, BRIP 28519* | Carica papaya | Australia | GU183331 | GQ849454 | GU183289 | GQ856735 | GQ856763 | |||
| C. tofieldiae | CBS 168.49 | Lupinus polyphyllus | Germany | GU227802 | GU227900 | GU228096 | GU228292 | GU228194 | |||
| CBS 495.85 | Tofieldia calyculata | Switzerland | GU227801 | GU227899 | GU228095 | GU228291 | GU228193 | ||||
| C. torulosum | CBS 102667 | Passiflora edulis, leaf blotch | New Zealand | JQ005165 | JQ005513 | JQ005599 | JQ005339 | JQ005252 | |||
| CBS 128544* | Solanum melongena | New Zealand | JQ005164 | JQ005512 | JQ005598 | JQ005338 | JQ005251 | ||||
| C. trichellum | CBS 217.64 | Hedera helix | Germany | GU227812 | GU227910 | GU228106 | GU228302 | GU228204 | |||
| CBS 118198 | Hedera sp. | UK | GU227813 | GU227911 | GU228107 | GU228303 | GU228205 | ||||
| C. tropicicola | BCC 38877, LC0598, L58* | Citrus maxima | Thailand | JN050240 | JN050218 | JN050246 | – | JN050229 | |||
| MFLUCC 100167, LC0957, BTL07 | Paphiopedilum bellatulum | Thailand | JN050241 | JN050219 | JN050247 | – | JN050230 | ||||
| C. truncatum | CBS 120709 | Capsicum frutescens | India | GU227877 | GU227975 | GU228171 | GU228367 | GU228269 | |||
| CBS 151.35* | Phaseolus lunatus | USA | GU227862 | GU227960 | GU228156 | GU228352 | GU228254 | ||||
| C. verruculosum | IMI 45525 | Crotalaria juncea | Zimbabwe | GU227806 | GU227904 | GU228100 | GU228296 | GU228198 | |||
| C. vietnamense | CBS 125477, BMT25(L3) | Coffea sp. | Vietnam | KF687720 | KF687791 | KF687876 | KF687768 | KF687831 | KF687854 | KF687815 | KF687752 |
| CBS 125478, LD16(L2)* | Coffea sp. | Vietnam | KF687721 | KF687792 | KF687877 | KF687769 | KF687832 | KF687855 | KF687816 | KF687753 | |
| C. yunnanense | CBS 132135, AS 3.9617* | Buxus sp. | China | JX546804 | JX519239 | JX519248 | JX519231 | JX546706 | |||
| Colletotrichum sp. CBS 159.50 | CBS 159.50 | Derris sp. | Indonesia | KF687724 | KF687800 | KF687867 | KF687778 | KF687823 | KF687845 | KF687804 | KF687738 |
| Monilochaetes infuscans | CBS 869.96* | Ipomoea batatas | South Africa | JQ005780 | JQ005843 | JQ005864 | JQ005801 | JX546612 | |||
1 AS, CGMCC: China General Microbiological Culture Collection; ATCC: American Type Culture Collection; BCC: BIOTEC Culture Collection, Thailand; BRIP: Plant Pathology Herbarium, Department of Employment, Economic, Development and Innovation, Queensland, Australia; CBS: Culture collection of the Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, Utrecht, the Netherlands; CPC: Working collection of Pedro W. Crous, housed at CBS, the Netherlands; ICMP: International Collection of Microorganisms from Plants, Auckland, New Zealand; IMI: Culture collection of CABI Europe UK Centre, Egham, UK; LC: Working collection of Lei Cai, housed at CAS, China; MAFF: MAFF Genebank Project, Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Japan; MFLUCC: Mae Fah Luang University Culture Collection, ChiangRai, Thailand; MUCL: BCCM/MUCL collection, Université catholique de Louvain, Belgium.
2 HIS3, CAL, GS genes were not used in multi-locus phylogenetic analysis.
3 sequencesdownloadedfromNIASGenBank (http://www.gene.affrc.go.jp/index_en.php)
* indicate ex-type strains.
Morphological analysis
To enhance sporulation, 5-mm-diam plugs from the margin of actively growing cultures were transferred to the centre of 9-cm-diam Petri dishes containing synthetic nutrient-poor agar medium (SNA) (Nirenberg 1976) amended with autoclaved filter paper and double-autoclaved stems of Anthriscus sylvestris placed onto the agar surface. Strains were also studied after growth on oatmeal agar (OA). Cultures were incubated for 10 d at 20 °C under near UV light with a 12 h photoperiod. Measurements and photographs of characteristic structures were made according to methods described by Liu et al. (2012). Appressoria on hyphae were observed on the reverse side of colonies grown on SNA plates. Microscopic preparations were made in clear lactic acid, with 30 measurements per structure, and observed with a Nikon Eclipse 80i microscope using differential interference contrast (DIC) illumination. Colony characters and pigment production on SNA and OA incubated at 20 °C were noted after 10 d. Colony colours were scored according to Rayner (1970). Growth rates were measured after 7 and 10 d.
Phylogenetic analyses
Genomic DNA of the isolates was extracted using the method of Damm et al. (2008). Eight loci including the 5.8S nuclear ribosomal gene with the two flanking internal transcribed spacers (ITS), a 200-bp intron of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a partial sequence of the actin (ACT), chitin synthase 1 (CHS-1), beta-tubulin (TUB2), calmodulin (CAL), glutamine synthetase (GS) and histon3 (HIS3) genes were amplified and sequenced using the primer pairs ITS1F (Gardes & Bruns 1993) + ITS4 (White et al. 1990), GDF1 + GDR1 (Guerber et al. 2003), ACT-512F + ACT-783R (Carbone & Kohn 1999), CHS-354R + CHS-79F (Carbone & Kohn 1999), T1 (O’Donnell & Cigelnik 1997) + Bt-2b (Glass & Donaldson 1995), CL1 + CL2A (O’Donnell et al. 2000), GSF1 + GSR1 (Stephenson et al. 1997) and CYLH3F + CYLH3R (Crous et al. 2004b), respectively. The PCR protocols were performed as described by Damm et al. (2009).
The DNA sequences obtained from forward and reverse primers were used to obtain consensus sequences using MEGA v. 5.1 (Tamura et al. 2011), and subsequent alignments were generated using MAFFT v. 6 (Katoh & Toh 2010), and manually edited using MEGA v. 5.1.
Sequences of the 21 Colletotrichum strains studied in this paper as well as sequences of 50 reference strains (Table 1) downloaded from GenBank (www.ncbi.nlm.nih.gov/genbank/) and NIAS GenBank (www.gene.affrc.go.jp/about_en.php) were included in individual alignments and eight single gene phylogenies were generated using a distance-based method. The ITS alignment included a further 22 sequences that were found in blastn searches in GenBank in addition to those in Table 1. Distance matrixes of the aligned sequences were calculated using the Kimura 2-parameter model (Kimura 1980), and analysed with the Neighbour-joining (NJ) algorithm (Saitou & Nei 1987) using MEGA v. 5.1, excluding positions with gaps. The reliability of the inferred trees was estimated by bootstrap analyses with 1 000 replicates.
A maximum parsimony analysis was performed on the multi-locus alignment including five of the eight loci (ACT, CHS-1, GAPDH, ITS, TUB2) of a total of 71 strains (Table 1) using PAUP v. 4.0b10 (Swofford 2002). Ambiguously aligned regions were excluded from all analyses. Unweighted parsimony (UP) analysis was performed. Trees were inferred using the heuristic search option with TBR branch swapping and 1 000 random sequence additions. Maxtrees were unlimited, branches of zero length were collapsed and all multiple parsimonious trees were saved. Clade stability was assessed in a bootstrap analysis with 1 000 replicates, each with 10 replicates of random stepwise addition of taxa. A second phylogenetic analysis of the concatenated alignment using a Markov Chain Monte Carlo (MCMC) algorithm was done to generate trees with Bayesian posterior probabilities in MrBayes v. 3.2.1 (Ronquist & Huelsenbeck 2003). Nucleotide substitution models were determined using MrModeltest v. 2.3 (Nylander 2004) for each gene region and included in the analyses. Two analyses of four MCMC chains were run from random trees for 10 000 000 generations and sampled every 1 000 generations. The first 25 % of trees were discarded as the burn-in phase of each analysis and posterior probabilities determined from the remaining trees. Monilochaetes infuscans strain CBS 869.96 was used as outgroup in all analyses. Sequences derived in this study were lodged in GenBank, the multi-locus alignment and tree in TreeBASE (http://www.treebase.org/treebase-web/search/studySearch.html) (S15175), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004a).
