Abstract
The transient receptor potential (TRP) superfamily consists of a large number of nonselective cation channels with variable degree of Ca2+-permeability. The 28 mammalian TRP channel proteins can be grouped into six subfamilies: canonical, vanilloid, melastatin, ankyrin, polycystic, and mucolipin TRPs. The majority of these TRP channels are expressed in different cell types including both excitable and nonexcitable cells of the cardiovascular system. Unlike voltage-gated ion channels, TRP channels do not have a typical voltage sensor, but instead can sense a variety of other stimuli including pressure, shear stress, mechanical stretch, oxidative stress, lipid environment alterations, hypertrophic signals, and inflammation products. By integrating multiple stimuli and transducing their activity to downstream cellular signal pathways via Ca2+ entry and/or membrane depolarization, TRP channels play an essential role in regulating fundamental cell functions such as contraction, relaxation, proliferation, differentiation, and cell death. With the use of targeted deletion and transgenic mouse models, recent studies have revealed that TRP channels are involved in numerous cellular functions and play an important role in the pathophysiology of many diseases in the cardiovascular system. Moreover, several TRP channels are involved in inherited diseases of the cardiovascular system. This review presents an overview of current knowledge concerning the physiological functions of TRP channels in the cardiovascular system and their contributions to cardiovascular diseases. Ultimately, TRP channels may become potential therapeutic targets for cardiovascular diseases.
Keywords: TRP channels, Ca2+ signaling, pathogenesis, heart diseases, vascular disorders
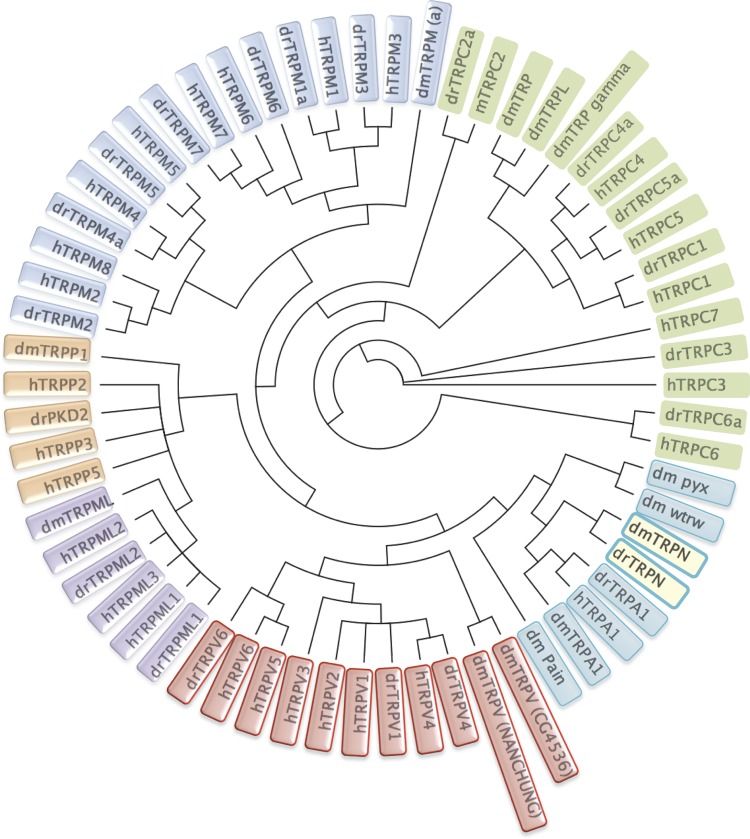
transient receptor potential (TRP) channels were first identified in Drosophila, where photoreceptors carrying Trp gene mutations exhibited a transient voltage response to continuous light causing impaired visual adaption (46, 189, 193). To date, 28 mammalian Trp genes have been identified, which are divided into six related subfamilies, consisting of the canonical TRP (TRPC), vanilloid TRP (TRPV), melastatin TRP (TRPM), ankyrin TRP (TRPA), polycystic TRP (TRPP), and mucolipin TRP (TRPML) groups (41, 192). The TRPC, TRPV, and TRPM subfamilies contain 7, 6, and 8 different channel proteins, respectively (41, 192, 214); the TRPA subfamily has only one gene (123, 281, 301); the TRPML subfamily contains three proteins, which are defined by the initially discovered gene mucolipin 1; and the TRPP subfamily has three members: TRPP2, TRPP3, and TRPP5, which are also known as polycystic kidney disease protein PKD2, PKD2-like proteins PKD2L1 (TRPP3), and PKD2L2 (TRPP5) (38, 41). PKD2 related proteins form Ca2+-permeable channel with PKD1, an 11 transmembrane protein, which is also known as TRPP1. Nonmammalian species, such as Drosophila and zebrafish, also have an additional subfamily, TRPN1 (191, 210). Thus there are seven subfamilies in the TRP channel superfamily (Fig. 1).
Fig. 1.
A phylogenetic tree of human, Zebrafish, and Drosophila transient receptor potential (TRP) channels. Sequence homology analyses show that all TRP channels fall into 7 subfamilies that comprise proteins with distinct channel properties. Because human TRPC2 is a pseudogene, mTRPC2 (mouse) is used for analysis. Aligned by ClustalW, the phylegenic tree was generated according to Jukes-Cantor Genetic Distance Model, and the tree was built by the Neighbor-Joining method. All calculation was done in Geneious software. h, homo sapiens; dr, Danio rerio; dm, Drosophila melanogaster. The TRP subfamilies are represented by different colors. Gene accession numbers are shown as follows: 1) Human TRP channels: canonical TRP (TRPC): hTRPC1 (EAW78963), hTRPC3 (NP_001124170), hTRPC4 (AAI04726), hTRPC5 (EAX02630), hTRPC6 (AAH93660), hTRPC7 (AAI28186); mTRPC2 (NP_001103367); vanilloid TRP (TRPV): hTRPV1 (NP_542437), hTRPV2 (NP_057197), hTRPV3 (NP_001245134), hTRPV4 (NP_067638), hTRPV5 (NP_062815), hTRPV6 (NP_061116); melastatin TRP (TRPM): hTRPM1 (NP_001238949), hTRPM2 (NP_003298), hTRPM3 (Q9HCF6), hTRPM4 (NP_060106), hTRPM5 (NP_055370), hTRPM6 (NP_060132), hTRPM7 (NP_060142), hTRPM8 (NP_076985); polycystic (TRPP): hTRPP2 (NP_000288), hTRPP3 (NP_057196), hTRPP5 (NP_055201); hTRPML1 (NP_065394), hTRPML2 (NP_694991), hTRPML3 (NP_060768); ankyrin TRP (TRPA): hTRPA1(NP_015628). 2) Zebrafish TRP channels: TRPC: drTRPC1 (AGW27444), drTRPC2a (AGW27445), drTRPC3 (AGW27447), drTRPC4a (AGW27448), drTRPC5a (AGW27450), drTRPC6a (AGW27452), drTRPC7a (AGW27454); TRPV: drTRPV1 (NP_001119871), drTRPV4 (NP_001036195), drTRPV6 (NP_001001849); TRPM: drTRPM1a (AGS55979), drTRPM2 (AGS55981), drTRPM3 (AGS55982), drTRPM4a (AGS55983), drTRPM5 (AGS55987), drTRPM6 (AGS55988), drTRPM7 (AGS55989); mucolipin TRP (TRPML): drTRPML1 (AAH54127), drTRPML2 (NP_957442); TRPA: drTRPA1 (NP_001007066); TRPP: drPKD2 (NP_001002310); TRPN: drTRPN (NP_899192). 3) Drosophila TRP channels (74): TRPC: dmTRP (NP_476768), dmTRPL (AAF58904), dmTRP γ (AAF53548); TRPV: dmTRPV_(NANCHUNG) (AAF49752), dmTRPV_(CG4536) (AAF46203); TRPM: dmTRPM (a) (NP_001137672); TRPML: dmTRPML (NP_649145); TRPN: dmTRPN (AAF52248); TRPA: dmTRPA1 (NP_476768), dm pain (AAF47293), dmwtrw (AHN57213), dmpyx (AAF47356); TRPP: dmTRPP1 (NP_609561).
Unique Features of TRP Channels
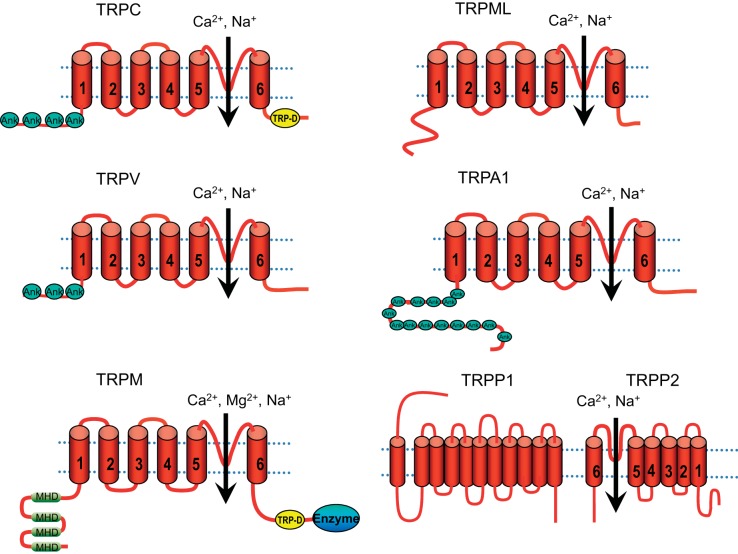
TRP channel primary structures predict six transmembrane domains (TM) (Fig. 2). The channel pore is between the TM5 and TM6, whereas the TM4 lacks the obvious voltage sensor in voltage-gated ion channels (28). Among different TRP subfamilies, the high level of primary amino acid sequence similarity is mostly limited to the transmembrane segments. Intracellular NH2- and COOH-termini are variable in length and consist of different domains (223). Many TRP channels have a variable number of ankyrin repeats at the NH2-termini: 3 to 4 in TRPCs, 6 in TRPVs, 14 to 15 in TRPAs, and about 29 in TRPNs (210). The NH2 terminus of TRPMs is characterized by four stretches of residues designated as the TRPM homology domain (MHD). In TRPCs and TRPMs, there is a small region that stretches from the COOH terminus to TM6, the so-called TRP domain, which is involved in PIP2 regulation of channel activation and desensitization (211, 247). Some TRP channels have an enzyme domain at the COOH-terminal tail. For example, TRPM2 has a Nudix hydrolase domain functioning as an ADP-ribose (ADPR) pyrophosphatase (230). TRPM6 and TRPM7, so-called the channel-kinases, have an atypical α-kinase domain involved in regulation of channel functions (200, 252) (Fig. 2). The majority of TRP channels are localized in the plasma membrane (PM); some TRP channels, including TRPV1 (307), TRPV2 (115, 128), TRPM2 (288), TRPM7 (146), and PKD2 (10, 26, 144), are localized in both PM and intracellular organelles such as sarcolemma reticulum (SR), endoplasmic reticulum (ER), synaptic vesicles, and lysosome. TRPMLs are localized in lysosomes (34) (Table 1).
Fig. 2.
Predicted structural topology of TRP channels. All TRP channels contain 6 transmembrane segments (S1 to S6) with a putative pore region (P) between S5 and S6. NH2- and COOH-termini are variable in length and contain different sets of domains. There are 4, 3, and 14 ankryrin (Ank) repeats in the TRPC subfamily, TRPV subfamily, and TRPA1, respectively. The NH2 terminus of TRPMs is characterized by 4 stretches of residues, designated as the TRPM homology domain (MHD). The TRP domain (TRP-D) is present in the members of the TRPC and TRPM subfamilies. An enzyme domain is present in some of the channels, e.g., TRPM2 has an ADP-ribose pyrophosphatase, whereas TRPM6 and TRPM7 contain an atypical protein kinase. TRPP2 interacts with TRPP1, the 11-transmembrane protein, to form a channel complex.
Table 1.
Properties and functions of TRP channels
| TRPs | Chromosome (Human) | PCa/PNa | Expression in Heart and Vasculature | Cellular Localization | Potential Functions in Cardiovascular System | References |
|---|---|---|---|---|---|---|
| TRPC1 | 3q22–3q24 | <10 | Myocytes, ECs, fibroblasts, SMCs | PM | Hypertrophy/heart failure, pulmonary hypertension | 41, 72, 179, 210, 287, 325, 358 |
| TRPC2 | 11p15.4–15.3 (pseudogene) | 2.7 | Myocytes (rodent) | PM | Pheromone sensing (in mice) | 41, 175 |
| TRPC3 | 4q27 | 1.6 | Myocytes, ECs, fibroblasts, SMCs | PM | Arrhythmia, hypertrophy/heart failure, vasodilation, atherosclerosis | 41, 72, 94, 210, 254, 287, 294, 325, 358 |
| TRPC4 | 13q13.1–q13.2 | ∼1 | Myocytes, ECs, fibroblasts, SMCs | PM | Hypertrophy/heart failure, vasoregulation, microvascular | 41, 72, 210, 287, 325, 358 |
| TRPC5 | Xq23 | ∼1 | Myocytes, ECs, fibroblasts, SMCs | PM | Heart farlure (?), mobility of SMCs, vasodilation | 41, 72, 210, 287, 325, 358 |
| TRPC6 | 11q21–q22 | 5 | Myocytes, ECs, fibroblasts, SMCs | PM | Arrhythmia, hypertrophy/heart failure, idiopathic pulmonary arterial hypertension, vasoregulation, Lung ischemia-reperfusion caused edema | 19, 41, 72, 151, 210, 278, 287, 325, 329, 356, 358 |
| TRPC7 | 5q31.1 | 0.5 ∼5.4 | Myocytes, ECs, fibroblasts, SMCs | PM | Hypertrophy/heart failure | 41, 52, 72, 210, 287, 325, 358 |
| TRPV1 | 17p13.3 | 10 | Myocytes, ECs, SMCs | SR, ER, PM | Hypertrophy/heart failure, vasoregulation, atherosclerosis, hypertension | 41, 72, 210, 287, 325, 327, 347, 358 |
| TRPV2 | 17p11.2 | ∼1–3 | Myocytes, ECs, fibroblasts, SMCs | SR, ER, PM | Cardiac structure and function, dilated cardiomyopathy | 41, 72, 115, 116, 128, 210, 287, 325, 347, 358 |
| TRPV3 | 17p13.3 | 6 | ECs | PM | Vasodilation | 41, 63, 64, 72, 210, 287, 325, 347, 358 |
| TRPV4 | 12q24.1 | ∼10 | Myocytes, ECs, fibroblasts, SMCs | PM | Vasodilation, BP regulation, pulmonary edema, fibroblast differentiation | 41, 72, 210, 277, 287, 296, 325, 347, 350, 358 |
| TRPV5 | 7q35 | >100 | ND | PM | ND | 41, 210, 325 |
| TRPV6 | 7q33–34 | >100 | Fibroblasts | PM | ND | 41, 210, 325 |
| TRPM1 | 15q13–q14 | >100 | Heart | PM | ND | 72, 287, 325, 358 |
| TRPM2 | 21q22.3 | <1 | Myocytes, ECs, fibroblasts, SMCs | PM, lysosome | Ischemic cardiomyopathy, endothelial permeability | 41, 59, 72, 73, 103, 188, 210, 287, 325, 347, 358 |
| TRPM3 | 9q21.13 | ∼1–20 | SMCs | PM | SMC phenotype switching, proliferation of SMCS | 41, 72, 210, 287, 325, 347, 358 |
| TRPM4 | 19q13.32 | 0.5–1.6 | Myocytes, ECs, fibroblasts, SMCs | PM | Cardiac conduction, vasoregulation, myogenic tone regulation, BP regulation | 41, 72, 147, 173, 183, 210, 280, 287, 325, 347, 358 |
| TRPM5 | 11p15.5 | <0.05 | Heart, aorta, pulmonary artery | PM | ND | 41, 72, 210, 287, 325, 347, 358 |
| TRPM6 | 9q21.13 | PMg/PNa: ∼6 | Fibroblasts, SMCs | PM | BP regulation? | 41, 72, 210, 287, 325, 347, 358 |
| TRPM7 | 15q21 | 0.3 | Myocytes, ECs, fibroblasts, SMCs | PM, synaptic vesicles | Heart development, cardiac fibrosis | 41, 72, 210, 256, 257, 287, 325, 347, 358 |
| TRPM8 | 2q37.1 | ∼1–3.3 | Aorta, SMCs | PM | Vasoregulation | 41, 72, 122, 210, 287, 325, 347, 358 |
| TRPA1 (ANKTM1) | 8q13 | 0.8–1.4 | ECs, SMCs | PM | Vasodilation | 41, 63, 64, 72, 210, 287, 325, 358 |
| TRPP2 (PKD2) | 4q21–23 | 1.0–5.0 | Myocytes, ECs, fibroblasts, SMCs | PM, ER, primary cilium, mitotic spindles and centrosome | Heart development, myogenic tone regulation, vascular stability, vascular leakage, intracranial aneurysm | 10, 15, 26, 29, 41, 49, 72, 80, 86, 92, 111, 126, 144, 177, 210, 251, 287, 315, 325, 331, 351, 358 |
| TRPP3 (PKD2L1) | 10q24–25 | ∼4 | Heart | PM, ER, primary cilium, mitotic spindles and centrosome | ND | 10, 15, 26, 29, 41, 49, 72, 80, 86, 92, 111, 126, 144, 177, 210, 251, 287, 325, 333, 351, 358 |
| TRPP5 (PKD2L2) | 5q31 | 1.0–5.0 | Myocytes | PM, ER, primary cilium, mitotic spindles and centrosome | ND | 10, 15, 26, 29, 41, 49, 72, 80, 86, 92, 111, 126, 144, 177, 210, 251, 287, 315, 325, 351, 358 |
| TRPML1 | 19p13.3–13.2 | PCa>PNa | ECs, fibroblasts | Late endosome, lysosome | ND | 34, 41, 210 |
| TRPML2 | 1p22 | PCa>PNa | Fibroblasts | Endosome, lysosome | ND | 34, 41, 210 |
| TRPML3 | 1p22.3 | PCa>PNa | Fibroblasts | PM, early and late endosome, lysosome | ND | 34, 41, 133, 210 |
TRPC, canonical transient receptor potential; TRPV, vanilloid transient receptor potential; TRPM, melastatin transient receptor potential; TRPA, ankyrin transient receptor potential; TRPP, polycystic transient receptor potential; TRPML, mucolipin transient receptor potential; PKD, polycystic kidney disease; SMC, smooth muscle cell; EC, endothelial cell; PM, plasma membrane; SR, sarcolamma reticulum; ER, endoplasmic reticulum; BP, blood pressure; ND, not detected (for expression) or not determined (for function).
Ca2+ permeation.
Most TRP channels are Ca2+-permeable nonselective cation channels (PCa/PNa<10), with the exceptions that TRPM4 and TRPM5 are monovalent selective (PCa/PNa<0.05) (41, 95, 105, 170, 212) and that TRPV5 and TRPV6 are highly Ca2+-selective (PCa/PNa>100) (41, 313, 357). The channel-kinases TRPM6 and TRPM7 are permeable to Mg2+, Ca2+, Na+, Zn2+, and other trace metals (Table 1).
TRP channels form homo- and heterotetrameric channels.
TRPC1 can form heteromeric channels with TRPC4 or TRPC5 (283, 284). TRPC3/6/7 can form heterotetrameric channels with each other (107). Moreover, in the presence of TRPC1, TRPC4 and TRPC5 can also form heteromeric channels with TRPC3 and TRPC6 (283). Within TRPV subfamily, TRPV1-4 channel subunits can assemble into heteromeric complexes (33, 101), whereas TRPV5 and TRPV6 form functional heteromeric channels (104). The heteromeric TRPM6 and TRPM7 channel complex exhibits distinct biophysical and pharmacological properties from TRPM6 and TRPM7 homomeric channels (165). In the TRPP subfamily, TRPP2 (PKD2) is the pore-forming subunit of the TRPP1-TRPP2 complex (238, 305). The intracellular channel TRPML1 interacts with TRPML3, leading to the translocation of TRPML3 to lysosomes (310).
Activation mechanisms.
Unlike voltage-gated ion channels, TRP channels do not have the typical voltage sensor at TM4, and thus are not gated by voltage (243). However, TRP channels can sense thermal, mechanical, chemical, nociceptive, and local cellular environmental stimuli and are gated in a polymodal activation manner (41, 311). TRPV1-V4 and TRPM3 are activated by high temperatures, whereas TRPM8, TRPA1, and TRPC5 are activated by low temperatures (208, 316, 368). TRPC channels require the phospholipase C (PLC) pathway for activation, either directly by diaglycerol (DAG), such as TRPC2, TRPC3, TRPC6, and TRPC7, or indirectly through a yet unknown mechanism, such as TRPC1, TRPC4, and TRPC5 (106, 312). Heterologously overexpressed TRPV5 and TRPV6 are considered constitutively open; however, the activation mechanisms of the endogenous TRPV5 and TRPV6 channels are unknown (208). The Mg2+-permeable TRPM6 and TRPM7 are activated by lowering intracellular free Mg2+ concentration (164, 165, 200, 252, 314), whereas a rise in intracellular Ca2+ ([Ca2+]i) is necessary to activate several TRP channels, including TRPM4 (158), TRPM5 (170, 236), TRPM2 (58), and TRPA1 (369). TRPM2 can be activated by multiple stimuli including ADPR, NAD+, and oxidative stress (93, 230, 261). TRPM3 is activated by hypo-osmotic cell swelling, rises in [Ca2+]i, d-erythro-sphingosine (D-SPH), and high temperatures (89, 90, 161, 316). The polymodal activation feature of TRPs confers diverse physiological and pathological functions.
Expression of TRP Channels in the Cardiovascular System
TRPs are ubiquitously expressed in various excitable and nonexcitable cells as detected by RT-PCR, Western blot, immunostaining, and functional current recordings. In the whole heart, TRPC1, TRPC3-7, TRPV2, TRPV4, TRPM2, TRPM4-5, TRPM7, and TRPP1/2 are detectable at mRNA levels (325). All TRPCs except TRPC5 are expressed in the sinoatrial (SA) nodal cells as detected by RT-PCR and immunostaining (124). The majority of TRP channels are detectable (RT-PCR) in cardiac myocytes and/or fibroblasts of different species (Table 1) (325, 358). TRPC6, TRPM4, and TRPM7 currents have been recorded in SA nodal cells and/or cardiac myocytes (50, 151, 256, 257, 365). TRPM2 currents are present in both cardiac myocytes and fibroblasts (103, 188, 291, 361), and PKD2 and PKD2L2 single channel currents have been recorded in rat cardiac myocytes (315). In vascular system, TRPC1, TRPV3, TRPC6, TRPV1-4, and TRPM2-8 were detected by RT-PCR in rodent aorta at the tissue level (70, 347). TRPC1, C3, and C6 channels are also detectable by RT-PCR in mouse portal veins (114), cerebral arteries (3, 330), smooth muscle cells (SMCs), and endothelial cells (ECs), whereas TRPC7 is only expressed in coronary artery SMCs (52). All TRPCs, TRPV1-4, most TRPMs except for TRPM5, TRPP1, and TRPP2, and TRPA1 are expressed at the mRNA level in SMCs or ECs of various vascular beds in different species (3, 287, 349). The native currents of TRPCs have been reported in SMCs and ECs. For example, in rabbit coronary SMCs, heteromeric TRPC1/C5 and TRPC3/7 channel activities operated by endothelin A receptor (ET-A) are mediated by phosphoinositide 3-kinase (PI3K) pathways (272). In rabbit mesenteric artery SMCs, TRPC6 channel activation by ANG II is inhibited by TRPC1/C5 channel activity through a Ca2+- and PKC-dependent mechanism (273). The detailed expression pattern of TRPs in various cell types is summarized in Table 1 and has also been summarized in previous reviews (3, 51, 72, 287, 325, 358).
REGULATION OF TRP CHANNELS
TRP channels are activated and regulated by a variety of stimuli including pressure, shear stress, mechanical stretch, oxidative stress, phospholipids, and the metabolites of phospholipids. In response to these stimuli, TRP channels integrate and transduce their activity to the downstream cellular amplification system via Ca2+ entry and membrane depolarization, thereby exhibiting an important role in regulating fundamental cell functions such as contraction, relaxation, proliferation, differentiation, and cell death.
Regulation of TRP Channels by Oxidative Stress
Several TRP channels are sensitive to oxidative stress. TRPM2 was initially identified as a redox-activated Ca2+ permeable channel (93). TRPC5 is also considered a redox factor since it can be activated by H2O2, the redox protein thioredoxin, and oxidized phospholipids (5, 341, 354). In addition, TRPC5, TRPC1, TRPC4, TRPV1, TRPV3, and TRPV4 are nitric oxide (NO) sensors in ECs (354). The NO-sensing (nitrosylation) cysteines are located at the NH2-terminal side of the TRPC5 pore and are conserved in these NO-sensing TRP channels (354). In ECs, TRPC3/4 heterotetrameric channels respond to oxidative stress stimulation (235). Oxidative stress increases TRPC6 activity through a cysteine oxidation-dependent pathway (55, 88) and by increasing surface expression of the channel (132). Oxidative stress sensitizes TRPV1 through covalent modification of conserved cysteines (36). The inward current of TRPM7 can be enhanced by H2O2 (1 mM) and the superoxide generator menadione (0.2 mM) after treatment for 10 to 30 min, whereas superoxide dismutase (SOD) mimic MnTBAP (0.2 mM) inhibits TRPM7 currents (1). However, a shorter (1 to 2 min) treatment of H2O2 does not influence TRPM7 currents. Moreover, MnTBAP fails to block TRPM7 induced cell rounding (285). Furthermore, TRPM7 overexpression can enhance levels of reactive oxygen species (ROS) and NO (285). H2O2 has also been demonstrated to inhibit TRPM6 (27) and to remove desensitization of TRPM4, which leads to increased vulnerability to necrotic cell death (274). TRPA1 can be activated by multiple products of oxidative stress including H2O2, alkenyl aldehydes such as 4-hydroxyhexenal (4-HE), and by cyclopentenone prostaglandin (8). Whereas most TRP channels are potentiated by oxidative stress, the TRPP2 channel activity is inhibited by ROS (190).
Regulation of TRP Channels by Mechanical Stretch
TRP channels play a key role in mechano-osmotic transduction, a process that is essential for various physiological functions such as myogenic regulation, vascular tone, muscle stretch, and volume regulation (91) (149). Several TRP channels are sensitive to various forms of mechanical stress. TRPC1 and TRPC6 were initially reported to be activated by mechanically or osmotically induced membrane stretch (180, 278). However, the mechanical gating of TRPC1 and TRPC6 was later challenged by other investigators (53, 87, 270). There is no report as to whether TRPC3 is sensitive to mechanical stretch, given that TRPC3 forms a heteromer with TRPC6. However, TRPC5 has been demonstrated to be activated by hypoosmotic- and pressure-induced membrane stretch (83). TRPV4 is activated by osmotic cell swelling (167, 282) via the generation of 5',6'-EET (5',6'-epoxyeicosatrienonic acid) (see Fig. 3) (215, 326). Similarly, TRPV2 is activated by osmotic cell swelling (14, 197). In the TRPM subfamily, TRPM3 (89) and TRPM4 (68) are activated by hypotonic cell swelling. However, Trpm4−/− mice are insensitive to pressure-induced myogenic response (270). TRPM7 overexpressed in HEK cells is potentiated by hypotonic stress at elevated intracellular Mg2+ and Mg-ATP but inhibited by hypertonic stress (17). Native TRPM7 current is potentiated by shear stress via translocation of TRPM7 to the cell membrane (219). Direct activation of TRPM7 by stretching and swelling has also been reported (217, 218). The role of TRPA1 in mechanical transduction in hair cells remains controversial (44, 45, 155), and it is unknown whether TRPA1 in ECs (63) is mechano-sensitive.
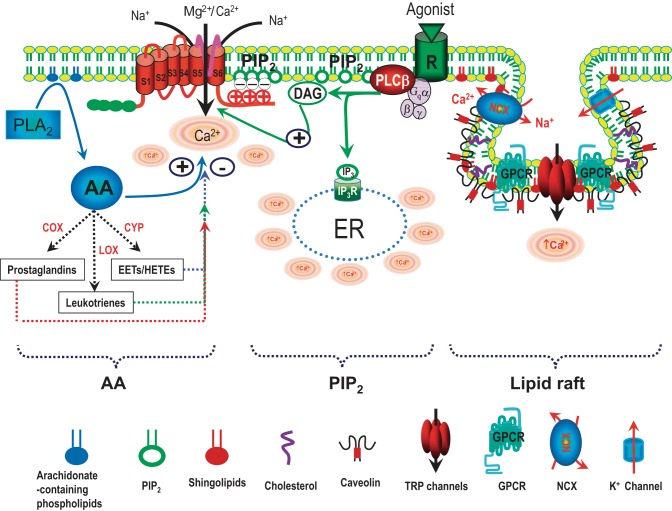
Fig. 3.
Schematic diagram of regulation of TRP channels by phospholipids and lipid rafts. TRP channels can be modulated by various phospholipids, including arachidonic acid (AA) generated by PLA2, phosphatidylinositol 4,5-bisphosphate (PIP2), and lipids in the lipid rafts. AA can be metabolized through 3 enzymatic pathways: 1) the cyclooxygenase (COX) pathway produces prostaglandins; 2) the lipoxygenase (LOX) pathway yields monohydroxy compounds and leukotrienes; and 3) the cytochrome P-450 (CYP) epoxygenase pathway generates hydroxyleicosatetraenoic acids (HETES) and epoxyeicosatrienoic acids (EETs). PIP2 is a co-activator of many TRP channels. Diaglycerol (DAG) generated by hydrolyzing PIP2 via Gq-linked receptor stimulation activates TRPC3 and TRPC6. Lipid rafts are specific microdomains orchestrating various signaling pathways, including GPCR, ion channels, and caveolin-1 (endothelial cells), or caveolin-3 (myocytes). Caveolae are a special type of lipid raft. Lipid rafts are enriched with cholesterol and sphingolipids. Disruption of lipid rafts by depletion of cholesterol or knockdown of caveolin alters TRP channel function within the rafts. Modified from Yue et al. (359).
PKD1 (TRPP1) and PKD2 (TRPP2) form flow-sensitive and mechanosensitive channel complexes in the primary cilia of different cell types (203, 204). In endothelial primary cilia, PKD1 and PKD2 are involved in fluid shear sensing and regulate Ca2+ signaling and NO release, thus contributing to vasodilation in response to an increase in blood flow (2, 204). PKD1 and PKD2 are also abundantly expressed in arterial SMCs (20, 239, 240), where they are involved in regulating stretch activated cation channels (SACs) activity (271). In response to mechanical stretch, PKD2 inhibits SACs and PKD1 reverses this inhibition by forming a PKD1/PKD2 complex (271). The inhibitory effect of PKD2 on SACs is mediated by interacting with the actin crosslinking protein FLNa (271). Thus, in the case of pressure sensing by arterial SMCs, PKD2 inhibits mechanosensitivity and PKD1 reverses this inhibition, whereas in the case of flow sensing by the primary cilia, both PKD1 and PKD2 promote mechanosensitivity (203, 204). Moreover, PKD2 can form channel complexes with TRPV4, sensing mechanical and thermal stimuli (143).
Regulation of TRP Channels by Phospholipids and Lipid Rafts
Phospholipids, the metabolites of phospholipids, and lipid rafts exert a variety of functions in the cardiovascular system, at least partially through regulating the activities of various ion channels, including TRP channels (Fig. 3).
AA regulation of TRP channels.
Arachidonic Acid (AA) and its metabolites have multiple functions in living cells. Under normal conditions, the concentration of AA in the plasma ranges from 2 to 16 μM (24, 244, 309), which can be increased by 10- to 13-fold in response to tissue injury or ischemia (187). AA is generated via several different pathways. For example, AA can be produced by cleaving off the fatty acid from phospholipids through cytosolic phospholipase A2 (cPLA2) and secretory phospholipase A2 (sPLA2) (82, 199). AA can also be generated from DAG by DAG lipase (6, 279). The metabolites of AA also exert multiple cellular functions. AA can be metabolized through three enzymatic pathways (117, 187): 1) the cyclooxygenase (COX) pathway produces prostaglandins; 2) the lipoxygenase (LOX) pathway yields monohydroxy compounds and leukotrienes; and 3) the cytochrome P-450 (CYP) epoxygenase pathway generates hydroxyleicosatetraenoic acids (HETES) and epoxyeicosatrienoic acids (EETs) (Fig. 3).
Several TRP channels are regulated by AA and its metabolites (187). AA directly potentiates the response of TRPV3 to 2-aminoethoxydiphenyl borate (2-APB) (109). The epoxygenase products of AA, including 12-, 15-, and 5-HETE and leukotriene, activate TRPV1 (110). TRPV4 is also activated by the epoxygenase products 5',6'- and 8',9'-EETs, but not by lipoxygenase products (326). TRPC6 channels are activated by the P-450-epoxygenase product 20-HETE (12, 43). AA also activates TRPA1 (11), TRPM2 (93, 300), and TRPM5 (221) but inhibits TRPM8 (9). The effect of AA may be mediated by AA binding to membrane molecules, or through altering bilayer mechanical properties by inserting itself between the membrane molecules. Alternatively, AA effects can be mediated by the activation of PKC after it is released by receptor-mediated phospholipases A2 (PLA2) activation (199, 342). Moreover, AA can exert its effects by changing the affinity of the channels to PIP2 (276, 317).
PIP2 regulation of TRP channels.
Most TRP channels are regulated by phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2, or PIP2) (211, 246). PIP2 either activates or is required for activation by a large number of TRP channels (Fig. 3). For TRPCs, the hydrolysis product DAG activates TRPC3, TRPC6, and TRPC7 (106). Thus it was proposed that PIP2 exhibits inhibitory effects so that hydrolysis of PIP2 and production of DAG activate TRPC channels (157, 258). The effects of PIP2 on TRPVs and TRPMs are mainly mediated by direct or indirect interaction with the positively charged residues at either the NH2- or COOH terminus (57, 211, 246, 286). The interaction of PIP2 with the NH2 terminus of TRPV4 is required for TRPV4 activation induced by hypotonicity and heat (78). PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+ (160). PIP2 hydrolysis induced by Ca2+ entry is responsible for Ca2+-dependent inactivation of TRPV6 (297) and desensitization of TRPV2 (186). In contrast, PIP2 hydrolysis potentiates voltage- and temperature-dependent TRPV3 activation (56). PIP2 was originally reported as an inhibitor of TRPV1 (37), but it is now believed that PIP2 is an activator of TRPV1 (138, 169, 176, 308, 348). PIP2 regulates or activates TRPM channels including TRPM2 (302), TRPM4 (209), TRPM5 (171), TRPM6 (338), TRPM7 (253), and TRPM8 (169, 247). For example, PIP2 regulates the channel activities of TRPM2, TRPM4, and TRPM5 by changing Ca2+ affinity (302) or Ca2+ dependence (171, 209). Furthermore, PIP2 is required for TRPM6 and TRPM7 activation, since depletion of PIP2 inactivates TRPM6 and TRPM7 (253, 338). Activation of TRPM8 by PIP2 is synergized by menthol and low temperature (247). PIP2 modulates TRPA1 by preventing its desensitization (129).
Regulation of TRP channels by lipids and lipid rafts.
A variety of ion channels are regulated by lipid rafts, the lipid microdomains in the plasma membrane (47, 162, 178). Several TRP channels, including TRPC1, TRPC4, TRPC5, TRPV1, TRPM7, and TRPM8, are associated with lipid rafts (22, 195, 352). Lipid rafts are enriched with cholesterol, sphingolipids, and caveolin (Fig. 3). Cholesterol depletion inhibits TRPC1 currents in ECs (156, 226) but increases TRPM8 currents induced by menthol and cooling stimuli in sensory neurons and overexpression systems (195, 237), and likely SMCs (122). Cholesterol was proposed to be essential for membrane targeting of TRPV1 (174, 289). Moreover, cholesterol binds to TRPV1 and inhibits TRPV1 current (233). TRPM3 in SMCs is partially suppressed by endogenous cholesterol, and foam cells were loaded with cholesterol lack TRPM3 activity (205).
Sphingosine (SPH) and phosphorylated sphingosine (S1P), the metabolites of sphingomyelin in lipid rafts, can regulate channel activity directly or through signaling pathways mediated by five different types of S1P receptors (S1PRs). Stimulation of S1PRs by S1P induces Ca2+ entry through TRPC5 and controls the motility of SMCs (340). TRPC5 is also regulated by oxidized phospholipids, 1-palmitoyl-2-glutaroyl-phosphatidylcholine (PGPC), and 1-palmitoyl-2-oxovaleroyl-phosphatidylcholine (POVPC), via activation of the G(i/o) pathway (5). TRPM7 can be inhibited directly by SPH (242) or indirectly by S1P through activating Gq-linked S1PRs (253). SPH can also directly activate TRPM3 (90). In lipid rafts, TRPM3 activity is determined by inhibition by cholesterol and activation by SPH (205).