RESULTS
Phylogeny
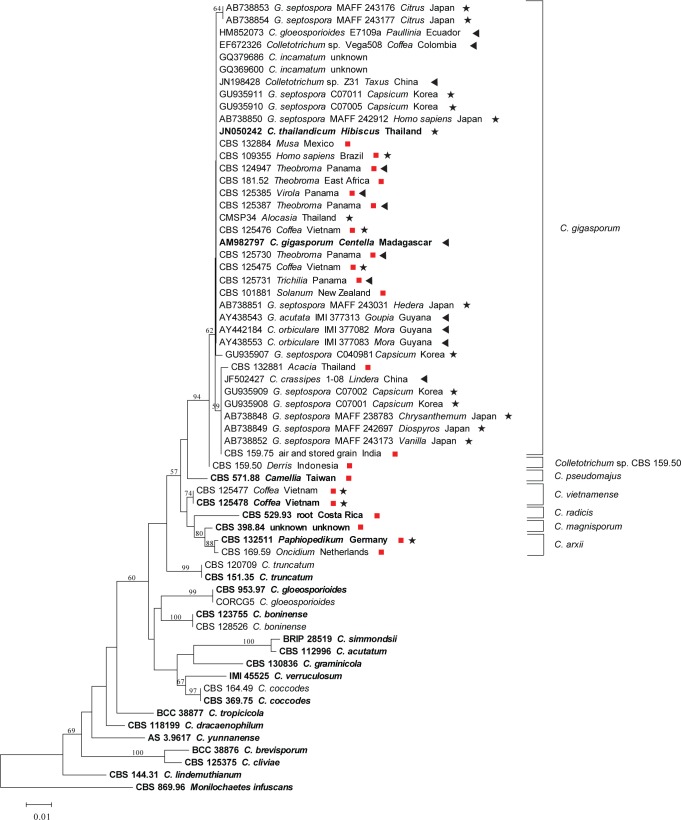
The eight NJ trees derived from the single gene sequence alignments (ACT, CAL, CHS-1, GAPDH, GS, HIS3, ITS, TUB2) confirmed that the 21 CBS isolates and the ex-type and other strains of C. gigasporum constituted a monophyletic lineage, distant from other known major clades of the genus Colletotrichum recognised by Cannon et al. (2012). The NJ trees are not shown in this study except for the phylogeny based on ITS data (Fig. 1). Isolates studied in this paper (marked with red squares) are separated into seven subclades, which could also be confirmed with the other seven single gene phylogenies.
Fig. 1.
Neighbour-joining tree of ITS sequences from 21 isolates generated in this study and 43 isolates from other studies, retrieved from GenBank. The tree was constructed using MEGA v.5.1 software. The Kimura-2-parameter method was used. Bootstrap support values (1 000 replicates) above 50 % are shown at the nodes. Ex-type cultures are emphasised in bold, and include the taxonomic name as originally described. Our isolates are marked with a red square, and the strain number is followed by host and country of origin. Stars indicate reported pathogens, triangles indicate reported endophytes, GenBank accessions are followed by taxonomic name as originally identified, strain number, host and country of origin. The tree is rooted with Monilochaetes infuscans.
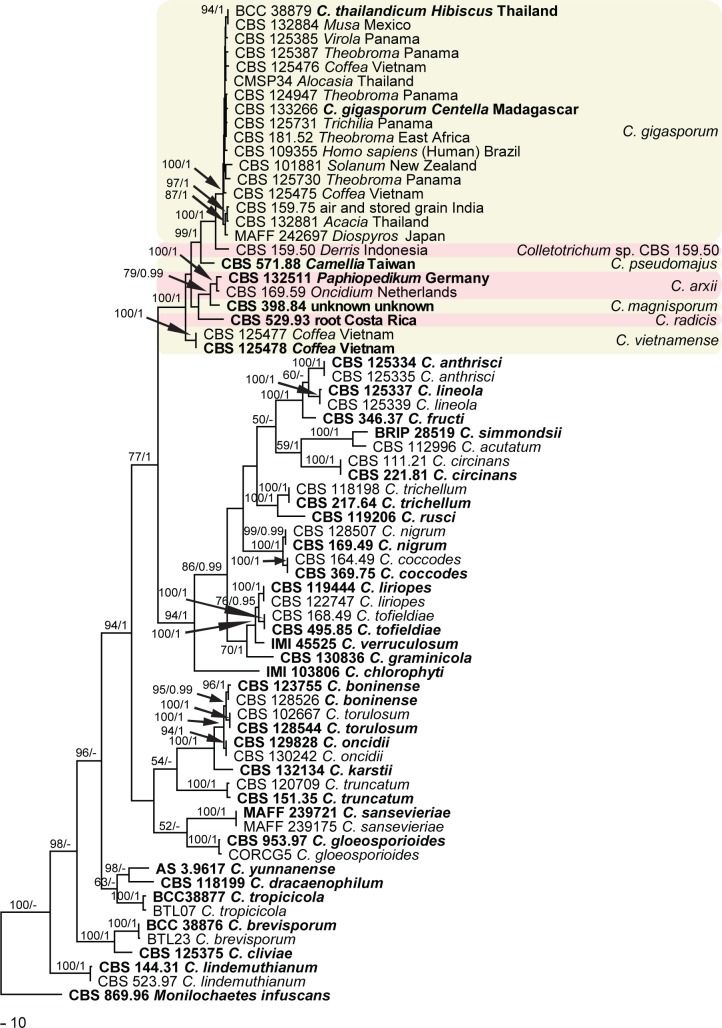
The multi-locus phylogenetic analysis included 70 ingroup strains, with Monilochaetes infuscans (CBS 869.96) as outgroup. The dataset of five loci comprised 1 512 characters including the alignment gaps, of which 699 characters were parsimony-informative, 85 parsimony-uninformative and 728 constant. Parsimony analysis resulted in 94 most parsimonious trees, one of them (length = 3417, CI = 0.438, RI = 0.798, RC = 0.349, HI = 0.562) is shown in Fig. 2, where the 21 strains studied belong to a major clade consisting of seven subclades. More than half of the strains clustered in the largest subclade (C. gigasporum) with a high bootstrap support and Bayesian posterior probability value (100/1.00). The Bayesian tree confirmed the tree topology of the trees obtained with maximum parsimony.
Fig. 2.
One of 206 most parsimonious trees obtained from a heuristic search of combined ACT, CHS-1, GAPDH, ITS and TUB2 gene sequences of Colletotrichum species. Bootstrap support values (1 000 replicates) above 50 % and Bayesian posterior probability values above 0.95 are shown at the nodes. Numbers of ex-type strains are emphasised in bold. Strain numbers studied are followed by host and country of origin. The tree is rooted with Monilochaetes infuscans.
Taxonomy
Based on the results of the single and multi-locus phylograms, we accept seven species within the C. gigasporum species complex, including six species that are new to science. In addition, two recently described species are shown to be synonymous. All novel species are characterised and illustrated below except for a species which is represented by a single strain, CBS 159.50. Since this strain is sterile, we designate it as Colletotrichum sp. CBS 159.50.
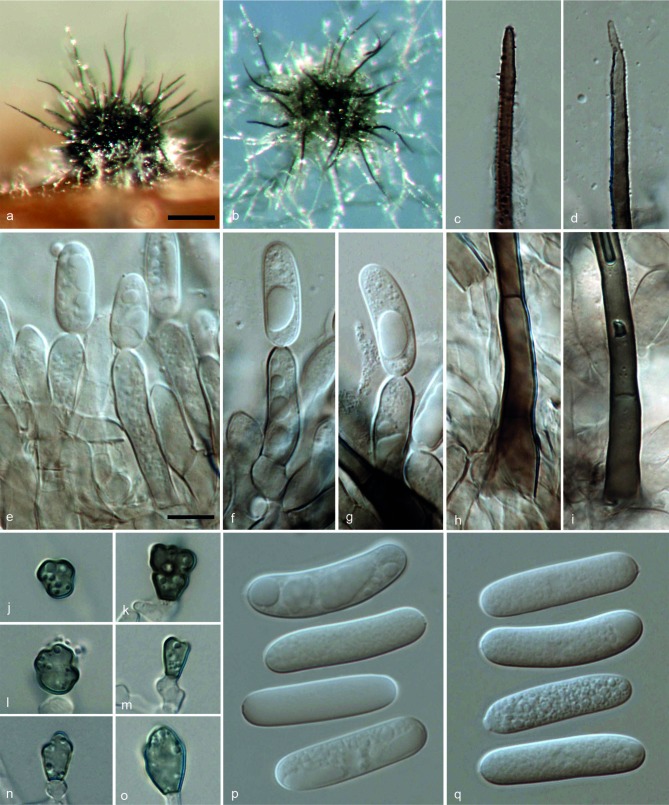
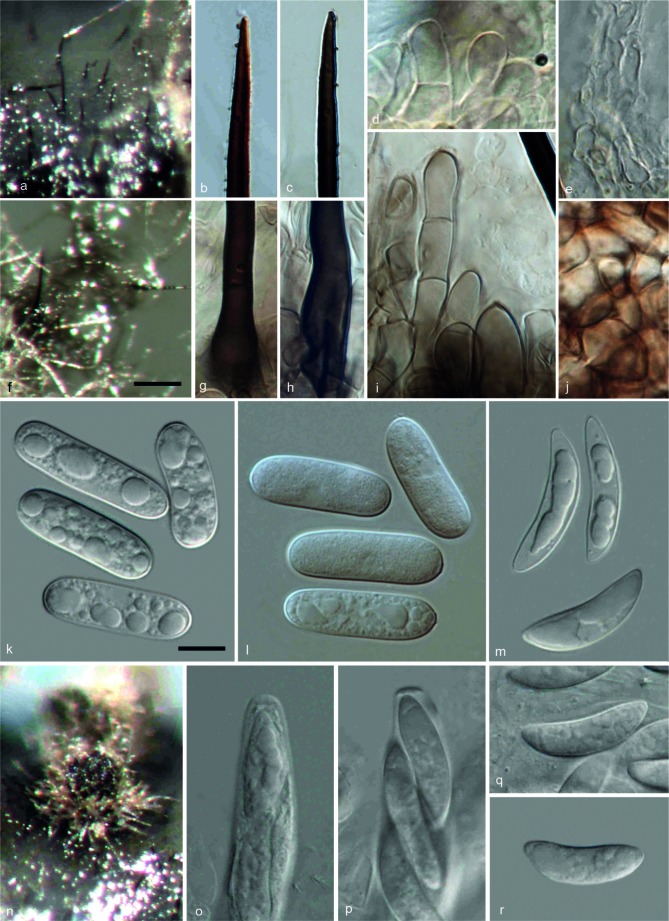
ColletotrichumarxiiF. Liu, L. Cai, Crous & Damm, sp. nov. — MycoBank MB807164; Fig. 3
Fig. 3.