ROLE OF TRP CHANNELS IN THE HEART
TRP channels play an essential role in heart function. For example, deletion of TRPM7 and TRPP1/TRPP2 in mice can cause abnormal heart development. Patients carrying mutations of TRPM4 develop cardiac conduction defects (CCDs). Other TRP channels have been shown to play important roles in the pathogenesis of other heart diseases, although deletion of these channels normally does not cause obvious heart defects under normal physiological conditions.
TRP Channels in Hypertrophy and Heart Failure
Role of TRPC channels in hypertrophy and heart failure.
The function of TRPC channels in the heart under normal physiological conditions are elusive, since knockout of several TRPC genes does not produce any abnormality in mouse hearts. However, TRPC channels have been shown to play an essential role under pathological conditions. In cultured neonatal rat cardiomyocytes, hypertrophic agonist stimulation with endothelin-1 (ET-1), phenylephrine, and ANG II leads to upregulation of TRPC1 (220), TRPC3 (21), and TRPC7 (262). In the pressure overload mouse and rat models, TRPC3 was upregulated (25). In heart failure patients, TRPC5 expression is induced to express (25), whereas TRPC6 is significantly upregulated (151). With the use of siRNA, it was demonstrated that TRPC3 and TRPC6 are required for ANG II-induced NFAT translocation in cultured rat myocytes (222), a crucial step in cardiac hypertrophy (100). Moreover, overexpression of TRPC7 increased apoptosis of rat cardiomyocytes induced by ANG II (262); and TRPC3 overexpression increased apoptosis by activating calpain in adult mouse myocytes subjected to ischemia/reperfusion injury (269). In in vivo studies, transgenic mice overexpressing TRPC3 showed increased calcineurin/NFAT (Cn/NFAT) activation, cardiomyopathy, and increased hypertrophy when subjected to ANG II/phenylephrine infusion or pressure overload stimulation (201). Transgenic mice overexpressing TRPC6 at an intermediate level develop hypertrophy and heart failure without stimulation, whereas mice with a high level of TRPC6 expression die from cardiomyopathy (151). Knocking out TRPC1 protects mice against pressure overload induced hypertrophy by altering mechanosensitive signaling through calcineurin-Nuclear factor of activated T-cells (Cn/NFAT), mammalian target of rapamycin, and Akt (267). Transgenic mice expressing myocyte-specific dominant-negative TRPC3, TRPC6, or TRPC4 attenuate the cardiac hypertrophic response following either neuroendocrine agonist infusion or pressure-overload stimulation through Cn/NFAT activation, suggesting that TRPCs, either TRPC3/6/7 or TRPC1/4/5 complexes, are important mediators of pathological hypertrophy and may serve as potential therapeutic targets (69, 335). Indeed, Pyr3, a BTP2 derivative that specifically inhibits TRPC3, was reported to block hypertrophy in mice subjected to pressure overload (136). The recently developed TRPC3/6 dual blockers inhibit isolated cultured myocyte hypertrophy signaling triggered by ANG II or ET-1 (266). Moreover, the protective effects against pressure-overload hypertrophy was only observed in TRPC3/6 double KO mice, but not in single TRPC3−/− or TRPC6−/− mice (266), supporting the value of dual blockage.
TRPC3 and TRPC6 channel activities can be inhibited when phosphorylated by protein kinase G (PKG) at T11/ and T70/S322, respectively (135, 141, 152, 153). The cGMP/PKG-dependent inhibition of TRPC3 and TRPC6 negatively regulates Cn/NFAT activation and contributes to antihypertrophic effects of natriuretic peptides-Guanylyl Cyclase-A (GC-A) signaling in the heart (135, 141). In mice lacking GC-A, the constitutively active Cn/NFAT pathway can be inhibited by the selective TRPC channel blocker BTP2, thereby attenuating hypertrophy. Conversely, overexpression of TRPC6 in mice lacking GC-A exacerbated cardiac hypertrophy. Moreover, it was found that inhibition of TRPC6 by PKG phosphorylation underlies the mechanism for antihypertrophic effects of the cardiac ANP/BNP GC-A pathway (137).
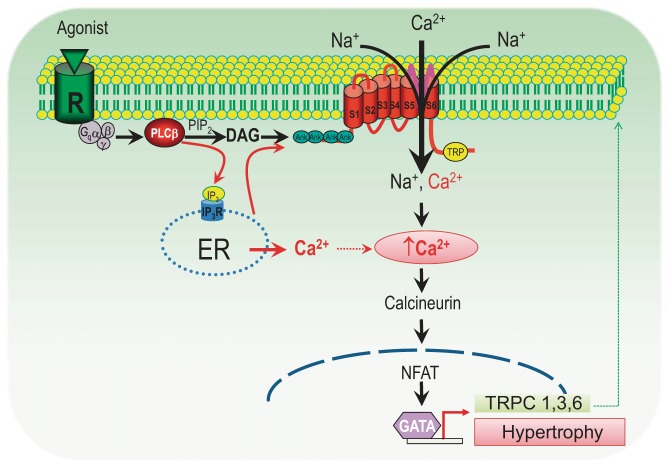
It appears that the mechanism by which TRPC channels-mediated hypertrophy is through activation of the Cn/NFAT pathway. When activated by Gq-linked receptor agonists, stretch, or other stimuli, TRPC channels mediated Ca2+ influx activates calcineurin (Cn), leading to NFAT translocation and activation of the hypertrophy cascade (Fig. 4). Meanwhile, activation of the Cn/NFAT signaling pathway also contributes to the upregulation of several TRPC channels, including TRPC1, TRPC3, and TRPC6, which have conserved NFAT consensus sites in their promoters (25, 151, 220). This positive feedback mechanism causes long-termed hypertrophic remodeling in the heart (Fig. 4).
Fig. 4.
Proposed mechanism underlying cardiac hypertrophy via TRPC/calcineurin/Nuclear factor of activated T-cells (NFAT) pathway. Activation of Gq-linked receptors by hypertrophic stimuli, such as ANG II, phenylephrine (PE), and endothelial-1 (ET-1), leads to the production of DAG and IP3 via hydrolysis of PIP2 by PLC activation. DAG activates TRPC3 and TRPC6, and IP3-induced store depletion releases Ca2+ and subsequently activates TRPC channels. The resultant rise in [Ca2+]i activates calcineurin (Cn) and causes translocation of NFAT, leading to the activation of hypertrophic gene expression, including TRPC1, 3, and 6. Upregulated TRPC channels will further activate the Cn/NFAT pathway, thereby perpetuating the TRPC-Ca2+-Cn/NFAT hypertrophy cascade (69, 151, 325).
Role of TRPV1 and TRPV2 in hypertrophy and heart failure.
TRPV1 is mainly expressed in sensory neurons (41). The capsaicin-sensitive cardiac nerves regulate a series of complex cellular events contributing to physiological and pathological myocardial function, including regulation of normal cardiac function and development of cardiac adaptation to ischemic stress (225, 370). Deletion of Trpv1 impairs post-ischemic recovery in the isolated perfused mouse heart (320). Activation of TRPV1 under ischemic conditions protects mouse hearts from injury possibly via increasing substance P (SP) release from the capsaicin sensory neurons (320); Trpv1 deletion also impairs preconditioning protection against myocardial injury (367). Moreover, activation of TRPV1 by endogenous activator 12-lipoxygenase-derived eicosanoids protects hearts against myocardial ischemia/reperfusion injury (268).
In contrary with the protective effect of TRPV1 in ischemic injury, systemic capsaicin pretreatment resulted in cardiac dysfunction characterized by elevation of left ventricular end-diastolic pressure (370). Mice lacking TRPV1 were also protected from pressure overload induced cardiac hypertrophy (23). Administration of TRPV1 antagonist BCTC (4-(3-Chloro-2-pyridinyl)-N-[4-(1,1-dimethylethyl)phenyl]-1-piperazinecarboxamide) prevents loss of heart function in a cardiac hypertrophy mouse model (108), suggesting that antagonizing TRPV1 can be a new treatment option for cardiac hypertrophy and heart failure (108).
TRPV2 is localized intracellularly and can translocate to the plasma membrane when stimulated by receptor agonists (115, 128) or mechanical stress (115). It was demonstrated that whereas TRPV2 is localized to the intracellular compartments and intercalated discs in normal ventricles, it is extensively localized to the ventricular sarcolemma in dilated cardiomyopathy (DCM) patients as well as in animal models of heart failure, including DCM hamsters and DCM mice (116). Overexpression of the NH2-terminal TRPV2 domain significantly reduced the sarcolemmal accumulation of TRPV2 and simultaneously ameliorated cardiac dysfunction, preventing DCM progression and improving survival of DCM mice (116). Moreover, the TRPV2 inhibitor tranilast effectively prevented DCM progression in DCM hamsters (116), suggesting that the sarcolemmal TRPV2 accumulation plays a crucial role in Ca2+-induced myocyte degeneration in DCM. Interestingly, a recent study demonstrated that TRPV2 is essential to maintain normal cardiac structure and function (131). Trpv2 deletion leads to reduced heart performance (131, 250) and significantly increased death rate (131). Therefore, TRPV2 plays an important role in the heart under physiological and pathological conditions.
Role of TRPM2 in ischemic cardiomyopathy.
TRPM2 is highly expressed in immunocytes (93, 230, 261, 343). In contrast, the expression level of TRPM2 in the heart detected by quantitative PCR is relatively low (59, 73). The level of TRPM2 mRNA in the heart was ∼36-fold lower than that of the polymorphonuclear leukocytes (PMNs) (103). In cardiac myocytes, the potential role of TRPM2 was implicated by evaluating the effects of TRPM2 blocker clotrimazole on myocyte apoptosis (345). Yang and colleagues (345) demonstrated that inhibition of TRPM2 by clotrimazole significantly reduced neonatal rat myocyte apoptosis induced by H2O2. Detailed characterization of TRPM2, such as current recording or evaluation of expression level, was not reported in rat cardiac myocytes (345). In rat cardiac fibroblasts, however, Takahashi and colleagues found that TRPM2 expression was enhanced by hypoxia treatment for 24 h, and clotrimazole sensitive TRPM2-like currents were also significantly larger in hypoxia-treated fibroblasts. Moreover, TRPM2 in cardiac fibroblasts were upregulated in atrial fibrillation (AF) patients in comparison with control patients (361). Recently, there were two controversial reports about pathophysiological functions of TRPM2 in the mouse heart (103, 188). Hiroi and colleagues (103) reported that neutrophil TRPM2, but not myocardial TRPM2, exacerbates myocardial ischemia/reperfusion (I/R) injury, and that knocking out TRPM2 protects the heart against I/R injury. Not only was the infarct size smaller in the TRPM2-KO heart 24 h after I/R injury but contractile function was also improved (103). In contrast, Miller and colleagues demonstrated that at 2 or 3 days after I/R injury, there was no difference between WT and TRPM2-KO mouse hearts in infarct size and the areas at risk. Moreover, TRPM2-KO mice exhibited impaired heart performance (188). It is unclear why there was such a large discrepancy between the two research groups' findings, given that both groups used the I/R injury mouse model, global TRPM2-KO, and assessed heart function at 24 h or 48–72 h after reperfusion. Further investigation is required to clarify the function of TRPM2 in I/R myocardial injury as well as the underlying mechanisms.
Role of PKD1 and PKD2 in hypertrophy.
PKD1 and PKD2 are two proteins associated with autosomal dominant polycystic kidney disease (ADPKD), the most common inherited form of kidney failure. Patients with ADPKD have various clinical complications including hypertension and hypertrophy (15, 29, 111). PKD1 and PKD2 form a functional channel complex (92, 238, 305), which can be located in the plasma membrane (49, 86, 92), endoplasmic reticulum (ER) (10, 26, 144), SR (10, 26, 144), primary cilium (80, 177, 351), mitotic spindles (251), and centrosome (126). The majority of PKD2 proteins are located in the ER (26), and plasma membrane targeting of PKD2 is dynamically regulated by interacting proteins, posttranslational modifications, interactions with other channel subunits, and trafficking between the ER and plasma membrane (306). Although the detailed mechanisms by which PKD2 dysfunction causes malfunction in various systems remain elusive, it has been shown that PKD2 regulates IP3R-mediated Ca2+ signaling (166) and ryanodine receptor (RyR2)-dependent Ca2+ signaling (10) by directly interacting with inositol trisphosphate (IP3) receptor (IP3R) and RyR2. The NH2 terminus of PKD2 binds to RyR2, whereas the COOH terminus only binds to RyR2 in its open state (10). The COOH terminus of PKD2 functionally inhibits RyR2 channel activity. Pkd2−/− cardiomyocytes displayed a higher frequency of spontaneous Ca2+ oscillations, reduced Ca2+ release from the SR, and reduced Ca2+ content as well as reduced Ca2+ transient when compared with Pkd2+/+ cardiomyocytes (10). This decrease in SR Ca2+ load and altered RyR2 function may activate a series of molecular events and thereby contribute to the cardiovascular abnormalities observed in patients with ADPKD.
Role of TRP Channels in Heart Development
Role of PKD2 in heart development.
Defective cardiac valve formation is one of the clinical complications of ADPKD (15, 29, 111). With the use of Pkd2−/− mice, it was further confirmed that PKD2 is essential to normal development of the interventricular and interatrial septa (334). Pkd2−/− mice die in utero between embryonic day (E) 13.5 and parturition, with structural defects in cardiac septation (334). Although it is known that PKD2 has a stable expression during heart development, the mechanisms by which PKD2 is involved in cardiac development have remained unknown (30). Moreover, the Pkd2−/−-caused cardiac development phenotype resembles the phenotype of tolloid-like I (TLL1) deletion, a bone morphogenetic protein-1-related metalloprotease (42). Thus it is plausible that the signaling pathways that are involved in TLL1-deletion induced heart defects also underlie the mechanism of heart defects in Pkd2 −/− mice (324). Nonetheless, further studies are required to better understand the mechanism of Pkd2−/−-induced heart defects.
Role of TRPM7 in heart development.
TRPM7 is essential for early embryonic development (120, 121). Global deletion of Trpm7 in mice results in embryonic lethality before embryonic day 7 (E7). Deletion of Trpm7 before and during organogenesis results in severe tissue-specific defects in the kidney and brain, whereas deletion of Trpm7 after E10.5 does not alter normal brain development (121). TRPM7 also plays an essential role in the early, but not late, developmental stages of the heart (256, 257). Cardiomyocyte-specific deletion of Trpm7 at about E13 by αMHC-Cre produces viable mice with normal adult ventricular size and function (256). However, cardiomyocyte-specific deletion of Trmp7 before E9 by TnT/Isl1-Cre results in impaired compact myocardium development with consequent congestive heart failure and embryonic death by E11.5 (256). Interestingly, if a second copy of Trmp7 is deleted at an intermediate time point by using αMHC-Cre in mice with Trpm7 already deleted in one allele, it will result in 50% of mice with normal heart function, and 50% of mice with penetrant adult cardiomyopathy characterized by ventricular dysfunction, disrupted atrioventricular conduction, dispersed ventricular repolarization, and ventricular arrhythmia (256). This cardiomyopathy is associated with upregulated hypertrophy genes (Nppa/Actal/Postn/TGF-β2/Timpl) and downregulated genes expressed in the conduction system (TRPM4, HCN4, KCNJ3) as well as genes for the repolarizing K+ channels (KCNK3, KCNA1, KCND2, KCNJ3, KCNV2). Interestingly, HCN4 expression in the SAN can be regulated by Trpm7 deletion in adult mice, likely due to the “fetal-like” and “embryonic” nature of SAN cells (257). Nonetheless, further investigation is required to understand how TRPM7 or the kinase domain of TRPM7 (145) exhibits transcriptional regulation of various ion channels in the heart and in other systems.
Role of TRP Channels in Arrhythmogenesis
TRPM4 and CCD.
Several TRP channels have been shown to be involved in arrhythmogenesis. A mutation of TRPM4 in the NH2 terminus (Asn7lys substitution, E7K) was identified in a large African family with autosomal-dominant progressive familial heart block type 1 (PFHB1) (147). Although the biophysical properties of TRPM4E7K are similar to those of WT TRPM4, TRPM4E7K are constitutively SUMOylated, resulting in impaired endocytosis and enhanced TRPM4 current density (147). However, the precise mechanism by which gain-of-function of TRPM4 causes conduction block remains unknown.
Several other mutations of TRPM4 have been identified recently in families with autosomal dominant isolated cardiac conduction block (173, 280). Similar to the E7K mutant, the A432T, G844D, R164W identified in three unrelated families with autosomal dominant isolated cardiac conduction block are also gain-of-function mutations (173). Moreover, mutants Q133H, Q293R, G582S, Y790H, K914R, and P970S have recently been identified in eight familial or sporadic cases with CCD by analysis of 160 unrelated probands with CCD, albeit the biophysical functional changes of these mutants are unknown (280). There are also 8 polymorphisms, including two in-frame deletions (R762-G765del and K487-L498del) identified in Brugada syndrome (BrS), right bundle branch block (RBBB) and control individuals (280). The majority of mutations are located at the NH2-terminal of TRPM4, Y790H is located within TM2, K914R is located at the TM3 and TM4 linker, and P970S is a pore mutant (Fig. 5). Moreover, the mutation K914R is located within a proposed SUMOylation site, and Q131H is located within the calmodulin binding site (280). It will be crucial to understand the mechanism by which TRPM4 mutations cause CCD.
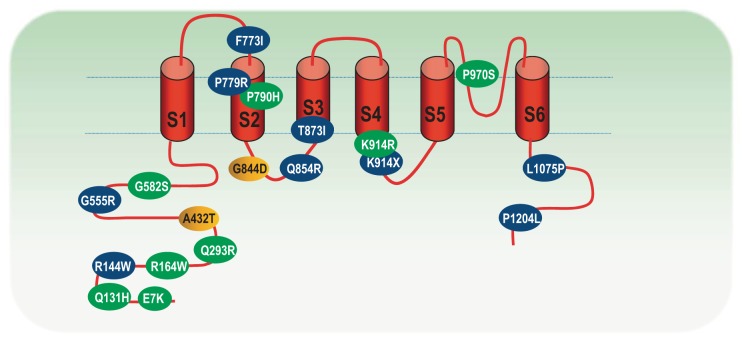
Fig. 5.
Localization of the identified mutations in the TRPM4 channel. Mutations resulting in cardiac conduction defect are in green; mutations identified in Brugada syndrome (BrS) are in blue; and mutations identified in both are in yellow (147, 172, 173, 280). There are also 8 polymorphisms (not shown), including A101T, Y103C, R252H, K487-L498del, D561A, R762-G765del, Q854R, and P1204L identified in BrS, right bundle branch block, and control individuals (280). Modified from Stallmeyer et al. (280).
TRPM4 and BrS.