Colletotrichum arxii (CBS 132511). a, b. Acervuli; c, d. tips of setae; e–g. conidiophores; h, i. basal parts of setae; j–o. appressoria; p, q. conidia (a, d, f–g, i, q: from Anthriscus stem; b, c, e, h, j–p: from SNA. – a, b: DM; c–q: DIC). — Scale bars: a = 100 μm (applies to a, b); e = 10 μm (applies to c–q).
Etymology. Named after Josef Adolf von Arx for his very substantial contribution to the classification of the genus Colletotrichum.
On Anthriscus stem. Vegetative hyphae hyaline, smooth-walled, septate, branched. Conidiomata acervular, conidiophores and setae formed on a cushion of roundish to angular brown cells. Setae pale to medium brown, smooth-walled to verruculose, 1–5-septate, 80–260 μm long, base cylindrical, 3.5–6 μm diam, tip acute to obtuse. Conidiophores pale brown, septate, branched. Conidiogenous cells pale brown, cylindrical to clavate, 17.5–24 × 5–7 μm, opening 1–2.5 μm diam. Conidia hyaline, aseptate, smooth-walled, cylindrical to slightly curved, both ends rounded, 21–32 × 5.5–7.5 μm, av. ± SD = 28.1 ± 2.6 × 6.8 ± 0.5 μm, L/W ratio = 4.1; the other isolate CBS 169.59 forms relatively shorter conidia, 20–26.5 × 5.5–7.5 μm, av. ± SD = 23.1 ± 2 × 6.4 ± 0.5 μm, L/W ratio = 3.6.
On SNA. Vegetative hyphae hyaline to medium brown, smooth-walled, septate, branched. Conidiomata acervular. Setae pale to medium brown, smooth-walled to verruculose, 1–3-septate, 120–180 μm long, base cylindrical to inflated, 4.5–7.5 μm diam, tip acute. Conidiophores hyaline to pale brown, septate, branched. Conidiogenous cells hyaline to pale brown, cylindrical to clavate, 10–21.5 × 5.5–7.5 μm, opening 1.5–3 μm diam. Conidia hyaline, aseptate, smooth-walled, cylindrical to slightly curved, both ends rounded, (20–)24.5–30 × 5.5–7.5 μm, av. ± SD = 27.0 ± 1.8 × 6.7 ± 0.5 μm, L/W ratio = 4; the other isolate CBS 169.59 forms relatively shorter conidia, 15.5–24 × 5–7.5 μm, av. ± SD = 21.4 ± 2 × 6.3 ± 0.5 μm, L/W ratio = 3.4. Appressoria (few observed) pale brown, aseptate, solitary, with a ellipsoidal to irregular outline and a crenate or lobed margin, 4–11.5 × 4–9 μm, av. ± SD = 8.5 ± 2.5 × 6.0 ± 1.5 μm, L/W ratio = 1.4.
Culture characteristics — Colonies on OA flat with undulate margin, surface white, aerial mycelium lacking; reverse white; colonial diam 54–63 mm in 7 d, > 90 mm in 10 d. Colonies on SNA flat with erose or dentate margin, medium hyaline, buff around Anthriscus stem, aerial mycelium lacking; colonial diam 68–77 mm in 7 d, > 90 mm in 10 d.
Specimens examined. GERMANY, Berlin, glasshouse, on living leaves of Paphiopedilum sp., Dec. 2010, U. Damm (holotype CBS H-21492, culture ex-type CBS 132511 = Paphi 2-1). – NETHERLANDS, Baarn, Cantonspark, on Oncidium excavatum, unknown collection date and collector (isolated by J.A. von Arx in 1956), culture CBS 169.59 = IMI 304050 = IMI 309371.
Notes — Although there are many Colletotrichum speciesreported from orchids, which include C. boninense (s.lat.), C. cinctum, C. cliviae, C. crassipes, C. cymbidiicola, C. gloeoporioides (s.lat.), C. liriopes, C. lujae, C. macrosporum, C. oncidii, C. orchidearum, C. orchidophilum, C. siamense, C. stanhopeae, C. vanillae (Stoneman 1898, Allescher 1902, Patel et al. 1953, von Arx 1957, Sutton 1980, Li 1999, Moriwaki et al. 2003, Talubnak & Soytong 2010, Yang et al. 2011, Damm et al. 2012a), C. arxii can be distinguished from these species either from phylogenetic data or morphological characteristics. Colletotrichum arxii is phylogenetically distinct from the C. acutatum, C. boniense and C. gloeosporioides complexes, as well as C. cliviae and C. liriopes (Fig. 2), and could be morphologically distinguished from the other species that presently still lack molecular data.
Colletotrichum arxii differs from C. macrosporum, a species from an orchid from Brazil, by forming narrower conidia (C. macrosporum 28–32 × 8–10 μm) (Saccardo 1896). Although C. orchidearum was originally described by Allescher (1902) from Munich, Germany, the same location as our strain CBS 132511, they can be differentiated from each other based on conidial size, with C. arxii forming significantly longer conidia than C. orchidearum (C. orchidearum (13.5–)15.5–19.5 × 5–6 μm, av. ± SD = 17.2 ± 1.6 × 5.5 ± 0.3 μm) (Damm et al. 2012a).
Colletotrichum cinctum (Berk. & M.A. Curtis) Stoneman was originally described from orchids, Oncidium sp. and Maxillaria sp. (Stoneman 1898) and also identified from Paphiopedilum insigne (specimen BPI 397219) in the USA (collected by J. Rubinger on 14 July 1921, unpubl.). Colletotrichum stanhopeae was described from Stanhopea sp. in Brazil (Hennings 1908), C. vanillae from Vanilla odorata in Italy (Saccardo 1906) and C. lujae from Luja in Belgium (Verplancke 1935). However, the conidia of these four species, C. cinctum (12–15 × 3–4 μm), C. stanhopeae (10–16 × 3.5–4 μm), C. vanillae (18–21 × 5.5–7 μm), C. lujae (9.3–10.5 × 2–3.1 μm) are significantly smaller than those of C. arxii (20–30 × 5.5–7.5 μm).
Closest match in a blastn search with the ITS sequence of strain CBS 132511 (with 99 % identity, 8 bp differences) was an endophytic isolate (DQ780412) from Magnolia liliifera probably in Thailand (Promputtha et al. 2007) and an endophytic isolate (FJ205460) from an orchid in Taiwan (Wang et al. unpubl. data). The closest match with the TUB2 sequence (with 97 % identity, 16 bp differences) was isolate MUCL 41702 from Orchis in Singapore (FN599826; Rakotoniriana & Munaut, unpubl. data).
Colletotrichum gigasporumE.F. Rakotoniriana & Munaut, Mycol. Progr. 12: 407. 2013
= Colletotrichum thailandicum Phouliv., Noireung, L. Cai & K.D. Hyde, Cryptog. Mycol. 33: 354. 2012.
Notes — Colletotrichum gigasporum is characterised by large conidia ((22–)25–29(–32) × (6–)7–9 μm). Phylogenetic analyses by Rakotoniriana et al. (2013) based on the ITS and TUB2 sequences placed it in a distinct clade far from the currently accepted Colletotrichum species. Another species with large conidia (27–30 × 9–10 μm), C. thailandicum, was described from diseased Alocasia sp. and Hibiscus rosa-sinensis from Thailand (Noireung et al. 2012). Colletotrichum thailandicum is morphologically similar to C. gigasporum; the ITS and β-tubulin sequences of both fungi are identical or near-identical (differed in two nucleotide position in β-tubulin). In addition, phylogenetic analyses of single locus data, including ITS (Fig. 1), and multi-locus data (Fig. 2), show that the ex-type strains of the two species cluster together in one strongly supported clade. Since C. gigasporum was published online earlier (8 August 2012) than C. thailandicum (September 2012), we regard C. thailandicum as a synonym of C. gigasporum.
Strain CBS 109355, isolated from a phaeohyphomycotic cyst from a Brazilian man, was originally identified as C. crassipes, mainly based on morphology of the appressoria with crenate or deeply lobed margins and its size of conidia (Castro et al. 2001). In addition, strains CBS 159.75 and IMI 302450, which were deposited as C. crassipes in the CBS and IMI culture collections, were compared morphologically with CBS 109355 by Castro et al. (2001). However, strains CBS 159.75 and CBS 109355 were reidentified as C. gigasporum in the present study (Fig. 2). Hitherto, the taxonomic status of C. crassipes as well as the genetic relationship between C. gigasporum and C. crassipes remain unclear due to the lack of an ex-type culture and DNA sequence data. Thus, an epitype is needed to stabilise the nomenclature of C. crassipes.
In addition to being a disease-causing agent of humans, C. gigasporum is also associated with Musa sp. (Fig. 1, 2), the anthracnose of which is commonly considered to be caused by C. musae that belongs to the C. gloeosporioides species complex (Weir et al. 2012).However, C. gigasporum is phylogenetically distinct from C. musae, and its conidia are significantly larger than those of C. musae. Additional Colletotrichum species associated with Musa spp. include C. cavendishii, C. liukiuensis and C. paxtonii. Colletotrichum gigasporum differs from C. liukiuensis (Sawada 1959), a species on leaves of M. liukiuensis in Taiwan, and C. cavendishii (Petrak 1925), a species on living leaves of M. cavendishii by producing larger conidia (20.5–25.5 × 6–9 μm vs 12–14 × 4.8–5.5 μm and 10–19 × 4.5–7 μm, respectively). Colletotrichum paxtonii, a species associated with banana in St. Lucia, belongs to the C. acutatum complex (Johnston & Jones 1997, Damm et al. 2012a) and is therefore not closely related to C. gigasporum.
Our 5-locus phylogram shows that several strains from diverse countries and hosts cluster with C. gigasporum (syn. C. thailandicum). Based on our blastn search in GenBank, the results of which are included in the ITS phylogeny, 22 additional ITS sequences from GenBank cluster with the ex-type strain of C. gigasporum,including sequences derived from strains isolated from plants as endophytes or pathogens and even strains that were isolated from human tissue (Fig. 1). This is in accordance with the conjecture that ecologically C. gigasporum can occur as either endophyte or pathogen (Rakotoniriana et al. 2013). The isolates from which most of these GenBank sequences were generated had been previously identified as C. crassipes, C. gloeosporioides, C. incarnatum, C. orbiculare or C. taiwanense (sexual morph Glomerella septospora) (Fig. 1).
The ascospores and conidia of C. gigasporum resemble those of C. taiwanense with respect to their size. However, C. gigasporum produces aseptate conidia and 0–1-septate ascospores (Rakotoniriana et al. 2013), while the conidia of C. taiwanense may become 1–5-septate with age and ascospores are mostly 3-septate and may become up to 6- or 8-septate when old (Sivanesan & Hsieh 1993). Colletotrichum taiwanense, originally described from Styrax formosanus in Taiwan, is currently poorly characterised using molecular methods (Hyde et al. 2009, Cannon et al. 2012). Unfortunately, a subculture from the ex-type isolate of C. taiwanense (IMI 353024) is contaminated; the original strain could not be recovered. Several plant pathogenic strains from various hosts (none of them from Styrax) that were previously identified as C. taiwanense were reidentified as C. gigasporum based on the ITS-rDNA phylogram in this study (Fig. 1). Colletotrichum gigasporum differs from C. incarnatum (Zimmermann 1901), a species first described from Coffea liberica in Java, by producing larger conidia (20.5–25.5 × 6–9 μm vs 14–19 × 5 μm).
Some strains from Mora excelsa in Guyana had been previously identified as C. orbiculare (Lu et al. 2004) and grouped with C. gigasporum in our ITS tree. However, C. orbiculare was recently redefined and shown to belong to a different species complex together with C. lindemuthianum (Damm et al. 2013).
Although the ITS-rDNA phylogram revealed that C. gigasporum strains formed two subclades (Fig. 1), the bootstrap values are too low to support two distinct species, which could also be verified by the multi-locus phylogram (Fig. 2).
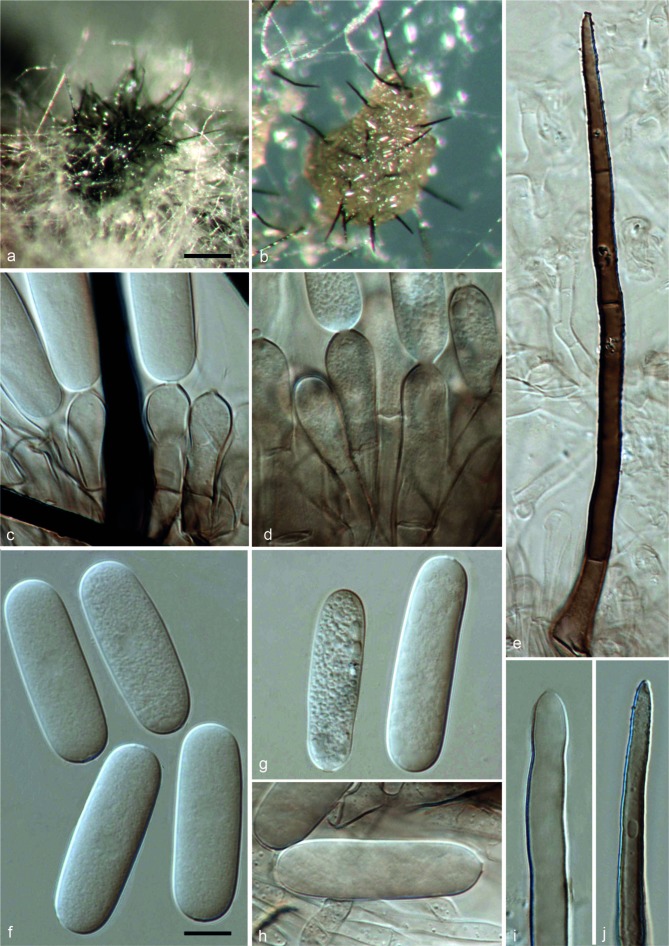
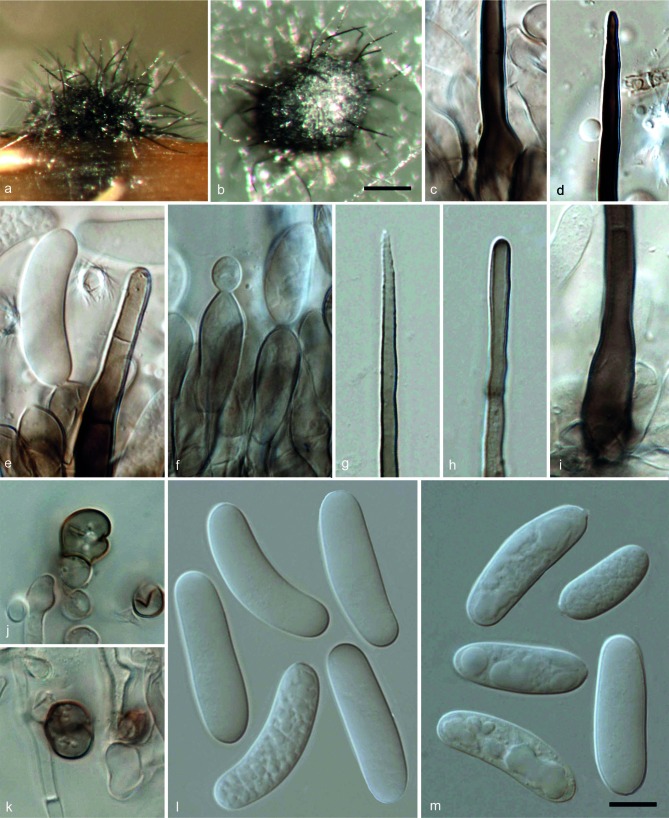
Colletotrichum magnisporumF. Liu, L. Cai, Crous & Damm, sp. nov. — MycoBank MB807163; Fig. 4
Fig. 4.
Colletotrichum magnisporum (CBS 398.84). a, b. Acervuli; c, d. conidiophores; e, i, j. setae; f–h. conidia (a, d, g–j: from Anthriscus stem; b, c, e, f: from SNA. – a, b: DM; c–m: DIC). — Scale bars: a = 100 μm (applies to a, b); f = 10 μm (applies to c–j).
Etymology. Referring to the large size of its conidia.
On Anthriscus stem. Vegetative hyphae hyaline to brown, smooth-walled, septate, branched. Conidiomata acervular, conidiophores and setae formed on a cushion of angular brown cells. Setae medium to dark brown, smooth-walled to verruculose, 0–4-septate, 42.5–105 μm long, base cylindrical to inflated, 5.5–11.5 μm diam, tip acute to obtuse. Conidiophores hyaline to brown, septate, branched. Conidiogenous cells hyaline to medium brown, cylindrical or clavate, 18–33.5 × 5.5–10 μm, opening 1.5–2.5 μm diam. Conidia hyaline, aseptate, smooth-walled, cylindrical with rounded ends, 28–39 × 8.5–10.5 μm, av. ± SD = 33.8 ± 4.1 × 9.9 ± 0.6 μm, L/W ratio = 3.4.
On SNA. Vegetative hyphae hyaline to medium brown, smooth-walled, septate, branched. Conidiomata acervular. Setae medium to dark brown, smooth-walled to verruculose, 1–4-septate, 91.5–230.5 μm long, base cylindrical to inflated, 5–12.5 μm diam, tip ± acute. Conidiophores hyaline to medium brown, septate, branched. Conidiogenous cells hyaline to pale brown, cylindrical to clavate, 17.5–26.5 × 7.5–9.5 μm, opening 1.5–2.5 μm diam. Conidia hyaline, aseptate, smooth-walled, cylindrical with rounded ends, 28.5–40.5 × 8.5–11 μm, av. ± SD = 34.3 ± 2.7 × 9.7 ± 0.5 μm, L/W ratio = 3.5. Appressoria not observed.
Culture characteristics — Colonies on OA flat with entire margin, surface iron-grey with a white margin, aerial mycelium lacking; reverse olivaceous-grey to iron-grey; colonial diam 56–60 mm in 7 d, > 90 mm in 10 d. Colonies on SNA flat with entire margin, medium hyaline, buff around Anthriscus stem, aerial mycelium lacking; colonial diam 64–65 mm in 7 d, > 90 mm in 10 d.