Whereas TRPM4 mutations were not identified in heritable arrhythmia entities such as sinus dysfunction (SND), BrS, or congenital LQTS in a cohort reported by Stallmeyer et al. (280), Liu and colleagues (172) recently identified 14 heterozygous variants of TRPM4 in 20 unrelated individuals in a large cohort of 248 BrS cases with no SCN5A mutation. The 14 TRPM4 variants include five new mutations (G555R, F773I, P779R, T873I, and L1075P), two known mutations (A432T, G844D), four putative BrS predisposing factors as defined by higher prevalence in the BrS cohort than the control population (R144W, Q854R, K914X, and P1204L), and three polymorphism variants (G582S, G737R, and K487-I498del) (172). In total, TRPM4 mutations account for about 2.7–6% of BrS cases (172). The functional change of these mutants is complex. The K914X is a nonfunctional channel mutant. The P779R exhibits reduced channel function due to decreased expression and shift of voltage dependence. The mutations T873I and L1075P show no change in whole cell current and single channel properties although their surface expression is increased. Nonetheless, the mechanisms linking TRPM4 functional changes and ECG perturbations observed in BrS remain elusive.
TRPC6 and arrhythmia.
TRPC6 may contribute to stretch associated AF. In rabbit hearts, dilation-induced AF potentiation can be inhibited by a nonselective cation channel blocker GxMTx4, a peptide isolated from tarantula venom (19). It is likely that TRPC6 underlies the nonselective cation channels, since TRPC6 activated by stretch as well as receptor stimulation in HEK cells can be blocked by GxMTx4 (278) and GxMTx4-sensitive TRPC6-like currents induced by myocyte deformation can be inhibited by TRPC6 antibody (60). TRPC6 is considered a stretch-sensitive ion channel and an important regulator of Ca2+ signaling in the endothelial cells of the atrial endocardial endothelium (207). It will be of importance to investigate whether stretch related AF is diminished in Trpc6−/− mice.
TRPC3 and arrhythmia.
In embryonic chick hearts, Sabourin and colleagues (255) demonstrate that TRPC1,3,4,5,6, and 7 are expressed in the atria, ventricles, and outflow tract of the developing chick heart. TRPC channels interact with the L-type Ca2+ channel α1C-subunit. Using ex vivo electrocardiograms, electrograms of isolated atria and ventricles, and ventricular mechanograms, the authors demonstrate that inhibition of TRPC channels by SKF-96365 leads to negative chronotropic, dromotropic, and inotropic effects; prolongs the QT interval; and provokes first- and second-degree atrioventricular blocks. Pyr3, a specific antagonist of TRPC3, affected atrioventricular conduction. Thus, TRPC channels, via interaction with the CaV1.2 channel, may play a key role in regulation of cardiac pacemaking, conduction, ventricular activity, and contractility during cardiogenesis (255). Indeed, TRPC3-specific blocker Pyr3 suppresses the A1-subtype of the adenosine receptor (A1AR) induced conduction disturbance (254), suggesting that TRPC3 may represent a potential therapeutic target for A1AR mediated conduction disorder. Moreover, TRPC3 is involved in fibrosis formation and inhibition of TRPC3 reduces the vulnerability to AF (94).
Role of TRP Channels in Cardiac Fibrosis and Fibrotic Heart Diseases
Fibrosis is associated with various forms of heart disease, including myocardial infarction, arrhythmia, hypertrophy, and heart failure (358, 362). Fibrosis is the accumulation of excessive extracellular matrix (ECM) proteins produced by cardiac fibroblasts and myofibroblasts. Fibroblasts are quiescent under normal conditions. When stimulated by various stimuli, fibroblasts proliferate, differentiate to myofibroblasts, synthesize ECM proteins and cytokines, and initiate the fibrogenesis cascade (358, 362). Ca2+ signaling has been shown to play a key role in fibroblast proliferation and differentiation; however, the ion channels responsible for Ca2+ entry remained unknown. Given that TRP channels are nonvoltage gated and Ca2+ permeable, they have been considered as candidate molecules responsible for Ca2+ influx in cardiac fibroblasts (59). Among the TRP channels expressed in fibroblasts as detected by RT-PCR (Table 1) (31, 59, 248, 361), TRPM7 currents can be readily recorded by patch-clamp (59, 253). TRPC3, TRPC3/6-like, TRPM2, and TRPV4 currents have also been recorded in cardiac fibroblasts (94, 97, 248, 291). Recent studies have demonstrated that Ca2+ signaling mediated by TRP channels plays a pivotal role in cardiac fibrogenesis and fibrotic heart diseases (4, 48, 59, 94) (Fig. 6).
Fig. 6.
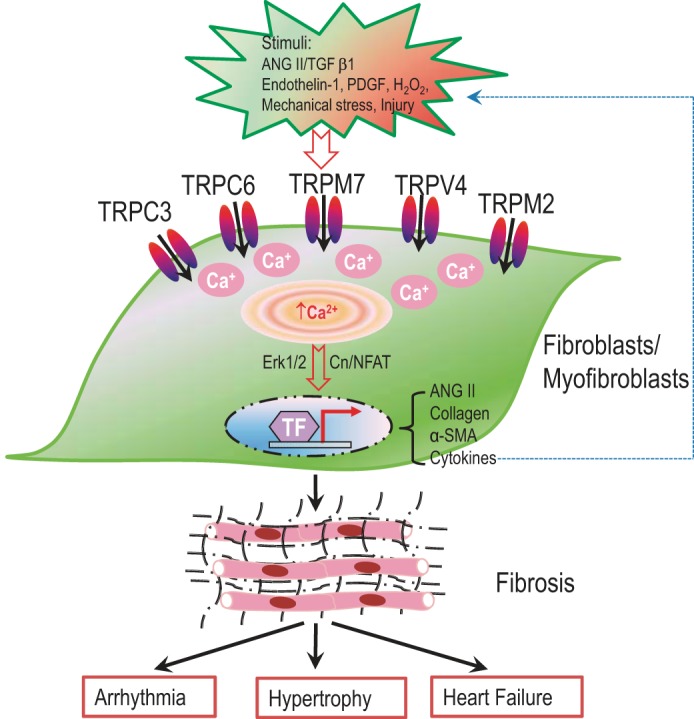
TRP channels and heart diseases associated with cardiac fibrosis. Several TRP channels, including TRPC3, TRPC6, TRPV4, TRPM2, and TRPM7, are functionally expressed in cardiac fibroblasts. Different TRP channels are activated when fibroblasts are stimulated by different stimuli, and mediate Ca2+ entry to support fibroblast proliferation, differentiation to myofibroblasts, and synthesis of extracellular matrix proteins and cytokines. Cytokines will in turn stimulate fibroblasts/myofibroblasts to perpetuate the fibrogenesis cascade. Fibrosis is the results of excessive extracellular matrix protein deposition, which contributes to the pathogenesis of various diseases, such as arrhythmia, hypertrophy, and heart failure.
TRPM7- and TGFβ1-induced fibrogenesis.
TRPM7 is significantly upregulated in human atrial fibroblasts from AF patients (59). TRPM7-mediated Ca2+ entry contributes to enhanced Ca2+ influx in AF fibroblasts. Knockdown of TRPM7 inhibits TGFβ1-induced fibroblast proliferation, differentiation, and collagen production (59). Moreover, TRPM7 is also upregulated by TGF-β1 in cultured fibroblasts. It appears that TRPM7-mediated Ca2+ signaling is essential for the TGF-β1 induced fibrogenesis cascade. Because fibrosis is one of the major detrimental factors for AF, and because TGF-β1 is a potent promoter of fibroblast differentiation and fibrogenesis, the effects of TRPM7 in TGF-β1-mediated fibrosis suggest that TRPM7 may serve as an effective therapeutic target for cardiac fibrosis. However, the mechanism by which TRPM7 is involved in the fibrogenesis cascade is still being intensely investigated.
TRPC3 Regulates Fibrosis and AF
An elegant study by Harada and colleagues (94) recently demonstrated that TRPC3 regulates fibroblast function, and thereby contributes to AF development. TRPC3 is highly expressed in freshly isolated fibroblasts, but largely diminishes in differentiated myofibroblasts (94). Inhibition of TRPC3 reduces ANG II-induced Ca2+ influx and suppresses extracellular-signal regulated kinase (ERK)-phosphorylation, resulting in reduced fibroblast proliferation. TRPC3 is upregulated in atria from AF patients, goats with electrically maintained AF, and tachypacing-induced heart-failure dogs (94). AF increases TRPC3 channel expression by causing NFAT-mediated downregulation of microRNA-26 and causes TRPC3-dependent enhancement of fibroblast proliferation and differentiation. Moreover, in in vivo experiments, TRPC3 blocker Pyrazole-3 can prevent the development of AF substrate in the electrically maintained dog model of AF. Thus reducing TRPC3-mediated Ca2+ signaling appears to reduce the susceptibility to AF. TRPC3 is likely a potential therapeutic target for fibrosis associated AF (94).
TRPC6 and fibroblast differentiation.
In cardiac myocytes, TRPC6-mediated Ca2+ entry activates the NFAT signaling pathway and induces hypertrophic gene expression (151) (Fig. 4). In cardiac fibroblasts, TRPC6-mediated Ca2+ entry by ET-1 stimulation activates NFAT, which acts as a negative regulator against ET-1-induced fibroblast differentiation (216). Recently, Davis and colleagues (48) demonstrated that TRPC6 is necessary and sufficient for fibroblast differentiation induced by ANG II. The authors showed that TRPC6 is upregulated by TGF-β1 and ANG II via the p38 MAPK (mitogen-activated protein kinase) serum response factor, and activation of TRPC6 induces fibroblast differentiation by activating the calcineurin/NFAT pathway. Dermal fibroblasts without TRPC6 (Trpc6−/−) failed to differentiate to myofibroblasts induced by TGF-β1 (48). Mice without TRPC6 display impaired dermal wound healing. Moreover, the impaired cardiac wound healing in Trpc6−/− mice significantly increases death rate after myocardial infarction (MI) surgery and weakens heart function (48).
TRPV4 and cardiac fibroblast differentiation.
Cardiac fibroblasts encounter a variety of stimuli, including hormones, cytokines, oxidative stress, and mechanical stretch. Although TRPC6, TRPV4, and TRPM7 are all sensitive to mechanical stretch, a recent study demonstrates that only TRPV4 is required for fibroblast differentiation induced by mechanical stimulation (4, 295). Using TRPV4 antagonist AB159908 and TRPV4 shRNA, the authors show that TGF-β1-induced myofibroblast differentiation is dependent on ECM stiffness and can be attenuated by inhibiting TRPV4. The role of TRPM7 in fibroblast differentiation was excluded based on the lack of effects of 500 μM carvacrol (4). It should be noted that carvacrol is a nonspecific inhibitor of TRPL and TRPM7 (232) and a nonspecific activator of TRPV3 and TRPA1 (339).
In summary, it appears that TRPC3-, TRPC6-, TRPV4-, and TRPM7-mediated Ca2+-signals contribute to fibroblast differentiation induced by TGF-β1 (Fig. 6). Because these channels are activated by different stimuli, it is plausible that each channel plays its role under different physiological/pathological conditions. It would not be surprising if more TRP channels are found to be involved in fibroblasts differentiation as well as fibrosis-associated heart diseases, since most TRP channels expressed in fibroblasts are upregulated by TGF-β1 (59).
TRP CHANNEL AND VASCULAR PHYSIOLOGY/PATHOLOGY
Many TRP channels are highly expressed in ECs and vascular SMCs (VSMCs) (Fig. 7 and Table 1) (51, 52, 102, 287, 348). TRP channel-mediated Ca2+ entry has been demonstrated to play an essential role in regulation of vascular tone, vascular permeability, mechano-sensing, secretion, angiogenesis, cell proliferation, cell death, and various vascular disorders.
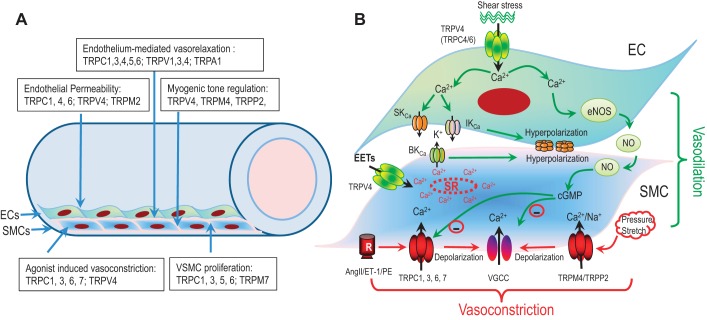
Fig. 7.
Role of TRP channels in the vascular system. A: TRP channels expressed in endothelial cells (ECs) and smooth muscle cells (SMCs) are involved in various functions of ECs and SMCs, including vasorelaxation, vasoconstriction, endothelial permeability, myogenic tone regulation, and SMC proliferation. B: schematic diagram illustrating endothelium-dependent vasorelaxation, and agonists as well as stretch/pressure-induced vasoconstriction. Activation of TRPV4 or other TRP channels in ECs by shear stress induces vasorelaxation through at least 2 pathways: 1) Ca2+ entry-mediated by TRPV4 activates SKCa and IKCa, leading to hyperpolarization of ECs and relaxation of SMCs through myoendothelial coupling (gap junctions); 2) Ca2+ influx via TRP channels in ECs enhances the synthesis and release of vasodilator factors such as nitric oxide (NO), which can inhibit voltage-gated Ca2+ channels (VGCC) and TRPC6 via cGMP-dependent pathway to induce relaxation. In SMCs, TRPV4 activation by EETs or other agonists triggers SR Ca2+ release and activation of BKCa, leading to hyperpolarization and vasorelaxation. Activation of TRPC channels such as TRPC6 or TRPC3 in SMCs via Gq-linked GPCRs stimulation by agonists (ANG II, ET-1, or PE) causes depolarization, and subsequent activation of VGCC leading to vasoconstriction. Moreover, stretch or pressure-induced activation of TRP channels, such as TRPM4 and TRPP2, can also depolarize SMCs, resulting in vasoconstriction.
TRP Channels and Vascular Physiology
TRP channels and endothelium-mediated vasorelaxation.
Changes in [Ca2+]i in ECs regulate vascular tone by releasing vasodilatory mediators such as nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor (EDHF) or by inducing endothelium-dependent-hyperpolarization (EDH). Ca2+ entry through a variety of TRP channels, including TRPC1, TRPC3, TRPC4, TRPC5, TRPC6, TRPV1, TRPV3, TRPV4, and TRPA1, may contribute to endothelium-dependent vasodilation (75, 287, 354, 363). It has been demonstrated that TRPC4 is involved in NO-mediated vasorelaxation (75), since Trpc4−/− mouse aortic ECs display reduced ACh-induced Ca2+ entry and decreased endothelium-dependent NO-mediated vasorelaxation of blood vessels (75). Similarly, Trpc1−/− and Trpc3−/− mouse aortas have diminished Ca2+ influx and aortic relaxation induced by carbachol (CCh) (139), consistent with the notion that TRPC3 channel activity can stimulate endothelium-dependent hyperpolarization to induce relaxation (265). Moreover, TRPC5 and several other TRP channels including TRPC1, TRPC4, TRPV1, and TRPV3-4 are considered as NO sensors in ECs (354). Activation of TRPC5 by S-nitosylation upon GPCR stimulation elicits Ca2+ entry into ECs, which in turn amplifies NO production (292). NO from ECs diffuses into adjacent SMCs and suppresses TRPC6 and VDCC activity via NO/cGMP/PKG pathway, leading to relaxation of SMCs (Fig. 7).
The role of TRPV4 in the vasculature has been extensively investigated. Earley and colleagues (65) demonstrate that in cerebral artery myocytes, activation of TRPV4 by 11,12-EET increases the frequency of Ca2+ sparks, which activates BKCa and thereby hyperpolarizes the SMCs. The authors propose that TRPV4 forms a Ca2+ signaling complex with ryanodine receptors (RyRs) and BKCa channels that elicits SMC hyperpolarization and arterial dilation via Ca2+-induced Ca2+ release in response to an endothelial-derived factor (65). Similar vasodilatory effects were also observed in mesenteric artery (66). Studies using TRPV4 KO mice suggested that TRPV4 is involved in endothelium-dependent vasodilation in response to shear stress and ACh stimulation (96, 185). TRPV4 KO impairs endothelial-dependent relaxation (260, 277). Using transgenic mice that express a genetically encoded Ca2+ biosensor (GCaMP2), Sonkusare and colleagues (277) demonstrated that activation of a few TRPV4 channels in ECs can hyperpolarize SMC membrane potential by about 10 mV. The authors elegantly demonstrate that activation of TRPV4 by the agonist GSK1016790A elicits Ca2+ sparklets, which activate BKCa and SKCa channels, leading to TRPV4 induced hyperpolarization in ECs (277). The hyperpolarized ECs cause hyperpolarization of SMCs through gap junctions within the myoendothelial junctions (Fig. 7), leading to vasodilation. Whereas low-level activation of TRPV4 channels by GSK (3 to 10 nM) or via muscarinic receptor stimulation causes vasodilation, higher-level activation of TRPV4 (100 nM GSK) leads to rapid global Ca2+ overload in ECs and oscillations of blood-vessel diameter (277), consistent with the systemic activation of TRPV4-induced reduction in blood pressure and circulatory failure (332).
TRPA1 and TRPV3 are also involved in endothelium-dependent dilation of cerebral artery (63, 64). TRPV3-mediated endothelium-dependent relaxation can be blocked by inhibition of KCa and Kir channels (63). TRPA1 is present in membrane projections of ECs proximal to VSMCs in cerebral artery (63) and perivascular nerve (13). TRPA1-elicited vasodilation can be mediated by releasing calcitonin gene-related peptide at the perivascular nerves (13) or by activation of SKCa, IKCa, and inward rectifier (61, 63, 79, 241).
TRP channels and agonist-induced vasoconstriction.
Vasoconstriction can be induced by various agonists including norepinephrine, vasopressin, ET-1, ANG II, and UTP, by binding to receptors on the SMC to induce depolarization and constriction of arterial SMCs. Recent studies have demonstrated that TRPC channels are likely the mediator of receptor activation-induced vascular constriction. TRPC6 is responsible for α1-adrenoceptor-induced Ca2+ entry (114) and seems to be the vasopressin-activated cation channel in A7R5 cells (125). However, TRPC6-KO mice exhibit elevated blood pressure and enhanced agonist-induced contractility of aortic rings and cerebral arteries. The unexpected high blood pressure and enhanced response to agonist are likely caused by the upregulation of TRPC3 (54). ANG II activates TRPC6 at low concentration but activates TRPC1 at high concentration (100 nM) in mesenteric artery myocytes (259). ET-1 activates TRPC7, possibly as a heterotetramer with TRPC3 in rabbit coronary artery myocytes (229). A recent study also demonstrated that TRPV4 contributes to pulmonary vasoconstriction induced by serotonin (5-HT) (336). Thus different TRP channels may contribute to vasoconstriction in response to different vasoconstrictors (Fig. 7). The differential activation of these TRP channels by various excitatory stimuli may have important implications for the development of new therapeutic strategies targeting to specific vasoconstrictor mechanisms in vascular disease.