Specimen examined. Unknown collection details (deposited in CBS culture collection in June 1984) (holotype CBS H-21491, culture ex-type CBS 398.84).
Notes — Although C. magnisporum is represented by only a single strain in this study, it could be distinguished from the related species C. arxii based on its phylogenetic distance (Fig. 2) and its morphology. The two species differ by 40 bp differences in five genes totally, as well as a long insertion (174 bp) in GAPDH sequences in C. arxii that is missing in C. magnisporum. In addition, the conidia of C. arxii (24.5–30 × 5.5–7.5 μm, av. = 27 × 6.7 μm) are shorter and narrower than C. magnisporum (28.5–40.5 × 8.5–11 μm, av. = 34.3 × 9.7 μm).For other comments see C. radicis.
The closest matches in a blastn search in GenBank with the ITS sequence of strain CBS 398.84 were with 100 % identity EF672323 from the endophytic isolate VegaE4-36 from Coffea arabica from Hawaii, USA (Vega et al. 2010), EU686812 from an endophytic isolate from Rhipidocladum racemiflorum from Panama (Higgins et al. 2011), as well as KF436311 from the endophytic isolate TK780 from a tropical woody plant from Panama (Higginbotham et al. 2013). The closest match with the TUB2 sequence (with 96 % identity, 16 bp differences) was isolate MUCL 41702 from Orchis in Singapore (FN599826; Rakotoniriana & Munaut unpubl. data).
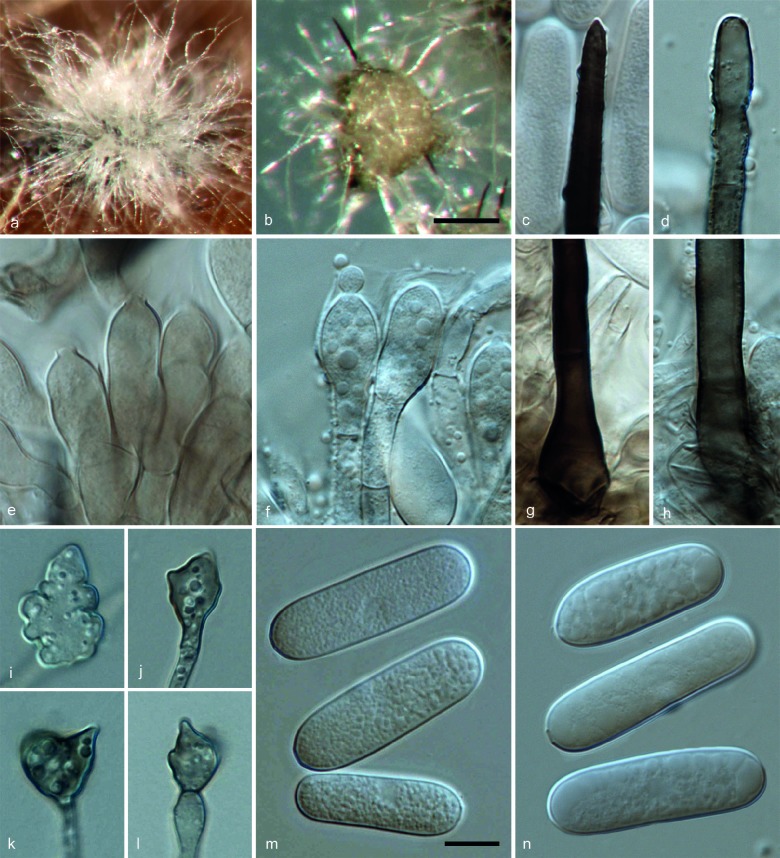
Colletotrichum pseudomajusF. Liu, L. Cai, Crous & Damm, sp. nov. — MycoBank MB807165; Fig. 5
Fig. 5.
Colletotrichum pseudomajus (CBS 571.88). a, f. Acervuli; b, c. tips of setae; d, i. conidiophores; e. paraphyses; g, h. basal parts of setae; j. outer surface of peridium; k, l. conidia; m, q, r. ascospores; n. ascomata; o, p. asci (a, b, d, e, g, j, k, m, n, p: from OA; c, f, h, i, l, o, q, r: from SNA. – a, f, n: DM; b–e, g–m, o–r: DIC). — Scale bars: f = 100 μm (applies to a, f, n); k = 10 μm (applies to b–e, g–m, o–r).
Etymology. Referring to its morphology, which resembles that of Glomerella major.
On OA. Vegetative hyphae medium brown, smooth-walled, septate, branched. Conidiomata acervular, conidiophores and setae formed on a cushion of roundish brown cells. Setae medium to dark brown, smooth-walled to verruculose, 0–3-septate, 100–215 μm long, base inflated to cylindrical, 4–8 μm diam, tip acute. Conidiophores hyaline to medium brown, septate, branched. Conidiogenous cells hyaline to pale brown, cylindrical to clavate, 12–18 × 4–8 μm, opening 1.5–2 μm diam. Conidia hyaline, aseptate, smooth-walled, cylindrical with rounded ends, occasionally slightly curved, 21.5–27 × 6–9 μm, av. ± SD = 24.3 ± 1.5 × 7.8 ± 0.6 μm, L/W ratio = 3.1.
Sexual morph developed on OA. Ascomata globose, sometimes subconical, black, surrounded with brown hairs, 95–165 μm diam, ostiolate; neck, when present, 35–60 μm long; outer wall composed of angular brown cells, 6–20 μm diam. Interascal tissue composed of paraphyses, thin-walled, hyaline, septate, the apex rounded. Asci cylindrical, 93–123.5 × 10.5–12.5 μm, 8-spored. Ascospores uni- or biseriately arranged, hyaline, aseptate, smooth-walled, lunate, tip ± acute, 20–27.5 × 5–7 μm, av. ± SD = 24.2 ± 1.6 × 6.2 ± 0.4 μm, L/W ratio = 3.9.
On Anthriscus stem. Remaining sterile.
On SNA. Vegetative hyphae hyaline to medium brown, smooth-walled, septate, branched. Conidiomata acervular. Setae dark brown, smooth-walled to verruculose, 0–3-septate, 125–190 μm long, base cylindrical to inflated, 5.5–8 μm diam, tip acute. Conidiophores pale brown, septate, branched. Conidiogenous cells pale brown, cylindrical, clavate to bullet-shaped, 14.5–18 × 4–8 μm, opening 1.5–2 μm diam. Conidia hyaline, aseptate, smooth-walled, cylindrical with rounded ends, 22–30.5 × 6.5–9.5 μm, av. ± SD = 26.3 ± 1.7 × 8.1 ± 0.5 μm, L/W ratio = 3.2. Appressoria not observed.
Sexual morph developed on SNA. Ascomata globose, subconical to obpyriform, black, surrounded with hyaline to medium brown hairs, 260–360 μm diam, ostiolate; neck when present, 60–200 μm long; outer wall composed of angular brown cells, 5–15 μm diam. Interascal tissue composed of paraphyses, thin-walled, hyaline, septate, the apex rounded. Asci cylindrical, 73.5–98.5 × 10–12.5 μm, 8-spored. Ascospores uni- or biseriately arranged, hyaline, aseptate, smooth-walled, lunate, tip ± acute, 18.5–25 × 4.5–7.5 μm, av. ± SD = 21.2 ± 1.5 × 6.0 ± 0.7 μm, L/W ratio = 3.5.
Culture characteristics — Colonies on OA umbonate with entire margin, surface iron-grey to greenish black, white aerial mycelium; reverse olivaceous-grey; colonial diam 42–45 mm in 7 d, 65–68 mm in 10 d. Colonies on SNA flat with entire margin, medium hyaline; colonial diam 40–47 mm in 7 d, 66–74 mm in 10 d.
Specimen examined. TAIWAN, on twig of Camellia sinensis, unknown collection date and collector (isolated by J. Chen) (holotype CBS H-21493, culture ex-type CBS 571.88).
Notes — Several Colletotrichum species have been reported from tea plants, which include C. camelliae described on living leaves of tea plants (Camellia sinensis) from Sri Lanka (Massee 1899), Glomerella cingulata f. sp. camelliae described from ornamental camellia from New Zealand (Dickens & Cook 1989) and Glomerella major described from healthy wood in the vicinity of rotting lesions on Camellia sinensis from North-East India (Tunstall 1934).
Weir et al. (2012) clarified the taxonomic status of G. cingulata f. sp. camelliae based on molecular analysis and pathogenicity tests, showing it to belong to the C. gloeosporioides complex. The phylogenetic analysis shows that strain CBS 571.88 (here referred as C. pseudomajus) is phylogenetically distinct from the C. gloeosporioides complex. Additionally, C. pseudomajus differs from G. cingulata f. sp. camelliae in producing much larger conidia and ascospores (C. pseudomajus: conidia 22–30.5 × 6.5–9.5 μm and ascospores 18.5–25 × 4.5–7.5 μm vs G. cingulata f. sp. camelliae: conidia 11.3–21.8 × 3.5–6.9 μm and ascospores 10–13 × 3.5–4.5 μm) (Dickens & Cook 1989).
The name C. camelliae, although not listed by Hyde et al. (2009) and Cannon et al. (2012), is widely used for the causal agent of the brown blight disease of tea (Sosa de Castro et al. 2001, Muraleedharan & Baby 2007). However, the status of C. camelliae and its taxonomic relationship with G. cingulata f. sp. camelliae remain unresolved (Weir et al. 2012). There are 11 ITS sequences of Colletotrichum sp. from tea in GenBank (EF063686, FJ515007, EU732732, FJ216456, HQ832797, JQ809665, HQ832801, AB548281, AB218993, GQ916544, HE655519), of which sequence HQ832801 associated strain nested within the C. boninense complex in the ITS phylogenetic tree, while the others belong to several clades within the C. gloeoporioides complex (data not shown). Appropriate fresh collections associated with brown blight symptoms of tea from Sri Lanka are needed for epitypification to clarify the phylogenetic relationships of this taxon. Colletotrichum pseudomajus can be distinguished from C. camelliae by its significantly larger conidia (22–30.5 × 6.5–9.5 μm vs 15–17 × 4–5 μm).
Colletotrichum pseudomajus is morphologically similar to G. major except for the presence of paraphyses and the shape of its ascospores. Paraphyses were reported to be absent in G. major, but thin-walled, hyaline and septate paraphyses are present in C. pseudomajus; ascospores of G. major are ellipsoid, not allantoid, with obtuse or subacute tips (Tunstall 1935), while those of C. pseudomajus are lunate, with more or less acute tips (Fig. 5). Currently, the phylogenetic position of G. major is unresolved due to the lack of an ex-type isolate. Thus, an epitype is needed to stabilise the nomenclature of G. major and to clarify the relationship between C. pseudomajus and G. major.
The closest matches in a blastn search with the ITS sequence of CBS 571.88 with 100 % identity were JX009424, the sequence generated from the same isolate by Weir et al. (2012), and JQ809667 from the endophytic isolate JD08-18 from Camellia sinensis in China (Fang et al. 2013), as well as JN418782 from the endophytic isolate E10202g from Otoba parvifolia in Ecuador (Barba et al. unpubl. data). Closest match with the TUB2 sequence (with 93 % identity, 32 bp differences) was isolate MUCL 41702 from Orchis in Singapore (FN599826; Rakotoniriana & Munaut unpubl. data). The blastn search with the GAPDH sequence of CBS 571.88 showed similarity with JN050231 (85 % identity, 34 bp differences) from isolate BCC 38879 from Hibiscus rosa-sinensis in Thailand (Noireung et al. 2012) which is here referred to C. gigasporum, and JX009422 (99 % identity, 1 bp difference), a sequence generated from the same isolate. The only base difference in the end of the sequence was due to sequencing error by Weir et al. (2012).
ColletotrichumradicisF. Liu, L. Cai, Crous & Damm, sp. nov. — MycoBank MB807166; Fig. 6
Fig. 6.
Colletotrichum radicis (CBS 529.93). a, b. Acervuli; c, i. basal parts of setae; d, g, h. tips of setae; e. conidiogenous cells with conidia; f. conidiophores; j, k. appressoria-like structures; l, m. conidia (a, f–i, m: from Anthriscus stem; b–e, j–l: from SNA. – a, b: DM; c–m: DIC). — Scale bars: b = 100 μm (applies to a, b); m = 10 μm (applies to c–m).
Etymology. Referring to the host organ, a plant root, from which it was isolated.
On Anthriscus stem. Vegetative hyphae hyaline to medium brown, smooth-walled, septate, branched. Conidiomata acervular, conidiophores and setae formed on a cushion of angular brown cells. Setae brown, smooth-walled, 0–3-septate, 77–192 μm long, base cylindrical to inflated, 5.5–6.5 μm diam, tip acute to obtuse. Conidiophores hyaline to brown, septate, branched. Conidiogenous cells hyaline to medium brown, cylindrical to clavate, 14–23 × 5.5–8.5 μm, opening 1.5–2 μm diam. Conidia hyaline, aseptate, smooth-walled, cylindrical to slightly curved, both ends rounded, 15.5–28 × 5.5–9.5 μm, av. ± SD = 22.6 ± 3.4 × 7.8 ± 0.7 μm, L/W ratio = 2.9.
On SNA. Vegetative hyphae hyaline to medium brown, smooth-walled, septate, branched. Chlamydospores not observed (but see below). Conidiomata acervular. Setae medium to dark brown, smooth-walled, 0–3-septate, 43–230 μm long, base cylindrical to inflated, 3.5–8.5 μm diam, tip acute to obtuse. Conidiophores brown, septate, branched. Conidiogenous cells medium brown, cylindrical to clavate, 11.5–24 × 5–9 μm, opening 1–2.5 μm diam. Conidia hyaline, aseptate, smooth-walled, cylindrical to slightly curved, 25.5–32.5 × 6.5–9.5 μm, av. ± SD = 28.2 ± 1.7 × 7.9 ± 0.6 μm, L/W ratio = 3.6. Appressoria not observed on the undersurface of the medium, but in old cultures appressoria-like structures that possibly function as chlamydospores were observed within the medium; these are single or in small dense clusters, light to medium brown, smooth-walled, globose, subglobose, elliptical to clavate in outline, with an entire or undulate margin, 4–8.5 μm diam.
Culture characteristics — Colonies on OA flat with entire margin, aerial mycelium lacking; colonial diam 64–71 mm in 7 d, > 90 mm in 10 d. Colonies on SNA flat with entire margin, aerial mycelium lacking, medium hyaline, buff around Anthriscus stem; colonial diam 64–75 mm in 7 d, > 90 mm in 10 d.
Specimen examined. COSTA RICA, La Selva, host plant unknown (isolated from a plant root), unknown collection date and collector (isolated by G. Weber in Mar. 1993) (holotype CBS H-21494, culture ex-type CBS 529.93).
Notes — Colletotrichum radicis is phylogenetically close to but clearly differentiated from C. magnisporum based on multi-locus and single gene phylogenetic analyses (Fig. 1, 2). Furthermore, C. radicis produces relatively short and narrow conidia (25.5–32.5 × 6.5–9.5 μm, av. = 28.2 × 7.9 μm) compared to those of C. magnisporum (28.5–40.5 × 8.5–11 μm, av. = 34.3 × 9.7 μm). In addition, many conidia of C. radicis are slightly curved, while those of C. magnisporum are straight.
The closest match in a blastn search with the ITS sequence of CBS 529.93 was FJ205460 (with 97 % identity, 18 bp differences) from a root associated isolate from an orchid in Taiwan (Wang et al. unpubl. data). Closest matches with the TUB2 sequence were FN599817 (with 95 % identity, 22 bp differences) from isolate CBS 169.59 from Oncidium excavatum in the Netherlands, which is here referred to as C. arxii (Munaut et al. unpubl. data) and FN599826 (with 95 % identity, 23 bp differences; Rakotoniriana & Munaut unpubl. data) from isolate MUCL 41702 from Orchis in Singapore.
ColletotrichumvietnamenseF. Liu, L. Cai, Crous & Damm, sp. nov. — MycoBank MB807167; Fig. 7
Fig. 7.
Colletotrichum vietnamense (CBS 125478). a, b. Acervuli; c, d. tips of setae; e, f. conidiophores; g, h. basal parts of setae; i–l. appressoria; m, n. conidia (a, d, f, h, n: from Anthriscus stem; b, c, e, g, i–m: from SNA. a, b: DM; c–n: DIC). — Scale bars: b = 100 μm (applies to a, b); m = 10 μm (applies to c–n).
Etymology. Referring to the country where the fungus was collected.
On Anthriscus stem. Vegetative hyphae hyaline to medium brown, smooth-walled, septate, branched. Conidiomata acervular, conidiophores and setae formed on a cushion of angular brown cells. Setae medium to dark brown, smooth-walled to verruculose, 1–3-septate, 100–180 μm long, base cylindrical to inflated, 6–9.5 μm diam, tip subacute to rounded. Conidiophores hyaline to brown, septate, branched. Conidiogenous cells hyaline to medium brown, cylindrical, clavate to pyriform, 17–26.5 × 7–9.5 μm, opening 2–3.5 μm diam, collarette (few observed) 0.5 μm long. Conidia hyaline, aseptate, smooth-walled, cylindrical, occasionally slightly curved, both ends rounded, 19.5–40 × 8–10.5 μm, av. ± SD = 32.3 ± 4.9 × 9.5 ± 0.6 μm, L/W ratio = 3.4.
On SNA. Vegetative hyphae hyaline to medium brown, smooth-walled, septate, branched. Conidiomata acervular. Setae medium to dark brown, smooth-walled to verruculose, 1–7-septate, 118–176 μm long, base cylindrical to inflated, 7.5–9.5 μm diam, tip subacute. Conidiophores hyaline to brown, septate, branched. Conidiogenous cells hyaline to medium brown, cylindrical, clavate, to pyriform, 13–20.5 × 7.5–10 μm, opening 2–3 μm diam, collarette 0.5 μm long. Conidia hyaline, aseptate, smooth-walled, cylindrical, occasionally slightly curved, both ends rounded, 24–39 × 7.5–11.5 μm, av. ± SD = 31.2 ± 3.6 × 9.6 ± 0.7 μm, L/W ratio = 3.3. Appressoria (only few observed) pale brown, solitary, irregular outline with crenate or lobed margin, 9–17 × 5.5–12.5 μm, av. ± SD = 13.2 ± 2.7 × 9.1 ± 2.7 μm, L/W ratio = 1.2.