TRP channels and myogenic tone regulation.
Elevation of intravascular pressure causes depolarization and constriction (myogenic tone) of small arteries, and this myogenic response is a key element in tightly regulating blood flow in coronary, mesenteric, renal, and cerebral arteries. Although it is known that L-type VGCC-mediated Ca2+ influx is required for myogenic contraction secondary to SMC depolarization (206), it remains unknown how a change in intraluminal pressure is coupled to the initial depolarization of VSMCs. Recent studies implicate that several mechanically sensitive TRP channels, including TRPC1, TRPC5, TRPC6, TRPV1, TRPV2, TRPV4, TRPM4, TRPM7, and TRPP1/TRPP2, may play a role in pressure-induced depolarization of VSMCs (113). Moreover, TRPM8 expressed in SMCs can also cause constriction or vasodilatation, depending on previous vasomotor tone (122).
TRPC6.
TRPC6 has been shown to be directly (278) or indirectly activated by mechanical stretch (184), or potentiated by mechanical stretch (112). Regardless of these conflicting results, it was suggested that TRPC6 may play an important role in regulating vascular tone via the PLC/DAG and PLA2/20-HETE pathways (112, 113). Indeed, antisense of TRPC6 decreases TRPC6 protein expression and greatly attenuates arterial smooth muscle depolarization and contraction caused by pressure changes in intact rat cerebral arteries (330). However, the pressure-induced constriction of cerebral arteries was not impaired in Trpc6−/− mice (53, 54), implicating that TRPC6 may be involved in myogenic tone regulation within the context of other mechanosensitive TRP channels and/or upstream as well as downstream signaling pathways (102).
TRPM4.
TRPM4 has been shown to be involved in myogenic tone regulation. The pressure-induced depolarization of SMCs and myogenic vasoconstriction of intact cerebral artery can be attenuated by treatment with TRPM4 antisense (68). Knockdown of TRPM4 by anti-sense in pial artery SMCs in vivo leads to impaired adjustment of cerebral blood flow at elevated mean artery pressure (MAP) (245), indicating that TRPM4 is crucial for autoregulation of cerebral blood flow in response to pressure change in vivo (245). Moreover, inhibition of TRPM4 by 9-phenanthrol causes hyperpolarization and reduction of myogenic tone in rat cerebral artery (84). Although it appears that TRPM4 is involved in myogenic tone, it is unknown how intraluminal pressure can enhance TRPM4 channel activity (62), since the notion of direct mechanosensitivity of TRPM4 remains controversial (62, 196). It is likely that TRPM4 is activated indirectly by stretch through PKC-dependent regulation (67) and PLC-dependent activation (62). A recent study demonstrated that pressure-induced TRPM4 activation is through PLCγ1-mediated TRPC6 activation (85). In Trpm4−/− mice, however, the pressure-induced increase in vascular resistance in isolated hind limbs of Trpm4−/− mice is similar to that of WT mice (183). Further investigation is required to clarify the mechanosensitivity of TRPM4 and its physiological/pathological functions.
PKD1 and PKD2.
PKD1 and PKD2 colocalize at the membrane of the primary cilia of renal epithelial cells and ECs where they are proposed to transduce luminal shear stress into a Ca2+ signal (203, 204). PKD1 and PKD2 are also abundantly expressed in arterial myocytes and regulate myogenic tone (20, 240). In Pkd1−/− mice, the mesenteric myogenic response was significantly impaired (271). Interestingly, deletion of PKD2 in PKD1-deficient VSMCs restored stress activated channel activity (SAC) and myogenic tone, indicating that PKD2 inhibits SAC and myogenic tone. The mechanism by which PKD2 inhibits SAC activity is through interacting with filamin A, since the crosslinking of filamin A is critical for SAC regulation (271). The inhibition of PKD2 on SAC and myogenic tone can be reversed by overexpression of PKD1 (271), indicating that the PKD1/PKD2 ratio regulates pressure sensing. In cerebral arteries, however, where PKD2 is the predominant PKD isoform, knockdown PKD2 inhibits SAC currents and reduces myogenic tone (202). In non-SMC types such as kidney epithelium primary cilia and perinodal crown cells (142, 203, 353), PKD2 is stimulated by mechanical stretch. Thus it seems that PKD2 differentially regulates the myogenic response in cerebral and mesenteric arteries (202, 271), depending on cell types as well as the expression level of interacting partners such as PKD1.
TRP channels and vascular permeability.
The permeability of the endothelial barrier is balanced by the contraction force of ECs and adhesive force that hold the cells in a flattened state. An increase in the contraction force or decrease in the adhesive force enlarges the inter-endothelial gap, resulting in the loss of the selective vascular barrier to circulating macromolecules (299). A rise in [Ca2+]i initiates signaling cascades, which lead to EC contraction, actin polymerization, and disassembly of vascular cadherin at the adherens junctions, leading to endothelial barrier dysfunction, vascular leakage, and vascular edema (299). Several TRP channels, including TRPC1, TRPC4, TRPC6 (275), TRPV4 (7, 119), and TRPM2, have been implicated in regulation of vascular permeability (40, 99, 119, 227, 275, 298, 299). Trpc4−/− mice display impaired store operated Ca2+ entry (SOCE) and reduced lung vascular permeability in response to PAR-1 agonist thrombin (298). Inflammatory agonists thrombin and bradykinin also induce permeability changes in lung ECs through activation of TRPC6 (275), TRPC1 (227, 228), and TRPC1/4 (40). TNF-α-induced increase in TRPC1 expression in human pulmonary artery endothelial cells (HPAECs) resulted in marked endothelial barrier dysfunction in response to thrombin (228). Moreover, TRPC1-, TRPC3-, and TRPC6-mediated Ca2+ entry is likely responsible for VEGF-induced endothelial hyperpermeability (32, 118). TRPV4 has been implicated in endothelial hyperpermeability and lung injury (7, 119). Activation of TRPV4 by 4α-PDD and cytochrome P-450-derived EETs (Fig. 3) increases permeability specifically in the endothelial and epithelial layers of the alveolar septal wall of the lung (7). It appears that P-450-dependent activation of TRPV4 induces disruption of the alveolar septal barrier and underlies high vascular pressure-induced lung injury such as alveolar flooding and impairment of gas-exchange (7). Thus it seems that TRPC1 and TRPC4 channels most prominently influence the lung extra-alveolar endothelial barrier, whereas TRPV4 influences the lung capillary endothelial barrier (39).
ROS are important mediators of vascular barrier dysfunction in settings such as ischemia/reperfusion and hypoxic conditions. Although several TRP channels expressed in ECs are sensitive to oxidative stress, it was demonstrated that H2O2 increases endothelial permeability by activating TRPM2 in human pulmonary artery ECs (99). Moreover, excessive expression of TRPM2 also causes apoptosis in response to oxidative stress (99). However, the function of TRPM2 in endothelial permeability has not been demonstrated in Trpm2−/− cells or mice.
TRP channels and SMC proliferation.
Under pathological conditions, SMCs can switch from a contractile to proliferative phenotype, resulting in overgrowth of SMCs. Overgrowth of SMCs is associated with a variety of vascular diseases, including hypertension, atherosclerosis, and restenosis (18). TRPC1, TRPC3, TRPC6, TRPV1, TRPV4, TRPM3, and TRPM7 have all been implicated in phenotype switching and enhanced proliferation of SMCs. In human coronary artery SMCs (hCASMCs), upregulation of TRPC1 contributes to ANG-II mediated hCASMC proliferation (293). TRPC1 is also upregulated in human neointimal hyperplasia after vascular injury (148) and in rodent vascular injury models (16, 148). TRPC5-mediated Ca2+ signaling activated by S1P controls SMC motility (340). TRPC6 is upregulated in pulmonary artery smooth muscle cells (PASMCs) by PDGF and ET-1 in in vitro studies, and enhanced TRPC6 expression increases PASMC growth (150, 355). Moreover, the expression of TRPC3 and TRPC6 mRNA and protein is much higher in PASMCs from patients with IPAH than that from normotensive patients or patients with secondary pulmonary hypertension (355). TRPC3 blocker Pyr3 can inhibit SMC proliferation and prevent stent-induced arterial remodeling (140). In addition, TRPC1 and TRPC6 are upregulated by hypoxia-inducible factor (HIF-1) in PASMCs (319), whereas TRPC4 is upregulated in ECs induced by hypoxia (71). TRPV1 and TRPV4 in pulmonary artery SMCs are upregulated by hypoxia (322) and are involved in migration of SMCs (182). TRPV1 is also involved in SMC proliferation (322). TRPM3 is expressed in both contractile and proliferative SMCs (205). TRPM7 has been shown to be involved in SMC phenotype switching induced by ANG II (366). Given the important role of Ca2+ in SMC growth and phenotype switching, TRP channels can be potential targets for vascular remodeling caused by hyperplasia of SMCs.
Role of TRP Channels in Vascular Diseases
TRP channels and BP regulation.
Changes in Ca2+ homeostasis are associated with systemic hypertension and pulmonary arterial hypertension. Several TRP channels have been implicated to play an important role in blood pressure regulation.
TRPV1 and hypertension.
TRPV1 is expressed in the sensory nerve endings, endothelial cells, and SMCs. Activation of TRPV1 in in vitro preparations can induce relaxation (234, 371), constriction (264), or biphasic responses (130). Activation of TRPV1 at the sensory nerve ending by ananamide leads to the release of calcitonin-gene-related peptide (CGRP), resulting in vasodilatation in isolated arteries (371). Release of CGRP also underlies the mechanism by which TRPV1 regulates blood pressure in a high-salt rat model (163). TRPV1 also induces vasodilation by producing NO in ECs (234). However, in SMCs, activation of TRPV1 by 20-hydroxyeicosatetraenoic acid (20-HETE) released from the arterial wall by increased intraluminal pressure leads to the release of the vasoactive neuropeptide substance P, which binds to tachykinin NK1 receptor and induces SMC contraction and vasoconstriction (264). Moreover, it was reported that low concentrations of capsaicin (1–10 nM) induced relaxation through activation of TRPV1 in ECs, whereas high concentrations of capsaicin (0.1–1 μM) induced constriction via activation of TRPV1 in SMCs (130). Activation of TRPV1 by 1–10 nM capsaicin can also cause constriction in the coronary artery by releasing endothelin from nerve endings (290).
In in vivo studies, Trpv1 deletion does not alter MAP under normal conditions (224). However, TRPV1 seems involved in blood pressure regulation under pathological conditions. Long-term activation of TRPV1 by dietary capsaicin increases the phosphorylated levels of protein kinase A (PKA) and endothelial NO synthase in mesenteric arteries as well as plasma levels of NO metabolites, leading to endothelium-dependent relaxation and lowered blood pressure in hypertensive rats (344). TRPV1 also exhibits protective effects against endotoxin-induced hypotension and mortality in rats (321). Moreover, deletion of TRPV1 is associated with protective effects against obesity-induced hypertension (181). Therefore, it appears that TRPV1 plays different roles in regulating blood pressure under various pathological conditions.
TRPM4 and systemic BP regulation.
Although TRPM4 is associated with myogenic vasoconstriction (68, 245), Trpm4−/− mice are hypertensive (183). Further analysis indicated that the hypertension in Trpm4−/− mice is caused by elevated circulating catecholamine levels due to increased ACh-induced exocytosis in chromaffin cells (183). Moreover, agonist-induced contraction of aortic rings and pressure induced increases in vascular resistance of hind limbs are similar in Trpm4−/− and WT mice (183). Thus TRPM4 regulates BP by controlling catecholamine release from chromaffin cells (183).
TRPV4 and systemic BP regulation.
In vitro evidence indicates that TRPV4 is involved in EDHF-induced vasodilation. In in vivo studies, TRPV4-specific agonist GSK1016790A induces reduction in blood pressure and circulatory collapse (332), whereas TRPV4 KO mice do not exhibit changes in basal MAP (66, 332, 364). However, hypertension induced by inhibition of NO synthase was greater in TRPV4 KO mice than WT mice (66), suggesting a vascular protective effect of endothelial TRPV4. Moreover, TRPV4 agonists reduce MAP in mice and several other animal models (66, 363, 364), and the hypotensive effect is significantly enhanced in rats fed with a high-salt diet (76, 77). Thus endothelial TRPV4 activation may exert a protective effect against pathological hypertension.
Other TRP channels and BP regulation.
The role of TRP channels in regulating blood pressure seems more complicated than initially thought. Although inhibition of TRPC6 attenuates SMC depolarization and contraction caused by pressure in intact cerebral arteries (330), the basal MAP in TRPC6 KO mice is about 7 mm Hg higher than in WT mice (54). This unexpected increase in MAP is presumably caused by compensatory overexpression of TRPC3 (52, 54). In TRPC1 KO mice, the blood pressure is moderately decreased (263), and EDHF-dependent vasodilatation is enhanced, suggesting that TRPC1 is a negative regulator of EDHF-dependent vasodilatation. Moreover, a low Mg2+ level is associated with hypertension in animal models and in patients (159, 303), suggesting that Mg2+-permeable TRPM6 and TRPM7 may play a role in regulating BP. However, although it was shown that TRPM6 and TRPM7 are differentially regulated by ANG II in spontaneously hypertensive rats (98, 304), there is no convincing evidence to suggest that TRPM6 and TRPM7 are involved in BP regulation to date (323).
TRP channels and pulmonary hypertension: TRPC6 mutation and pulmonary hypertension (PH).
PH is a life-threatening disease, characterized by pulmonary vascular remodeling. A functional single-nucleotide polymorphism (SNP) has been identified in patients with idiopathic pulmonary artery hypertension (PAH) (356). The −254 (C to G) SNP in the promoter region of TRPC6 creates a binding sequence for NF-κB, leading to enhanced NF-κB-mediated promoter activity, which stimulates TRPC6 expression in PAMSCs (356). There are 6.3% of idiopathic PAH patients carrying homozygous −254(C to G), suggesting that this SNP may predispose individuals to a high-risk of developing idiopathic PAH by upregulating TRPC6 in PASMC (72).
TRP channels and hypoxic pulmonary hypertension.
Hypoxia is one of the most frequent inducers of chronic PH (81). Abnormal SMC proliferation is a primary hallmark of chronic hypoxia-induced PH, and [Ca2+]i is essential for SMC proliferation. Both TRPC6 and TRPC1 are predominantly expressed in precapillary pulmonary SMCs, which control pulmonary vascular resistance. TRPC6 is upregulated in hypoxia-induced PH (168) and in patients with idiopathic PAH (355). However, although TRPC6 is essential for acute hypoxic vasoconstriction, it is not involved in chronic hypoxia-induced PH, as evidenced by studies demonstrating that Trpc6−/− mice were not protected from hypoxia-induced vascular remodeling, increased right ventricular systolic pressure, or right heart hypertrophy (328). Because both TRPC6 and TRPC1 were upregulated in hypoxia-induced PH in mice and rats (319), a recent study demonstrated that TRPC1 plays an essential role in hypoxia-induced PH, as TRPC1 KO mice are at least partially protected from hypoxia-induced PH (179).
Several TRPVs and TRPMs are also expressed in pulmonary artery SMCs, among which TRPV4 is the only one that is significantly upregulated by chronic hypoxia (346). TRPV4 contributes to the enhanced myogenic response and to the full development of hypoxic PH (336, 346). Deletion of TRPV4 largely delays and attenuates PH, right heart hypertrophy, and vascular remodeling (346). Moreover, TRPV4 is suggested to be the major Ca2+ pathway for vasoconstriction induced by 5-HT (336), which is significantly upregulated in chronic hypoxic PH (336).
TRP channels and pulmonary edema.
Pulmonary edema represents a major cause of morbidity and mortality in heart failure (HF) patients. Dysfunction of the left ventricle in HF patients causes elevated pulmonary vascular pressure (PVP), leading to pressure-induced leakage of fluid and resultant edema. Activation of TRPV4 by synthetic activators, CYP metabolites of AA, or high vascular pressure causes increased lung permeability and lung injury (7, 119). Administration of TRPV4 activator GSK1016790A induces a dose-dependent reduction in blood pressure followed by circulatory collapse in dogs, rats, and WT mice but not in Trpv4−/− mice (332). Activation of TRPV4 elicited circulatory collapse is mediated by NO-independent failure of the endothelial-epithelial permeability barrier in the lung and selected tissues (332). TRPV4 blocker GSK2193874 prevents the increased vascular permeability and pulmonary edema caused by PVP in isolated rodent and canine lungs (296). Moreover, GSK2193874 pretreatment inhibited the formation of pulmonary edema and enhanced arterial oxygenation in both acute and chronic HF models. Furthermore, GSK2193874 treatment can reverse pulmonary edema already established by myocardial infarction in mice (296). Because TRPV4 is significantly upregulated in HF patients, inhibition of TRPV4 may serve as a novel therapeutic strategy for preventing and reversing HF associated pulmonary edema (296, 350).
Lung ischemia/reperfusion caused edema (LIRE) is another life-threatening lung injury that occurs after lung embolism, lung transplantation, and thrombarterectomy (329). Weissmann et al (328) demonstrated that TRPC6 plays an essential role in LIRE. ECs from Nox-2- and TRPC6-deficient mice displayed reduced ischemia-induced Ca2+ entry and attenuated impairment of barrier function. TRPC6-KO mice or Nox2 deletion mice are protected from ischemia/reperfusion-induced LIRE. However, deletion of TRPC1 and TRPC4 does not protect the mice from ischemia/reperfusion-induced LIRE (329). The working model for the potential role of TRPC6 in LIRE is that superoxide produced in ECs via Nox-2 activates PLC-γ, leading to generation of DAG and subsequently activation of TRPC6 as well as Ca2+ influx. Increased Ca2+ enhances endothelial permeability and results in edema (329).
TRP channels and atherosclerosis.
Atherosclerosis is a major cause of coronary artery disease, periphery vascular disorders, and stroke. Although the pathogenesis of atherosclerosis is not fully understood, dysfunction of ECs and SMCs contributes to atherosclerosis and monocytes/macrophages also play an essential role. Although many TRP channel are expressed in ECs and SMCs, little is known about the function of TRP channels in atherosclerosis. TRPC3 appears to be involved in atherosclerosis in Apoe−/− mice on high-fat diet (HFD), since Apoe−/− mice transplanted with bone marrow of Trpc3−/− mice showed slowed lesion progression and reduced necrotic core (294). Thus TRPC3 is likely involved in both initiation and progression of atherosclerosis. TRPV1 also seems to be involved in atherosclerosis. It has been shown that evodiamine produced protective effects on atherosclerosis in Apoe−/− mice through activation of TRPV1 (327). Moreover, deletion of TRPV1 in Apoe−/− mice promotes the progression of atherosclerosis (327). The mechanism by which TRPV1 exhibits protective effects in atherosclerosis is unknown and requires further investigation.