Culture characteristics — Colonies on OA flat with entire margin, rosy-buff pigmented, aerial mycelium white to grey, sparse; reverse olivaceous-grey; colonial diam 56–61 mm in 7 d, > 90 mm in 10 d. Colonies on SNA flat with entire margin, medium hyaline, buff around Anthriscus stem, aerial mycelium lacking; colonial diam 61–63 mm in 7 d, > 90 mm in 10 d.
Specimens examined. VIETNAM, Lam Dong Province, Dalat, from anthracnose on leaf of Coffea sp., unknown collection date, P. Nguyen & E. Lijeroth (holotype CBS H-21512, culture ex-type CBS 125478 = LD16(L2)); Dak Lac Province, Buon Ma Thout, from anthracnose on leaf of Coffea sp., unknown collection date, P. Nguyen & E. Lijeroth, culture CBS 125477 = BMT25(L3).
Notes — Anthracnose of Coffea sp. can be caused by various Colletotrichum species, e.g., C. acutatum (Damm et al. 2012a), C. asianum (Prihastuti et al. 2009), C. coffeanum (Noack 1901), C. coffeophilum (Spegazzini 1919), C. costaricense (Damm et al. 2012a), C. fructicola (Prihastuti et al. 2009), C. incarnatum (Zimmermann 1901), C. kahawae (Waller et al. 1993), C. queenslandicum (Weir et al. 2012), C. siamense (Prihastuti et al. 2009) and C. walleri (Damm et al. 2012a). The newly described species C. vietnamense is morphologically and phylogenetically different from these species. Colletotrichum asianum, C. fructicola, C. kahawae, C. queenslandicum and C. siamense, belong to the C. gloeosporioides complex, and C. acutatum, C. costaricense and C. walleri, belong to the C. acutatum complex, all of them have much smaller conidia (Shivas & Tan 2009, Damm et al. 2012a, Weir et al. 2012).
Colletotrichum coffeanum was characterised by 1–2-septate setae; pyriform hyaline conidiophores, 18–20 × 4 μm; smooth, oblong with rounded ends, often curved conidia, 12–18 × 4–5 μm (Noack 1901). Colletotrichum coffeophilum produces aseptate setae, 25–50 × 4–6 μm; conidia ellipsoidal and hyaline, 1-guttulate, 13–15 × 6–8 μm (Spegazzini 1919). Colletotrichum incarnatum has dark brown setae, flat tipped, base cylindrical or somewhat swollen, 85 × 4–5 μm; conidia oblong, 14–19 × 5 μm (Zimmermann 1901). In contrast, C. vietnamense differs from these three species in forming much larger conidia and longer setae.
Another species known to occur on Coffea sp. from Vietnam in this complex is C. gigasporum (CBS 125476 and CBS 125475), which can be distinguished from C. vietnamense by each of the eight genes used in this study, including ITS (Fig. 1).
The closest matches with the ITS sequence of CBS 125478 were FJ968584 (with 100 % identity), a sequence generated from the same isolate by Nguyen et al. (2010), and EF672327 (with 100 % identity) from the endophytic isolate PR61F2, also from Coffea arabica , but from coffee berries in Puerto Rico, a country in Central America (Vega et al. unpubl. data). Closest match with the TUB2 sequence was KC293665 (with 96 % identity, 20 bp differences) from isolate gnqczg15 from China(Huang et al. unpubl. data).
DISCUSSION
Many of the strains included in the present study were deposited in the CBS culture collection as C. crassipes (Speg.) Arx. However, C. crassipes is a species with uncertain taxonomic status. There is significant confusion regarding its morphology in the literature. Spegazzini (1878) originally described this fungus as Gloeosporium crassipes from Vitis vinifera from Conegliano, Italy with conidia measuring 20–30 × 7–8 μm. Subsequently, von Arx (1957) combined Gloeosporium crassipes in Colletotrichum as C. crassipes along with 17 synonyms. The conidial size of C. crassipes was reported as 22–31 × 6–8 μm, broadly matching the original description; and the appressoria as irregular, usually lobed, measuring 8–12 μm (von Arx 1957). Sutton (1980) presented a different morphological concept of C. crassipes, which was characterised by conidia measuring 10–15 × 4.5–6.5 μm, long clavate or circular appressoria with crenate or deeply divided edges, 10.5–14 × 7–9.5 μm, and reduced another two names to synonymy with it. However, when Sutton summarised an accepted taxa list of Colletotrichum species, C. crassipes was characterised with conidia again with a different size (14–28 × 5–7 μm), and he suspected that this species may consist of a number of separate taxa (Sutton 1992). Moreover, several isolates identified as C. crassipes that have sequences lodged in GenBank actually belong to C. gloeosporioides s.lat. (Weir et al. 2012). Recollecting and epitypification of this taxon is required to stabilise the phylogenetic position of C. crassipes.
Although morphological features are not stable and change under different growth conditions and with repeated subculturing, species of the C. gigasporum species complex form larger conidia than most of the other species in the genus Colletotrichum, which provides a valuable character for species complex level diagnosis. Conidia of two other species with large conidia, C. euphorbiae and C. sansevieriae, differ in shape; they are slightly clavate with a round apex tapering to a truncate to slightly acute base (Nakamura et al. 2006, Crous et al. 2013). These two species do not belong to the C. gigasporum complex.
While single gene data, especially ITS data, are usually not sufficient for species recognition in most of the Colletotrichum species complexes or groups (Cannon et al. 2012) and multi-locus phylogenies are therefore now routinely used as the primary basis on which to describe new Colletotrichum species (Damm et al. 2012a, b, Weir et al. 2012, Liu et al. 2013a, b), species of the C. gigasporum species complex can be easily distinguished from each other using the individual gene data included in this study (Fig. 1).
Colletotrichum gigasporum appears to have a wide host range and geographic distribution. Isolates treated in this paper and those deposited in GenBank originate mainly from Africa (East Africa, Madagascar), Central and South America (Brazil, Chile, Columbia, Ecuador, Guyana, Mexico, Panama), Asia (China, India, Japan, Korea, Thailand, Vietnam) and New Zealand (Fig. 1). Besides, this species is associated with various host plants as pathogens and endophytes, from air and stored grain, indicating that it is not host-specific and apparently has different life styles. This character is not unique to C. gigasporum, manyother Colletotrichum species have been reported as both pathogens and endophytes, e.g. C. boninense, C. karstii and C. liriopes (Yang et al. 2011, Damm et al. 2012b, Tao et al. 2013). For instance, C. boninense causes diseases of Crinum asiaticum var. sinicum and Solanum lycopersicum, and is also an endophyte of Bletilla ochracea and Dacrycarpus dacrydioides (Damm et al. 2012b, Tao et al. 2013). The relationship between plant endophytic and pathogenic isolates of the same Colletotrichum species needs more research, as some endophytes may be latent pathogens (Lu et al. 2004).
Acknowledgments
We thank the curators of the CBS culture collection as well as Erick F. Rakotoniriana (Laboratory of Mycology, Université catholique de Louvain, Belgium) for kindly supplying isolates for this study. This study was financially supported by the External Cooperation Program of the Chinese Academy of Sciences (GJHZ1310) and the National Natural Science Foundation of China (NSFC 31110103906, NSFC 31322001).