TRP channels and vascular complications.
Mutations of PKD1 and PKD2 cause ADPKD, a systemic disorder associated with fatal vascular complications. Subarachnoid hemorrhage (SAH) caused by intracranial aneurysms (IAs) is the most significant vascular complication of ADPKD. ADPKD patients develop IAs at a frequency five times higher than that of the general population.
Mutations of PKD1 and PKD2 can impair the function of ECs and SMCs. ADPKD patients present an impaired endothelium-dependent relaxation response to ACh in resistance vessels (318). Moreover, a frequent polymorphism of endothelial NO synthase, Glu298Asp, has been found to be associated with renal disease progression in ADPKD patients (154, 231). PKD2 mutations in ADPKD patients alter [Ca2+]i regulation and lead to impaired VSMC contractility (239). In animal models, endothelium-dependent vasorelaxation is impaired in Pkd1+/− mice (134, 198). Homozygous Pkd1−/− mice exhibit edema, vascular leaks, and rupture of blood vessels. PKD1-KO mice die between embryonic day 14.5 and 15.5, most likely as the result of massive hemorrhage (134). Thus PKD1 seems to be essential for maintaining the integrity of blood vessels. In fact, vascular fragility and leakage have been observed in both Pkd1−/− and Pkd2−/− mouse models (334). Because PKD1 and PKD2 are expressed in ECs, SMCs, and epithelial cells, there are several potential mechanisms by which PKD1 and PKD2 mutations cause vascular instability, leakage, and IAs. First, haplo-insufficiency of human PKD2 function alters [Ca2+]i regulation and leads to decreased VSMC contractility (239), which is essential for maintaining vascular structural and functional integrity. Weakened VSMCs of blood vessel walls become apoptotic under fluid mechanical stress, resulting in vascular leakage and aneurysms including IAs (331). Second, PKD1 is also localized at the intercellular junction of epithelial and endothelial cells, which may also contribute to the vascular leakage in Pkd1−/− mice (134).
After SAH, cerebral vasospasm is the next major cause of morbidity and mortality in ADPKD patients. ET-1 has been suggested as a prominent factor to elicit powerful constriction of cerebral arteries. A recent study demonstrates that ET-1 significantly enhances Ca2+ entry mediated by TRPC1 and TRPC4 in SMCs from a dog model of SAH (337). Given that VGCC antagonists are relatively less effective in cerebral vasospasm (249), targeting TRPC channels may provide a better way to attenuate ET-1 elicited vasospasm.
CONCLUSIONS
As polymodal sensors, transducers, and effectors of a variety of stimuli, TRP channels are involved in numerous fundamental cellular functions and play important roles in the pathogenesis of various diseases in different systems including the cardiovascular system. The rapid progress in TRP channel research in the cardiovascular system using targeted deletion and transgenic mouse models in recent years has revealed that TRP channels play pivotal roles in the pathogenesis of heart diseases and vascular disorders. TRP channels have been considered drug targets in different systems (127, 194, 213). In the cardiovascular system, many questions remain unanswered and require future investigations. First, given that TRP channels are widely expressed in different cell types, using tissue-specific or cell-type specific knockout of TRP channel genes will provide more precise information about the causative role of a specific TRP channel in the development of cardiovascular diseases. Second, developing pharmacological tools, including channel-specific activators and inhibitors, which are not available for many TRP channels, will undoubtedly accelerate the progress in deciphering the physiological and pathological roles of TRP channels in the cardiovascular system. Third, genetic linkage analysis in patients with channelopathies will further shed light on TRP channel function in human cardiovascular disorders. Fourth, the function of some TRP channels, such as intracellular TRPML channels, which have been shown to be required for repair of the sarcolemma (35), has remained unexplored in the cardiovascular system. Investigating the potential role of these TRP channels may reveal novel functions of TRP channels in the cardiovascular system. Taken all together, TRP channel research is a rapidly evolving research field. Future work in this exciting field may lead to new mechanistic insights and therapeutic options for cardiovascular diseases.
GRANTS
This work was generously supported by the National Institutes of Health, National Heart, Lung, and Blood Institute Grant 2R01 HL078960, and American Heart Association Grant 12GRNT12050683 (to L. Yue).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.Y. and L.Y. drafted manuscript; Z.Y., J.X., A.Y., J.S., J.D., and L.Y. edited and revised manuscript; Z.Y., J.X., A.Y., J.S., J.D., and L.Y. approved final version of manuscript; J.X. and L.Y. prepared figures; L.Y. conception and design of research.
ACKNOWLEDGMENTS
We thank all current laboratory members and former laboratory members for the support of the work. Special thanks to Kenneth H. Koltermann at University of California, San Diego, for helping to get information for figures and Yi Feng at Boston University School of Medicine for editing and proofreading the manuscript.
REFERENCES
- 1.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell 115: 863–877, 2003. [DOI] [PubMed] [Google Scholar]
- 2.AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res 104: 860–869, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 23: 297–328, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adapala RK, Thoppil RJ, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, Thodeti CK. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol 54: 45–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O'Regan D, Porter KE, Li J, Beech DJ. Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arterioscler Thromb Vasc Biol 30: 1453–1459, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberts B, Jihnson A, Lewis J, Raff M, Roberts K, Walter P. Memberane structure. In: Molecular Biology Of The Cell, edited by Alberts B, Jihnson A, Lewis J, Raff M, Roberts K, and Walter PGarland Sceience 2002, p. 583. [Google Scholar]
- 7.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 28: 2485–2494, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci 27: 3347–3355, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anyatonwu GI, Estrada M, Tian X, Somlo S, Ehrlich BE. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci USA 104: 6454–6459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Basora N, Boulay G, Bilodeau L, Rousseau E, Payet MD. 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J Biol Chem 278: 31709–31716, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102: 12248–12252, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol 559: 685–706, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belz MM, Fick-Brosnahan GM, Hughes RL, Rubinstein D, Chapman AB, Johnson AM, McFann KK, Kaehny WD, Gabow PA. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int 63: 1824–1830, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, Beech DJ, Dreja K, Hellstrand P. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol 288: C872–C880, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Bessac BF, Fleig A. TRPM7 channel is sensitive to osmotic gradients in human kidney cells. J Physiol 582: 1073–1086, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bochaton-Piallat ML, Gabbiani G. Modulation of smooth muscle cell proliferation and migration: role of smooth muscle cell heterogeneity. Handb Exp Pharmacol 170: 645–663, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature 409: 35–36, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci USA 98: 12174–12179, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner JS, Dolmetsch RE. TrpC3 regulates hypertrophy-associated gene expression without affecting myocyte beating or cell size. PloS one 2: e802, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brownlow SL, Sage SO. Transient receptor potential protein subunit assembly and membrane distribution in human platelets. Thromb Haemost 94: 839–845, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Buckley CL, Stokes AJ. Mice lacking functional TRPV1 are protected from pressure overload cardiac hypertrophy. Channels 5: 367–374, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burtis CA, Ashwood ER, Bruns DE editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. Elsevier Health Sciences, 2005. [Google Scholar]
- 25.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem 281: 33487–33496, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 274: 28557–28565, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Cao G, Lee KP, van der Wijst J, de Graaf M, van der Kemp A, Bindels RJ, Hoenderop JG. Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J Biol Chem 285: 26081–26087, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26: 13–25, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Chapman AB, Johnson AM, Rainguet S, Hossack K, Gabow P, Schrier RW. Left ventricular hypertrophy in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 8: 1292–1297, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Chauvet V, Qian F, Boute N, Cai Y, Phakdeekitacharoen B, Onuchic LF, Attie-Bitach T, Guicharnaud L, Devuyst O, Germino GG, Gubler MC. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am J Pathol 160: 973–983, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JB, Tao R, Sun HY, Tse HF, Lau CP, Li GR. Multiple Ca2+ signaling pathways regulate intracellular Ca2+ activity in human cardiac fibroblasts. J Cell Physiol 223: 68–75, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell calcium 41: 51–61, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng W, Yang F, Takanishi CL, Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J Gen Physiol 129: 191–207, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng X, Shen D, Samie M, Xu H. Mucolipins: intracellular TRPML1-3 channels. FEBS letters 584: 2013–2021, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng X, Zhang X, Gao Q, Ali Samie M, Azar M, Tsang WL, Dong L, Sahoo N, Li X, Zhuo Y, Garrity AG, Wang X, Ferrer M, Dowling J, Xu L, Han R, Xu H. The intracellular Ca2+ channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat Med 20: 1187–1192, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang Hh, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci USA 106: 20097–20102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Chubanov V, Mederos YSM, Waring J, Plank A, Gudermann T. Emerging roles of TRPM6/TRPM7 channel kinase signal transduction complexes. Naunyn Schmiedebergs Arch Pharmacol 371: 334–341, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid Redox Signal 11: 765–776, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cioffi DL, Stevens T. Regulation of endothelial cell barrier function by store-operated calcium entry. Microcirculation 13: 709–723, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Clark TG, Conway SJ, Scott IC, Labosky PA, Winnier G, Bundy J, Hogan BL, Greenspan DS. The mammalian Tolloid-like 1 gene, Tll1, is necessary for normal septation and positioning of the heart. Development 126: 2631–2642, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Cloutier M, Campbell S, Basora N, Proteau S, Payet MD, Rousseau E. 20-HETE inotropic effects involve the activation of a nonselective cationic current in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 285: L560–L568, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Corey DP. What is the hair cell transduction channel? J Physiol 576: 23–28, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432: 723–730, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature 224: 285–287, 1969. [DOI] [PubMed] [Google Scholar]
- 47.Dart C. Lipid microdomains and the regulation of ion channel function. J Physiol 588: 3169–3178, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell 23: 705–715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J 18: 740–742, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Demion M, Bois P, Launay P, Guinamard R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res 73: 531–538, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther 112: 744–760, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Dietrich A, Kalwa H, Gudermann T. TRPC channels in vascular cell function. Thromb Haemost 103: 262–270, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch 455: 465–477, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25: 6980–6989, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wang Y, Hong JH, Gryczynski Z, Yang SH, Birnbaumer L, Ma R. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem 286: 31799–31809, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doerner JF, Hatt H, Ramsey IS. Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J Gen Physiol 137: 271–288, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P2 controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nature Commun 1: 38, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du J, Xie J, Yue L. Intracellular calcium activates TRPM2 and its alternative spliced isoforms. Proc Natl Acad Sci USA 107: 7239–7244, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res 106: 992–1003, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell cal 45: 38–54, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Earley S. TRPA1 channels in the vasculature. Br J Pharmacol 167: 13–22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Earley S. TRPM4 channels in smooth muscle function. Pflügers Arch 465: 1223–1231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res 104: 987–994, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol 77: 612–620, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–H1102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292: H2613–H2622, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ Res 108: 265–272, 2011. [DOI] [PubMed] [Google Scholar]
- 70.Facemire CS, Mohler PJ, Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol 286: F546–F551, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 285: L1233–L1245, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Firth AL, Remillard CV, Yuan JX. TRP channels in hypertension. Biochim Biophys Acta 1772: 895–906, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26: 159–178, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci 92: 394–403, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol 3: 121–127, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Gao F, Sui D, Garavito RM, Worden RM, Wang DH. Salt intake augments hypotensive effects of transient receptor potential vanilloid 4: functional significance and implication. Hypertension 53: 228–235, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao F, Wang DH. Impairment in function and expression of transient receptor potential vanilloid type 4 in Dahl salt-sensitive rats: significance and mechanism. Hypertension 55: 1018–1025, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia-Elias A, Mrkonjic S, Pardo-Pastor C, Inada H, Hellmich UA, Rubio-Moscardo F, Plata C, Gaudet R, Vicente R, Valverde MA. Phosphatidylinositol-4,5-biphosphate-dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc Natl Acad Sci USA 110: 9553–9558, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci 16: 23–30, 1995. [DOI] [PubMed] [Google Scholar]
- 80.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 119: 1383–1395, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Ghofrani HA, Voswinckel R, Reichenberger F, Weissmann N, Schermuly RT, Seeger W, Grimminger F. Hypoxia- and non-hypoxia-related pulmonary hypertension. Established and new therapies. Cardiovasc Res 72: 30–40, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Gijón MA, Leslie CC. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J Leukoc Biol 65: 330–336, 1999. [DOI] [PubMed] [Google Scholar]
- 83.Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol 586: 5633–5649, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzales AL, Garcia ZI, Amberg GC, Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol 299: C1195–C1202, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCgamma1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal 7: ra49, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflügers Arch 455: 1097–1103, 2008. [DOI] [PubMed] [Google Scholar]
- 88.Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, Yorio T, Ma H, Ma R. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem 285: 23466–23476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C. Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278: 21493–21501, 2003. [DOI] [PubMed] [Google Scholar]
- 90.Grimm C, Kraft R, Schultz G, Harteneck C. Activation of the melastatin-related cation channel TRPM3 [corrected] by D-erythro-sphingosine. Mol Pharmacol 67: 798–805, 2005. [DOI] [PubMed] [Google Scholar]
- 91.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev 81: 685–740, 2001. [DOI] [PubMed] [Google Scholar]
- 92.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000. [DOI] [PubMed] [Google Scholar]
- 93.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9: 163–173, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Harada M, Luo X, Qi XY, Tadevosyan A, Maguy A, Ordog B, Ledoux J, Kato T, Naud P, Voigt N, Shi Y, Kamiya K, Murohara T, Kodama I, Tardif JC, Schotten U, Van Wagoner DR, Dobrev D, Nattel S. TRPC3-dependent fibroblast regulation in atrial fibrillation. Circulation 126: 2051–2064, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol 37: 307–314, 2005. [DOI] [PubMed] [Google Scholar]
- 96.Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PloS one 2: e827, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hatano N, Itoh Y, Muraki K. Cardiac fibroblasts have functional TRPV4 activated by 4alpha-phorbol 12,13-didecanoate. Life Sci 85: 808–814, 2009. [DOI] [PubMed] [Google Scholar]
- 98.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells. Role of Angiotensin II. Circ Res 96: 207–215, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-Induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102: 347–355, 2008. [DOI] [PubMed] [Google Scholar]
- 100.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006. [DOI] [PubMed] [Google Scholar]
- 101.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci 118: 917–928, 2005. [DOI] [PubMed] [Google Scholar]
- 102.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiology 29: 343–360, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hiroi T, Wajima T, Negoro T, Ishii M, Nakano Y, Kiuchi Y, Mori Y, Shimizu S. Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc Res 97: 271–281, 2013. [DOI] [PubMed] [Google Scholar]
- 104.Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22: 776–785, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca2+-activated monovalent selective cation channel. Curr Biol 13: 1153–1158, 2003. [DOI] [PubMed] [Google Scholar]
- 106.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999. [DOI] [PubMed] [Google Scholar]
- 107.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 99: 7461–7466, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Horton JS, Buckley CL, Stokes AJ. Successful TRPV1 antagonist treatment for cardiac hypertrophy and heart failure in mice. Channels 7: 17–22, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, Zhu MX. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol 208: 201–212, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97: 6155–6160, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. Clin J Am Soc Nephrol 13: 2384–2398, 2002. [DOI] [PubMed] [Google Scholar]
- 112.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res 104: 1399–1409, 2009. [DOI] [PubMed] [Google Scholar]
- 113.Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther 123: 371–385, 2009. [DOI] [PubMed] [Google Scholar]
- 114.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)- permeable cation channel. Circ Res 88: 325–332, 2001. [DOI] [PubMed] [Google Scholar]
- 115.Iwata Y, Katanosaka Y, Arai Y, Komamura K, Miyatake K, Shigekawa M. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol 161: 957–967, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iwata Y, Ohtake H, Suzuki O, Matsuda J, Komamura K, Wakabayashi S. Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovasc Res 99: 760–768, 2013. [DOI] [PubMed] [Google Scholar]
- 117.Jenkins CM, Cedars A, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc Res 82: 240–249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, Malik AB. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ Res 96: 1282–1290, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Jian MY, King JA, Al-Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 38: 386–392, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322: 756–760, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jin J, Wu LJ, Jun J, Cheng X, Xu H, Andrews NC, Clapham DE. The channel kinase, TRPM7, is required for early embryonic development. Proc Natl Acad Sci USA 109: E225–E233, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol 296: H1868–H1877, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Ju YK, Chu Y, Chaulet H, Lai D, Gervasio OL, Graham RM, Cannell MB, Allen DG. Store-operated Ca2+ influx and expression of trpc genes in mouse sinoatrial node. Circ Res 100: 1605–1614, 2007. [DOI] [PubMed] [Google Scholar]
- 125.Jung S, Strotmann R, Schultz G, Plant TD. TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol 282: C347–C359, 2002. [DOI] [PubMed] [Google Scholar]
- 126.Jurczyk A, Gromley A, Redick S, San Agustin J, Witman G, Pazour GJ, Peters DJ, Doxsey S. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol 166: 637–643, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaneko Y, Szallasi A. TRP channels as therapeutic targets. Curr Top Med Chem 13: 241–243, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1: 165–170, 1999. [DOI] [PubMed] [Google Scholar]
- 129.Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflügers Arch 457: 77–89, 2008. [DOI] [PubMed] [Google Scholar]
- 130.Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, Papp Z, Edes I, Porszasz R, Toth A. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol 73: 1405–1412, 2008. [DOI] [PubMed] [Google Scholar]
- 131.Katanosaka Y, Iwasaki K, Ujihara Y, Takatsu S, Nishitsuji K, Kanagawa M, Sudo A, Toda T, Katanosaka K, Mohri S, Naruse K. TRPV2 is critical for the maintenance of cardiac structure and function in mice. Nat Commun 5: 3932, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim EY, Anderson M, Dryer SE. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am J Physiol Renal Physiol 302: F298–F307, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim HJ, Li Q, Tjon-Kon-Sang S, So I, Kiselyov K, Soyombo A, Muallem S. A novel mode of TRPML3 regulation by extracytosolic pH absent in the varitint-waddler phenotype. EMBO J 27: 1197–1205, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci USA 97: 1731–1736, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kinoshita H, Kuwahara K, Nishida M, Jian Z, Rong X, Kiyonaka S, Kuwabara Y, Kurose H, Inoue R, Mori Y, Li Y, Nakagawa Y, Usami S, Fujiwara M, Yamada Y, Minami T, Ueshima K, Nakao K. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-a signaling in the heart. Circ Res 106: 1849–1860, 2010. [DOI] [PubMed] [Google Scholar]
- 136.Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, Yoshida T, Wakamori M, Mori E, Numata T, Ishii M, Takemoto H, Ojida A, Watanabe K, Uemura A, Kurose H, Morii T, Kobayashi T, Sato Y, Sato C, Hamachi I, Mori Y. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci USA 106: 5400–5405, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klaiber M, Kruse M, Volker K, Schroter J, Feil R, Freichel M, Gerling A, Feil S, Dietrich A, Londono JE, Baba HA, Abramowitz J, Birnbaumer L, Penninger JM, Pongs O, Kuhn M. Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Res Cardiol 105: 583–595, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Klein RM, Ufret-Vincenty CA, Hua L, Gordon SE. Determinants of molecular specificity in phosphoinositide regulation. J Biol Chem 283: 26208–26216, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kochukov MY, Balasubramanian A, Noel RC, Marrelli SP. Role of TRPC1 and TRPC3 channels in contraction and relaxation of mouse thoracic aorta. J Vasc Res 50: 11–20, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Koenig S, Schernthaner M, Maechler H, Kappe CO, Glasnov TN, Hoefler G, Braune M, Wittchow E, Groschner K. A TRPC3 blocker, ethyl-1-(4-(2,3,3-trichloroacrylamide)phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-c arboxylate (Pyr3), prevents stent-induced arterial remodeling. J Pharmacol Exp Ther 344: 33–40, 2013. [DOI] [PubMed] [Google Scholar]
- 141.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF, Kass DA. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol 48: 713–724, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J 24: 705–716, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002. [DOI] [PubMed] [Google Scholar]
- 145.Krapivinsky G, Krapivinsky L, Manasian Y, Clapham DE. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell 157: 1061–1072, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 52: 485–496, 2006. [DOI] [PubMed] [Google Scholar]
- 147.Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Brink P, Pongs O. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest 119: 2737–2744, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res 98: 557–563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kung C. A possible unifying principle for mechanosensation. Nature 436: 647–654, 2005. [DOI] [PubMed] [Google Scholar]
- 150.Kunichika N, Landsberg JW, Yu Y, Kunichika H, Thistlethwaite PA, Rubin LJ, Yuan JX. Bosentan inhibits transient receptor potential channel expression in pulmonary vascular myocytes. Am J Respir Crit Care Med 170: 1101–1107, 2004. [DOI] [PubMed] [Google Scholar]
- 151.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116: 3114–3126, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kwan HY, Huang Y, Yao X. Protein kinase C can inhibit TRPC3 channels indirectly via stimulating protein kinase G. J Cell Physiol 207: 315–321, 2006. [DOI] [PubMed] [Google Scholar]
- 153.Kwan HY, Huang Y, Yao X. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc Natl Acad Sci USA 101: 2625–2630, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochim Biophys Acta 1772: 907–914, 2007. [DOI] [PubMed] [Google Scholar]
- 155.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50: 277–289, 2006. [DOI] [PubMed] [Google Scholar]
- 156.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol 70: 1174–1183, 2006. [DOI] [PubMed] [Google Scholar]
- 157.Large WA, Saleh SN, Albert AP. Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle. Cell calcium 45: 574–582, 2009. [DOI] [PubMed] [Google Scholar]
- 158.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109: 397–407, 2002. [DOI] [PubMed] [Google Scholar]
- 159.Laurant P, Robin S, Berthelot A. Magnesium deficiency increases vasoconstrictor activity without affecting blood pressure of aged spontaneously hypertensive rats. Magnes Res 10: 107–117, 1997. [PubMed] [Google Scholar]
- 160.Lee J, Cha SK, Sun TJ, Huang CL. PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+. J Gen Physiol 126: 439–451, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, Huang M, Lin JH, Feder JN, Janovitz EB, Levesque PC, Blanar MA. Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J Biol Chem 278: 20890–20897, 2003. [DOI] [PubMed] [Google Scholar]
- 162.Levitan I, Fang Y, Rosenhouse-Dantsker A, Romanenko V. Cholesterol and ion channels. Subcell Biochem 51: 509–549, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li J, Wang DH. Function and regulation of the vanilloid receptor in rats fed a high salt diet. J Hypertens 21: 1525–1530, 2003. [DOI] [PubMed] [Google Scholar]
- 164.Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem 282: 25817–25830, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol 127: 525–537, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li Y, Wright JM, Qian F, Germino GG, Guggino WB. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem 280: 41298–41306, 2005. [DOI] [PubMed] [Google Scholar]
- 167.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004. [DOI] [PubMed] [Google Scholar]
- 169.Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 25: 4835–4843, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 100: 15160–15165, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu D, Zhang Z, Liman ER. Extracellular acid block and acid-enhanced inactivation of the Ca2+-activated cation channel TRPM5 involve residues in the S3–S4 and S5-S6 extracellular domains. J Biol CHem 280: 20691–20699, 2005. [DOI] [PubMed] [Google Scholar]
- 172.Liu H, Chatel S, Simard C, Syam N, Salle L, Probst V, Morel J, Millat G, Lopez M, Abriel H, Schott JJ, Guinamard R, Bouvagnet P. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PloS one 8: e54131, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 3: 374–385, 2010. [DOI] [PubMed] [Google Scholar]
- 174.Liu M, Huang W, Wu D, Priestley JV. TRPV1, but not P2X, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci 24: 1–6, 2006. [DOI] [PubMed] [Google Scholar]
- 175.Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron 40: 551–561, 2003. [DOI] [PubMed] [Google Scholar]
- 176.Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci 27: 7070–7080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23: 2600–2607, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc Res 69: 798–807, 2006. [DOI] [PubMed] [Google Scholar]
- 179.Malczyk M, Veith C, Fuchs B, Hofmann K, Storch U, Schermuly RT, Witzenrath M, Ahlbrecht K, Fecher-Trost C, Flockerzi V, Ghofrani HA, Grimminger F, Seeger W, Gudermann T, Dietrich A, Weissmann N. Classical transient receptor potential channel 1 in hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med 20 188: 1451–1459, 2013. [DOI] [PubMed] [Google Scholar]
- 180.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7: 179–185, 2005. [DOI] [PubMed] [Google Scholar]
- 181.Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, Smillie SJ, Lalgi K, Fernandes ES, Gnudi L, Brain SD. A Role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension 61: 246–252, 2013. [DOI] [PubMed] [Google Scholar]
- 182.Martin E, Dahan D, Cardouat G, Gillibert-Duplantier J, Marthan R, Savineau JP, Ducret T. Involvement of TRPV1 and TRPV4 channels in migration of rat pulmonary arterial smooth muscle cells. Pflügers Arch 464: 261–272, 2012. [DOI] [PubMed] [Google Scholar]
- 183.Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londono JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest 120: 3267–3279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mederos y Schnitzler M., Storch U., Meibers S., Nurwakagari P., Breit A., Essin K., Gollasch M., and Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction The EMBO journal 27: 3092–3103, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mercado J, Gordon-Shaag A, Zagotta WN, Gordon SE. Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 30: 13338–13347, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Meves H. Arachidonic acid and ion channels: an update. Br J Pharmacol 155: 4–16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miller BA, Wang J, Hirschler-Laszkiewicz I, Gao E, Song J, Zhang XQ, Koch WJ, Madesh M, Mallilankaraman K, Gu T, Chen SJ, Keefer K, Conrad K, Feldman AM, Cheung JY. The second member of transient receptor potential-melastatin channel family protects hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 304: H1010–H1022, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Minke B. Drosophila mutant with a transducer defect. Biophys Struct Mech 3: 59–64, 1977. [DOI] [PubMed] [Google Scholar]
- 190.Montalbetti N, Cantero MR, Dalghi MG, Cantiello HF. Reactive oxygen species inhibit polycystin-2 (TRPP2) cation channel activity in term human syncytiotrophoblast. Placenta 29: 510–518, 2008. [DOI] [PubMed] [Google Scholar]
- 191.Montell C. The TRP superfamily of cation channels. Sci STKE 2005: 1–24, 2005. [DOI] [PubMed] [Google Scholar]
- 192.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell 108: 595–598, 2002. [DOI] [PubMed] [Google Scholar]
- 193.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230: 1040–1043, 1985. [DOI] [PubMed] [Google Scholar]
- 194.Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov 10: 601–620, 2011. [DOI] [PubMed] [Google Scholar]
- 195.Morenilla-Palao C, Pertusa M, Meseguer V, Cabedo H, Viana F. Lipid raft segregation modulates TRPM8 channel activity. J Biol Chem 284: 9215–9224, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Morita H, Honda A, Inoue R, Ito Y, Abe K, Nelson MT, Brayden JE. Membrane stretch-induced activation of a TRPM4-like nonselective cation channel in cerebral artery myocytes. J Pharmacol Sci 103: 417–426, 2007. [DOI] [PubMed] [Google Scholar]
- 197.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838, 2003. [DOI] [PubMed] [Google Scholar]
- 198.Muto S, Aiba A, Saito Y, Nakao K, Nakamura K, Tomita K, Kitamura T, Kurabayashi M, Nagai R, Higashihara E, Harris PC, Katsuki M, Horie S. Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Human Mol Genet 11: 1731–1742, 2002. [DOI] [PubMed] [Google Scholar]
- 199.Muzzio IA, Gandhi CC, Manyam U, Pesnell A, Matzel LD. Receptor-stimulated phospholipase A2 liberates arachidonic acid and regulates neuronal excitability through protein kinase C. J Neurophysiol 85: 1639–1647, 2001. [DOI] [PubMed] [Google Scholar]
- 200.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a MgATP-regulated divalent cation channel required for cell viability. Nature 411: 590–595, 2001. [DOI] [PubMed] [Google Scholar]
- 201.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J 20: 1660–1670, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Narayanan D, Bulley S, Leo MD, Burris SK, Gabrick KS, Boop FA, Jaggar JH. Smooth muscle cell transient receptor potential polycystin (TRPP)2 channels contribute to the myogenic response in cerebral arteries. J Physiol 591: 5031–5046, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 204.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117: 1161–1171, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Naylor J, Li J, Milligan CJ, Zeng F, Sukumar P, Hou B, Sedo A, Yuldasheva N, Majeed Y, Beri D, Jiang S, Seymour VA, McKeown L, Kumar B, Harteneck C, O'Regan D, Wheatcroft SB, Kearney MT, Jones C, Porter KE, Beech DJ. Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ Res 106: 1507–1515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol 259: C3–C18, 1990. [DOI] [PubMed] [Google Scholar]
- 207.Nikolova-Krstevski, Vesna WS, Friedrich O, Fatkin D. Transient receptor potential channel 6 (TRPC6) is an important mediator of mechanical stretch responses in the atrial endocardial endothelium. Circ Res 113: A044, 2013. [Google Scholar]
- 208.Nilius B. TRP channels in disease. Biochim Biophys Acta 1772: 805–812, 2007. [DOI] [PubMed] [Google Scholar]
- 209.Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 25: 467–478, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol 12: 218, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nilius B, Owsianik G, Voets T. Transient receptor potential channels meet phosphoinositides. EMBO J 27: 2809–2816, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T. The selectivity filter of the cation channel TRPM4. J Biol Chem 280: 22899–22906, 2005. [DOI] [PubMed] [Google Scholar]
- 213.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 66: 676–814, 2014. [DOI] [PubMed] [Google Scholar]
- 214.Nilius B, Voets T, Peters J. TRP Channels in Disease. Science's STKE 2005: 1–9, 2005. [DOI] [PubMed] [Google Scholar]
- 215.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol 286: C195–C205, 2004. [DOI] [PubMed] [Google Scholar]
- 216.Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, Inoue R, Mori Y, Kurose H. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem 282: 23117–23128, 2007. [DOI] [PubMed] [Google Scholar]
- 217.Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem 19: 1–8, 2007. [DOI] [PubMed] [Google Scholar]
- 218.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am J Physiol Cell Physiol 292: C460–C467, 2007. [DOI] [PubMed] [Google Scholar]
- 219.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res 98: 245–253, 2006. [DOI] [PubMed] [Google Scholar]
- 220.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol 42: 498–507, 2007. [DOI] [PubMed] [Google Scholar]
- 221.Oike H, Wakamori M, Mori Y, Nakanishi H, Taguchi R, Misaka T, Matsumoto I, Abe K. Arachidonic acid can function as a signaling modulator by activating the TRPM5 cation channel in taste receptor cells. Biochim Biophys Acta 1761: 1078–1084, 2006. [DOI] [PubMed] [Google Scholar]
- 222.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J 25: 5305–5316, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Owsianik G, D'Hoedt D, Voets T, Nilius B. Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol 156: 61–90, 2006. [PubMed] [Google Scholar]
- 224.Pacher P, Batkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol 558: 647–657, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pan HL, Chen SR. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation 110: 1826–1831, 2004. [DOI] [PubMed] [Google Scholar]
- 226.Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, Ambudkar IS. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci USA 106: 20087–20092, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Paria BC, Bair AM, Xue J, Yu Y, Malik AB, Tiruppathi C. Ca2+ influx induced by protease-activated receptor-1 activates a feed-forward mechanism of TRPC1 expression via nuclear factor-kappaB activation in endothelial cells. J Biol Chem 281: 20715–20727, 2006. [DOI] [PubMed] [Google Scholar]
- 228.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. Tumor necrosis factor-α-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol 287: L1303–L1313, 2004. [DOI] [PubMed] [Google Scholar]
- 229.Peppiatt-Wildman CM, Albert AP, Saleh SN, Large WA. Endothelin-1 activates a Ca2+-permeable cation channel with TRPC3 and TRPC7 properties in rabbit coronary artery myocytes. J Physiol 580: 755–764, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411: 595–599, 2001. [DOI] [PubMed] [Google Scholar]
- 231.Persu A, Stoenoiu MS, Messiaen T, Davila S, Robino C, El-Khattabi O, Mourad M, Horie S, Feron O, Balligand JL, Wattiez R, Pirson Y, Chauveau D, Lens XM, Devuyst O. Modifier effect of ENOS in autosomal dominant polycystic kidney disease. Hum Mol Genet 11: 229–241, 2002. [DOI] [PubMed] [Google Scholar]
- 232.Peters M, Trembovler V, Alexandrovich A, Parnas M, Birnbaumer L, Minke B, Shohami E. Carvacrol together with TRPC1 elimination improve functional recovery after traumatic brain injury in mice. J Neurotrauma 29: 2831–2834, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Picazo-Juárez G, Romero-Suárez S, Nieto-Posadas A, Llorente I, Jara-Oseguera A, Briggs M, McIntosh TJ, Simon SA, Ladrón-de-Guevara E, Islas LD, Rosenbaum T. Identification of a binding motif in the S5 helix that confers cholesterol sensitivity to the TRPV1 ion channel. J Biol Chem 286: 24966–24976, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol 568: 539–551, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem 281: 13588–13595, 2006. [DOI] [PubMed] [Google Scholar]
- 236.Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci USA 100: 15166–15171, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pristera A, Okuse K. Building excitable membranes: lipid rafts and multiple controls on trafficking of electrogenic molecules. Neuroscientist 18: 70–81, 2011. [DOI] [PubMed] [Google Scholar]
- 238.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183, 1997. [DOI] [PubMed] [Google Scholar]
- 239.Qian Q, Hunter LW, Li M, Marin-Padilla M, Prakash YS, Somlo S, Harris PC, Torres VE, Sieck GC. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum Mol Genet 12: 1875–1880, 2003. [DOI] [PubMed] [Google Scholar]
- 240.Qian Q, Li M, Cai Y, Ward CJ, Somlo S, Harris PC, Torres VE. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol 14: 2280–2287, 2003. [DOI] [PubMed] [Google Scholar]
- 241.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation 20: 138–148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Qin X, Yue Z, Sun B, Yang W, Xie J, Ni E, Feng Y, Mahmood R, Zhang Y, Yue L. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br J Pharmacol 168: 1294–1312, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 68: 619–647, 2006. [DOI] [PubMed] [Google Scholar]
- 244.Rapoport SI. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J Pediatr 143: S26–S34, 2003. [DOI] [PubMed] [Google Scholar]
- 245.Reading SA, Brayden JE. Central role of TRPM4 channels in cerebral blood flow regulation. Stroke 38: 2322–2328, 2007. [DOI] [PubMed] [Google Scholar]
- 246.Rohacs T. Regulation of TRP channels by PIP2. Pflügers Arch 453: 753–762, 2007. [DOI] [PubMed] [Google Scholar]
- 247.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8: 626–634, 2005. [DOI] [PubMed] [Google Scholar]
- 248.Rose RA, Hatano N, Ohya S, Imaizumi Y, Giles WR. C-type natriuretic peptide activates a non-selective cation current in acutely isolated rat cardiac fibroblasts via natriuretic peptide C receptor-mediated signalling. J Physiol 580: 255–274, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rothoerl RD, Ringel F. Molecular mechanisms of cerebral vasospasm following aneurysmal SAH. Neurol Res 29: 636–642, 2007. [DOI] [PubMed] [Google Scholar]