REFERENCES
- Allescher A. 1902. Fungi Imperfecti. In: Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz, 2nd edn1, 7: 385–704. [Google Scholar]
- Arx JA von . 1957. Die Arten der Gattung Colletotrichum Cda. Phytopathologische Zeitschrift 29: 413–468. [Google Scholar]
- Cannon PF, Damm U, Johnston PR, et al. 2012. Colletotrichum – current status and future directions. Studies in Mycology 73, 1: 181–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castro LGM, Silva Lacaz C da, Guarro J, et al. 2001. Phaeohyphomycotic cyst caused by Colletotrichum crassipes. Journal of Clinical Microbiology 39, 6: 2321–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004a. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Groenewald JZ, Risede JM, et al. 2004b. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2013. Fungal Planet description sheets: 154–213. Persoonia 31: 188–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Liu F, et al. 2013. The Colletotrichum orbiculare species complex: important pathogens of field crops and weeds. Fungal Diversity 61: 29–59. [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012a. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012b. The Colletotrichum boninense species complex. Studies in Mycology 73: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Mostert L, Crous PW, et al. 2008. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia 20: 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Woudenberg JHC, Cannon PF, et al. 2009. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Diversity 39: 45–87. [Google Scholar]
- Dickens J, Cook R. 1989. Glomerella cingulata on Camellia. Plant Pathology 38, 1: 75–85. [Google Scholar]
- Fang W, Yang L, Zhu X, et al. 2013. Seasonal and habitat dependent variations in culturable endophytes of Camellia sinensis. Journal of Plant Pathology & Microbiology 4: 169. doi:10.4172/2157-7471.1000169. [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes‐application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerber JC, Liu B, Correll JC, et al. 2003. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 95: 872–895. [PubMed] [Google Scholar]
- Hennings P. 1908. Fungi paraënses III. Hedwigia 48: 101–117. [Google Scholar]
- Higginbotham SJ, Arnold AE, Ibanez A, et al. 2013. Bioactivity of fungal endophytes as a function of endophyte taxonomy and the taxonomy and distribution of their host plants. Plos One 8, 9:e73192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins KL, Coley PD, Kursar TA, et al. 2011. Culturing and direct PCR suggest prevalent host generalism among diverse fungal endophytes of tropical forest grasses. Mycologia 103, 2: 247–260. [DOI] [PubMed] [Google Scholar]
- Hyde KD, Cai L, Cannon PF, et al. 2009. Colletotrichum-names in current use. Fungal Diversity 39: 147–182. [Google Scholar]
- Johnston PR, Jones D. 1997. Relationships among Colletotrichum isolates from fruit-rots assessed using rDNA sequences. Mycologia 89: 420–430. [Google Scholar]
- Katoh K, Toh H. 2010. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26: 1899–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16: 111–120. [DOI] [PubMed] [Google Scholar]
- Li JZ. 1999. Identification of anthracnose pathogen on vanilla in Xishuangbanna. Journal of Yunnan Tropical Crops Science and Technology 22: 1–3. [Google Scholar]
- Liu F, Cai L, Crous PW, et al. 2013a. Circumscription of the anthracnose pathogens Colletotrichum lindemuthianum and C. nigrum. Mycologia 105, 4: 844–860. [DOI] [PubMed] [Google Scholar]
- Liu F, Damm U, Cai L, et al. 2013b. Species of the Colletotrichum gloeosporioides complex associated with anthracnose diseases of Proteaceae. Fungal Diversity 61: 89–105. [Google Scholar]
- Liu F, Hu DM, Cai L. 2012. Conlarium duplumascospora gen. et. sp. nov. and Jobellisia guangdongensis sp. nov. from freshwater habitats in China. Mycologia 104, 5: 1178–1186. [DOI] [PubMed] [Google Scholar]
- Lu GZ, Cannon PF, Reid A, et al. 2004. Diversity and molecular relationships of endophytic Colletotrichum isolates from the Iwokrama Forest Reserve, Guyana. Mycological Research 108: 53–63. [DOI] [PubMed] [Google Scholar]
- Massee G. 1899. Tea and coffee disease. Bulletin of Miscellaneous Informations of the Royal Botanical Gardens Kew 151/152: 89–94. [Google Scholar]
- Moriwaki J, Sato T, Tsukiboshi T. 2003. Morphological and molecular characterization of Colletotrichum boninense sp. nov. from Japan. Mycoscience 44, 1: 47–53. [Google Scholar]
- Muraleedharan N, Baby UI. 2007. Tea disease: ecology and control. In:Pimentel D. (eds), Encyclopedia of Pest Management Vol.2 [electronic resource]. CRC Press, Boca Raton: 668–671. [Google Scholar]
- Nakamura M, Ohzono M, Iwai H, et al. 2006. Anthracnose of Sansevieria trifasciata caused by Colletotrichum sansevieriae sp. nov. Journal of General Plant Pathology 72: 253–256. [Google Scholar]
- Nguyen PTH, Vinnere Pettersson O, Olsson P, et al. 2010. Identification of Colletotrichum species associated with anthracnose disease of coffee in Vietnam. European Journal of Plant Pathology 127, 1: 73–87. [Google Scholar]
- Nirenberg H. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1–117. [Google Scholar]
- Noack F. 1901. Die Krankheiten des Kaffeebaumes in Brasilien. III. Colletotrichum coffeanum n. sp. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 2: 196–203. [Google Scholar]
- Noireung P, Phoulivong S, Liu F, et al. 2012. Novel species of Colletotrichum revealed by morphology and molecular analysis. Cryptogamie Mycologie 33: 347–362. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Nirenberg HI, Aoki T, et al. 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 41, 1: 61–78. [Google Scholar]
- Patel MK, Kamat MN, Pande CB. 1953. A new leaf blight of Crossandra infundibuliformis Nees. Indian Phytopathology 5, 2: 130–139. [Google Scholar]
- Petrak F. 1925. Mykologische Notizen. VIII. Annales Mycologici 23: 1–143. [Google Scholar]
- Prihastuti H, Cai L, Chen H, et al. 2009. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity 39: 89–109. [Google Scholar]
- Promputtha I, Lumyong S, Dhanasekaran V, et al. 2007. A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microbial Ecology 53, 4: 579–590. [DOI] [PubMed] [Google Scholar]
- Rakotoniriana EF, Scauflaire J, Rabemanantsoa C, et al. 2013. Colletotrichum gigasporum sp. nov., a new species of Colletotrichum producing long straight conidia. Mycological Progress 12, 2: 403–412. [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute, Kew. [Google Scholar]
- Rojas EI, Rehner SA, Samuels GJ, et al. 2010. Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panamá: multi-locus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia 102, 6: 1318–1338. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Saccardo PA. 1896. Fungi aliquot Brasilienses phyllogeni. Bulletin de la Société Royale de Botanique Belgique 35: 127–132. [Google Scholar]
- Saccardo PA. 1906. Sylloge fungorum vol. 18 Padova, Italy. [Google Scholar]
- Saitou N, Nei M. 1987. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: 406–425. [DOI] [PubMed] [Google Scholar]
- Sawada K. 1959. Descriptive catalogue of Taiwan (Formosan) fungi. Part XI. Special Publication College of Agriculture National Taiwan University 8: 1–268. [Google Scholar]
- Shivas RG, Tan YP. 2009. A taxonomic re-assessment of Colletotrichum acutatum, introducing C. fioriniae comb. et stat. nov. and C. simmondsii sp. nov. Fungal Diversity 39: 111–122. [Google Scholar]
- Sivanesan A, Hsieh WH. 1993. A new ascomycete, Glomerella septospora sp. nov. and its coelomycete anamorph, Colletotrichum taiwanense sp. nov. from Taiwan. Mycological Research 97, 12: 1523–1529. [Google Scholar]
- Sosa de Castro NT, Cabrera de Alvarez MG, Alvarez RE. 2001. Primera informatión de Colletotrichum camelliae como patógeno de Camellia japonica, en Corrientes. Retrieved 6 Oct. 2010 from www1.unne.edu.ar/cyt/2001/5-Agrarias/A-056.pdf. [Google Scholar]
- Spegazzini C. 1878. Ampelomiceti Italici, ossia enumerazione, diagnosi e storia dei principali parassiti della vite. Rivista di Viticoltura ed Enologia Italiana Anno 2: 405–411. [Google Scholar]
- Spegazzini C. 1919. Fungi costaricenses nonnulli. Boletýn de la Academia Nacional de Ciencias, Córdoba 23: 541–609. [Google Scholar]
- Stephenson SA, Green JR, Manners JM, et al. 1997. Cloning and characterisation of glutamine synthetase from Colletotrichum gloeosporioides and demonstration of elevated expression during pathogenesis on Stylosanthes guianensis. Current Genetics 31, 5: 447–454. [DOI] [PubMed] [Google Scholar]
- Stoneman B. 1898. A comparative study of the development of some anthracnoses. Botanical Gazette 26, 2: 69–120. [Google Scholar]
- Sutton B. 1992. The genus Glomerella and its anamorph Colletotrichum. In: Bailey JA, Jeger MJ. (eds), Colletotrichum Biology, Pathology and Control: 1–26 CAB International, Wallingford, UK. [Google Scholar]
- Sutton BC. 1980. The Coelomycetes. Commonwealth Mycological Institute, Kew, United Kingdom. [Google Scholar]
- Swofford D. 2002. PAUP 4.0 b10: Phylogenetic analysis using parsimony (* and other methods). Computer programme. Sinauer Associates, Sunderland, MA, USA. [Google Scholar]
- Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology Evolution 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talubnak C, Soytong K. 2010. Biological control of vanilla anthracnose using Emericella nidulans. Journal of Agricultural Technology 6, 1: 47–55. [Google Scholar]
- Tao G, Liu ZY, Liu F, et al. 2013. Endophytic Colletotrichum species from Bletilla ochracea (Orchidaceae), with descriptions of seven new species. Fungal Diversity 61: 139–164. [Google Scholar]
- Tunstall A. 1935. A new species of Glomerella on Camellia theae. Transactions of the British Mycological Society 19, 4: 331–336. [Google Scholar]
- Vega FE, Simpkins A, Aime MC, et al. 2010. Fungal endophyte diversity in coffee plants from Colombia, Hawai’i, Mexico and Puerto Rico. Fungal Ecology 3, 3: 122–138. [Google Scholar]
- Verplancke G.1935. Bijdrage tot de flora der woekerzwammen van België. Mededeelingen der Landbouwhoogeschool en der Opzoekingsstations van den Staat, te Gent III, 2: 83–112. [Google Scholar]
- Waller JM, Bridge PD, Black R, et al. 1993. Characterization of the coffee berry disease pathogen, Colletotrichum kahawae sp. nov. Mycological Research 97: 989–994. [Google Scholar]
- Weir BS, Johnston PR, Damm U. 2012. The Colletotrichum gloeosporioides species complex. Studies in Mycology 73: 115–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322 Academic Press, San Diego, California, USA. [Google Scholar]
- Wu LS, Han T, Li WC, et al. 2013. Geographic and tissue influences on endophytic fungal communities of Taxus chinensis var. mairei in China. Current Microbiology 66, 1: 40–48. [DOI] [PubMed] [Google Scholar]
- Yang YL, Cai L, Yu ZN, et al. 2011. Colletotrichum species on Orchidaceae in southwest China. Cryptogamie, Mycologie 32, 3: 229–253. [Google Scholar]
- Zimmermann A. 1901. Über einige an tropischen Kulturpflanzen beobachtete Pilze. I. Centralblatt für Bakteriologie und Parasitenkunde 7: 139–147. [Google Scholar]