- 250.Rubinstein J, Lasko VM, Koch SE, Singh VP, Carreira V, Robbins N, Patel AR, Jiang M, Bidwell P, Kranias EG, Jones WK, Lorenz JN. Novel role of transient receptor potential vanilloid 2 in the regulation of cardiac performance. Am J Physiol Heart Circ Physiol 306: H574–H584, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rundle DR, Gorbsky G, Tsiokas L. PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: role of mDia1 IN PKD2 localization to mitotic spindles. J Biol Chem 279: 29728–29739, 2004. [DOI] [PubMed] [Google Scholar]
- 252.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291: 1043–1047, 2001. [DOI] [PubMed] [Google Scholar]
- 253.Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol 4: 329–336, 2002. [DOI] [PubMed] [Google Scholar]
- 254.Sabourin J, Antigny F, Robin E, Frieden M, Raddatz E. Activation of transient receptor potential canonical 3 (TRPC3)-mediated Ca2+ entry by A1 adenosine receptor in cardiomyocytes disturbs atrioventricular conduction. J Biol Chem 287: 26688–26701, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sabourin J, Robin E, Raddatz E. A key role of TRPC channels in the regulation of electromechanical activity of the developing heart. Cardiovasc Res 92: 226–236, 2011. [DOI] [PubMed] [Google Scholar]
- 256.Sah R, Mesirca P, Mason X, Gibson W, Bates-Withers C, Van den Boogert M, Chaudhuri D, Pu WT, Mangoni ME, Clapham DE. Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation 128: 101–114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sah R, Mesirca P, Van den Boogert M, Rosen J, Mably J, Mangoni ME, Clapham DE. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc Natl Acad Sci USA 110: E3037–E3046, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Saleh SN, Albert AP, Large WA. Activation of native TRPC1/C5/C6 channels by endothelin-1 is mediated by both PIP3 and PIP2 in rabbit coronary artery myocytes. J Physiol 587: 5361–5375, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol 577: 479–495, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008. [DOI] [PubMed] [Google Scholar]
- 261.Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293: 1327–1330, 2001. [DOI] [PubMed] [Google Scholar]
- 262.Satoh S, Tanaka H, Ueda Y, Oyama J, Sugano M, Sumimoto H, Mori Y, Makino N. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol Cell Biochem 294: 205–215, 2007. [DOI] [PubMed] [Google Scholar]
- 263.Schmidt K, Dubrovska G, Nielsen G, Fesus G, Uhrenholt TR, Hansen PB, Gudermann T, Dietrich A, Gollasch M, de Wit C, Kohler R. Amplification of EDHF-type vasodilatations in TRPC1-deficient mice. Br J Pharmacol 161: 1722–1733, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circ Res 95: 1027–1034, 2004. [DOI] [PubMed] [Google Scholar]
- 265.Senadheera S, Kim Y, Grayson TH, Toemoe S, Kochukov MY, Abramowitz J, Housley GD, Bertrand RL, Chadha PS, Bertrand PP, Murphy TV, Tare M, Birnbaumer L, Marrelli SP, Sandow SL. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc Res 95: 439–447, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Seo K, Rainer PP, Shalkey Hahn V, Lee DI, Jo SH, Andersen A, Liu T, Xu X, Willette RN, Lepore JJ, Marino JP Jr, Birnbaumer L, Schnackenberg CG, Kass DA. Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc Natl Acad Sci USA 111: 1551–1556, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res 105: 1023–1030, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sexton A, McDonald M, Cayla C, Thiemermann C, Ahluwalia A. 12-Lipoxygenase-derived eicosanoids protect against myocardial ischemia/reperfusion injury via activation of neuronal TRPV1. FASEB J 21: 2695–2703, 2007. [DOI] [PubMed] [Google Scholar]
- 269.Shan D, Marchase RB, Chatham JC. Overexpression of TRPC3 increases apoptosis but not necrosis in response to ischemia-reperfusion in adult mouse cardiomyocytes. Am J Physiol Cell Physiol 294: C833–C841, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sharif-Naeini R, Dedman A, Folgering JH, Duprat F, Patel A, Nilius B, Honore E. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflügers Arch 456: 529–540, 2008. [DOI] [PubMed] [Google Scholar]
- 271.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ, Honore E. Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139: 587–596, 2009. [DOI] [PubMed] [Google Scholar]
- 272.Shi J, Ju M, Large WA, Albert AP. Pharmacological profile of phosphatidylinositol 3-kinases and related phosphatidylinositols mediating endothelin(A) receptor-operated native TRPC channels in rabbit coronary artery myocytes. Br J Pharmacol 166: 2161–2175, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shi J, Ju M, Saleh SN, Albert AP, Large WA. TRPC6 channels stimulated by angiotensin II are inhibited by TRPC1/C5 channel activity through a Ca2+- and PKC-dependent mechanism in native vascular myocytes. J Physiol 588: 3671–3682, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Simon F, Leiva-Salcedo E, Armisen R, Riveros A, Cerda O, Varela D, Eguiguren AL, Olivero P, Stutzin A. Hydrogen peroxide removes TRPM4 current desensitization conferring increased vulnerability to necrotic cell death. J Biol Chem 285: 37150–37158, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem 282: 7833–7843, 2007. [DOI] [PubMed] [Google Scholar]
- 276.Sohn JW, Lim A, Lee SH, Ho WK. Decrease in PIP2 channel interactions is the final common mechanism involved in PKC- and arachidonic acid-mediated inhibitions of GABA(B)-activated K+ current. J Physiol 582: 1037–1046, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res 50: 35–51, 2011. [DOI] [PubMed] [Google Scholar]
- 280.Stallmeyer B, Zumhagen S, Denjoy I, Duthoit G, Hebert JL, Ferrer X, Maugenre S, Schmitz W, Kirchhefer U, Schulze-Bahr E, Guicheney P, Schulze-Bahr E. Mutational spectrum in the Ca2+-activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat 33: 109–117, 2012. [DOI] [PubMed] [Google Scholar]
- 281.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. [DOI] [PubMed] [Google Scholar]
- 282.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000. [DOI] [PubMed] [Google Scholar]
- 283.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 278: 39014–39019, 2003. [DOI] [PubMed] [Google Scholar]
- 284.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645–655, 2001. [DOI] [PubMed] [Google Scholar]
- 285.Su LT, Chen HC, Gonzalez-Pagan O, Overton JD, Xie J, Yue L, Runnels LW. TRPM7 activates m-Calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-Terminal kinase. J Mol Biol 396: 858–869 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37: 175–195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sullivan MN, Earley S. TRP channel Ca2+ sparklets: fundamental signals underlying endothelium-dependent hyperpolarization. Am J Physiol Cell Physiol 305: C999–C1008, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sumoza-Toledo A, Lange I, Cortado H, Bhagat H, Mori Y, Fleig A, Penner R, Partida- Sánchez S. Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. Faseb 25: 3529–3542, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Szoke E, Borzsei R, Toth DM, Lengl O, Helyes Z, Sandor Z, Szolcsanyi J. Effect of lipid raft disruption on TRPV1 receptor activation of trigeminal sensory neurons and transfected cell line. Eur J Pharmacol 628: 67–74, 2010. [DOI] [PubMed] [Google Scholar]
- 290.Szolcsanyi J, Oroszi G, Nemeth J, Szilvassy Z, Blasig IE, Tosaki A. Functional and biochemical evidence for capsaicin-induced neural endothelin release in isolated working rat heart. Eur J Pharmacol 419: 215–221, 2001. [DOI] [PubMed] [Google Scholar]
- 291.Takahashi K, Sakamoto K, Kimura J. Hypoxic stress induces transient receptor potential melastatin 2 (TRPM2) channel expression in adult rat cardiac fibroblasts. J Pharmacol Sci 118: 186–197, 2012. [DOI] [PubMed] [Google Scholar]
- 292.Takahashi N, Kozai D, Mori Y. TRP channels: sensors and transducers of gasotransmitter signals. Front Physiol 3: 324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, Iijima T, Ito H. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis 195: 287–296, 2007. [DOI] [PubMed] [Google Scholar]
- 294.Tano JY, Solanki S, Lee RH, Smedlund K, Birnbaumer L, Vazquez G. Bone marrow deficiency of TRPC3 channel reduces early lesion burden and necrotic core of advanced plaques in a mouse model of atherosclerosis. Cardiovasc Res 101: 138–144, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thodeti CK, Paruchuri S, Meszaros JG. A TRP to cardiac fibroblast differentiation. Channels (Austin) 7: 211–214, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, Gordon E, Lozinskaya I, Elefante L, Qin P, Matasic DS, James C, Tunstead J, Donovan B, Kallal L, Waszkiewicz A, Vaidya K, Davenport EA, Larkin J, Burgert M, Casillas LN, Marquis RW, Ye G, Eidam HS, Goodman KB, Toomey JR, Roethke TJ, Jucker BM, Schnackenberg CG, Townsley MI, Lepore JJ, Willette RN. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 4: 159ra148.2012. [DOI] [PubMed] [Google Scholar]
- 297.Thyagarajan B, Lukacs V, Rohacs T. Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J Biol Chem 283: 14980–14987, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ Res 91: 70–76, 2002. [DOI] [PubMed] [Google Scholar]
- 299.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol 39: 173–185, 2002. [DOI] [PubMed] [Google Scholar]
- 300.Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J 25: 1804–1815, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol 61: 3–12, 2004. [DOI] [PubMed] [Google Scholar]
- 302.Toth B, Csanady L. Pore collapse underlies irreversible inactivation of TRPM2 cation channel currents. Proc Natl Acad Sci USA 109: 13440–13445, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Touyz RM. Role of magnesium in the pathogenesis of hypertension. Mol Aspects Med 24: 107–136, 2003. [DOI] [PubMed] [Google Scholar]
- 304.Touyz RM, He Y, Montezano AC, Yao G, Chubanov V, Gudermann T, Callera GE. Differential regulation of TRPM6/7 cation channels by ANG II in vascular smooth muscle cells from spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290: R73–R78, 2006. [DOI] [PubMed] [Google Scholar]
- 305.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94: 6965–6970, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cellular signalling 19: 444–453, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Turner H, Fleig A, Stokes A, Kinet JP, Penner R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. Biochem J 371: 341–350, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Localization of the PIP2 sensor of TRPV1 ion channels. J Biol Chem 286: 9688–9698, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.van der Vusse GJ, Roemen TH, Prinzen FW, Coumans WA, Reneman RS. Uptake and tissue content of fatty acids in dog myocardium under normoxic and ischemic conditions. Circ Res 50: 538–546, 1982. [DOI] [PubMed] [Google Scholar]
- 310.Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J Biol Chem 281: 17517–17527, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Venkatachalam K, Montell Channels CTRP. TRP channels. Annu Rev Chem Biomol Eng 76: 387–417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Venkatachalam K, Zheng F, Gill DL. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. The J Biol Chem 278: 29031–29040, 2003. [DOI] [PubMed] [Google Scholar]
- 313.Vennekens R, Hoenderop JGJ, Prenen J, Stuiver M, Willems PHGM, Droogmans G, Nilius B, Bindels RJM. Permeation and gating properties of the novel epithelial Ca2+c. J Biol Chem 275: 3963–3969, 2000. [DOI] [PubMed] [Google Scholar]
- 314.Voets T, Nilius B, Hoefs S, van der Kemp AWCM, Droogmans G, Bindels RJM, Hoenderop JGJ. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279: 19–25, 2004. [DOI] [PubMed] [Google Scholar]
- 315.Volk T, Schwoerer AP, Thiessen S, Schultz JH, Ehmke H. A polycystin-2-like large conductance cation channel in rat left ventricular myocytes. Cardiovasc Res 58: 76–88, 2003. [DOI] [PubMed] [Google Scholar]
- 316.Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70: 482–494, 2011. [DOI] [PubMed] [Google Scholar]
- 317.Wang C, Mirshahi UL, Liu B, Jia Z, Mirshahi T, Zhang H. Arachidonic acid activates Kir2.3 channels by enhancing channel-phosphatidyl-inositol 4,5-bisphosphate interactions. Mol Pharmacol 73: 1185–1194, 2008. [DOI] [PubMed] [Google Scholar]
- 318.Wang D, Iversen J, Strandgaard S. Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 11: 1371–1376, 2000. [DOI] [PubMed] [Google Scholar]
- 319.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Cir Res 98: 1528–1537, 2006. [DOI] [PubMed] [Google Scholar]
- 320.Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 112: 3617–3623, 2005. [DOI] [PubMed] [Google Scholar]
- 321.Wang Y, Novotny M, Quaiserova-Mocko V, Swain GM, Wang DH. TRPV1-mediated protection against endotoxin-induced hypotension and mortality in rats. Am J Physiol Regul Integr Comp Physiol 294: R1517–R1523, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wang YX, Wang J, Wang C, Liu J, Shi LP, Xu M, Wang C. Functional expression of transient receptor potential vanilloid-related channels in chronically hypoxic human pulmonary arterial smooth muscle cells. J Membr Biol 223: 151–159, 2008. [DOI] [PubMed] [Google Scholar]
- 323.Watanabe H, Iino K, Ohba T, Ito H. Possible involvement of TRP channels in cardiac hypertrophy and arrhythmia. Curr Top Med Chem 13: 283–294, 2013. [DOI] [PubMed] [Google Scholar]
- 324.Watanabe H, Murakami M, Ohba T, Ono K, Ito H. The pathological role of transient receptor potential channels in heart disease. Circulation 73: 419–427, 2009. [DOI] [PubMed] [Google Scholar]
- 325.Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther 118: 337–351, 2008. [DOI] [PubMed] [Google Scholar]
- 326.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003. [DOI] [PubMed] [Google Scholar]
- 327.Wei J, Ching LC, Zhao JF, Shyue SK, Lee HF, Kou YR, Lee TS. Essential role of transient receptor potential vanilloid type 1 in evodiamine-mediated protection against atherosclerosis. Acta physiologica 207: 299–307, 2013. [DOI] [PubMed] [Google Scholar]
- 328.Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA 103: 19093–19098, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M, Brandes RP, Grimminger F, Meissner M, Freichel M, Offermanns S, Veit F, Pak O, Krause KH, Schermuly RT, Brewer AC, Schmidt HH, Seeger W, Shah AM, Gudermann T, Ghofrani HA, Dietrich A. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nature Commun 3: 649, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002. [DOI] [PubMed] [Google Scholar]
- 331.Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension 41: 903–911, 2003. [DOI] [PubMed] [Google Scholar]
- 332.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther 326: 443–452, 2008. [DOI] [PubMed] [Google Scholar]
- 333.Wu G, Hayashi T, Park JH, Dixit M, Reynolds DM, Li L, Maeda Y, Cai Y, Coca-Prados M, Somlo S. Identification of PKD2L, a human PKD2-related gene: tissue-specific expression and mapping to chromosome 10q25. Genomics 54: 564–568, 1998. [DOI] [PubMed] [Google Scholar]
- 334.Wu G, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H Jr, Kucherlapati R, Edelmann W, Somlo S. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nature Genet 24: 75–78, 2000. [DOI] [PubMed] [Google Scholar]
- 335.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA 107: 7000–7005, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, Liedtke W, Sham JS. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol 305: C704–C715, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Xie A, Aihara Y, Bouryi VA, Nikitina E, Jahromi BS, Zhang ZD, Takahashi M, Macdonald RL. Novel mechanism of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab 27: 1692–1701, 2007. [DOI] [PubMed] [Google Scholar]
- 338.Xie J, Sun B, Du J, Yang W, Chen HC, Overton JD, Runnels LW, Yue L. Phosphatidylinositol 4,5-bisphosphate (PIP2) controls magnesium gatekeeper TRPM6 activity. Sci Rep 1: 12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nature Neurosci 9: 628–635, 2006. [DOI] [PubMed] [Google Scholar]
- 340.Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res 98: 1381–1389, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ. TRPC channel activation by extracellular thioredoxin. Nature 451: 69–72, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yaguchi T, Yamamoto S, Nagata T, Kanno T, Tanaka A, Nishizaki T. Effects of cis-unsaturated free fatty acids on PKC-epsilon activation and nicotinic ACh receptor responses. Brain Res Mol Brain Res 133: 320–324, 2005. [DOI] [PubMed] [Google Scholar]
- 343.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, Negoro T, Hiroi T, Kiuchi Y, Okada T, Kaneko S, Lange I, Fleig A, Penner R, Nishi M, Takeshima H, Mori Y. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nature Med 14: 738–747, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, Liu D, Arendshorst WJ, Huang Y, Tepel M, Zhu Z. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab 12: 130–141, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Yang KT, Chang WL, Yang PC, Chien CL, Lai MS, Su MJ, Wu ML. Activation of the transient receptor potential M2 channel and poly(ADP-ribose) polymerase is involved in oxidative stress-induced cardiomyocyte death. Cell Death Differ 13: 1815–1826, 2006. [DOI] [PubMed] [Google Scholar]
- 346.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L555–L568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006. [DOI] [PubMed] [Google Scholar]
- 348.Yao J, Qin F. Interaction with phosphoinositides confers adaptation onto the TRPV1 pain receptor. PLoS biology 7: e46, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res 97: 853–863, 2005. [DOI] [PubMed] [Google Scholar]
- 350.Yin J, Hoffmann J, Kaestle SM, Neye N, Wang L, Baeurle J, Liedtke W, Wu S, Kuppe H, Pries AR, Kuebler WM. Negative-feedback loop attenuates hydrostatic lung edema via a cGMP-dependent regulation of transient receptor potential vanilloid 4. Circ Res 102: 966–974, 2008. [DOI] [PubMed] [Google Scholar]
- 351.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002. [DOI] [PubMed] [Google Scholar]
- 352.Yogi A, Callera GE, Tostes R, Touyz RM. Bradykinin regulates calpain and proinflammatory signaling through TRPM7-sensitive pathways in vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol 296: R201–R207, 2009. [DOI] [PubMed] [Google Scholar]
- 353.Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, Nakai J, Dworniczak B, Ehrlich BE, Pennekamp P, Hamada H. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 338: 226–231, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2: 596–607, 2006. [DOI] [PubMed] [Google Scholar]
- 355.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang JY, Thistlethwaite PA, Channick RN, Robbins IM, Loyd JE, Ghofrani HA, Grimminger F, Schermuly RT, Cahalan MD, Rubin LJ, Yuan JX. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 119: 2313–2322, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yue L, Peng JB, Hediger MA, Clapham DE. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410: 705–709, 2001. [DOI] [PubMed] [Google Scholar]
- 358.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res 89: 744–753, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Yue L, Yue Z, Xie J. Phospholipid Regulation of Cardiac Ion Channels in Heart Disease. CRC press, 2013, p. 77–100. [Google Scholar]
- 361.Yue Z, Du J, Xie J, Qin X, Zhang Y, He Y, Fusco D, Liang B, Yue L. A potential role of TRPM2 mediated inflammation in heart disease (Abstract). Circulation 128: A18660, 2013. [Google Scholar]
- 362.Yue Z, Zhang Y, Xie J, Jiang J, Yue L. Transient receptor potential (TRP) channels and cardiac fibrosis. Curr Top Med Chem 13: 270–282, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zhang DX, Gutterman DD. Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol 57: 133–139, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53: 532–538, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhang YH, Sun HY, Chen KH, Du XL, Liu B, Cheng LC, Li X, Jin MW, Li GR. Evidence for functional expression of TRPM7 channels in human atrial myocytes. Basic Res Cardiol 107: 282, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zhang Z, Wang M, Fan XH, Chen JH, Guan YY, Tang YB. Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ Res 111: 1137–1146, 2012. [DOI] [PubMed] [Google Scholar]
- 367.Zhong B, Wang DH. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol 293: H1791–H1798, 2007. [DOI] [PubMed] [Google Scholar]
- 368.Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A, Clapham DE. Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci USA 108: 18114–18119, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10: 277–279, 2007. [DOI] [PubMed] [Google Scholar]
- 370.Zvara A, Bencsik P, Fodor G, Csont T, Hackler L Jr, Dux M, Furst S, Jancso G, Puskas LG, Ferdinandy P. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: a DNA microarray study. FASEB 20: 160–162, 2006. [DOI] [PubMed] [Google Scholar]
- 371.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999. [DOI] [PubMed] [Google Scholar]