Abstract
Background
This study aimed to evaluate whether treatment with sodium valproate (SV) was associated with reduced risk of stroke or myocardial infarction (MI).
Methods
Electronic health records data were extracted from Clinical Practice Research Database for participants ever diagnosed with epilepsy and prescribed antiepileptic drugs. A nested case–control study was implemented with cases diagnosed with incident non-haemorrhagic stroke and controls matched for sex, year of birth, and study start date (ratio of 1:6). A second nested study was implemented with MI as outcome. The main exposure variable was SV therapy assessed as: ever prescribed, pre-stroke year treatment, number of SV prescriptions, and cumulative time on SV drug therapy. Odds ratios were estimated using conditional logistic regression.
Results
Data were analysed for 2002 stroke cases and 13 098 controls. MI analyses included 1153 cases and 7109 controls. Pre-year stroke SV treatment (28%) was associated with increased stroke risk (odds ratio 1.22, 95% confidence interval (CI): 1.09 to 1.38, p < 0.001). No association was observed between ever being prescribed SV with ischemic stroke (OR = 1.01, 95% CI: 0.91 to 1.12, p = 0.875). A significant association was observed between ever being prescribed SV with MI (OR = 0.78, 95% CI: 0.67 to 0.90, p < 0.001). Patients in the highest quarter of SV treatment duration had lower odds of ischemic stroke (OR = 0.57, 95% CI: 0.44 to 0.72, p < 0.001) and MI (OR = 0.29, 95% CI: 0.20 to 0.44, p < 0.001).
Conclusion
Sodium valproate exposure was associated with the risk of MI, but not ischemic stroke. However, longer exposure to SV was associated with lower odds of stroke, but this might be explained by survivor bias.
Keywords: epilepsy, histone deacetylase (HDAC) inhibitors, ischemic stroke, myocardial infarction, sodium valproate, antiepileptic drugs, pharmacoepidemiology
INTRODUCTION
Recently, a genetic variant on chromosome 7p21.1 has been associated with ischaemic stroke in a large genome wide association study (GWAS),1 and this result has been confirmed in further populations.2 The underlying gene is thought to be histone deacetylase 9 (HDAC9). The association was confined to large artery stroke, not being present with other stroke subtypes.1 Consistent with a role in atherosclerosis the same variant was associated with increased carotid intima-media thickness and asymptomatic carotid plaque.3 HDAC9 is upregulated in symptomatic carotid atherosclerotic plaques,3 and drugs inhibiting HDAC activity might offer a novel preventative treatment for larger artery stroke.
The commonly used antiepileptic drug (AED) sodium valproate (SV) is a non-specific HDAC inhibitor.4 SV has been shown to attenuate atherosclerosis in apoE deficient mice.5 A Danish cohort study suggested that while AED treated epilepsy was associated with an increased risk of stroke, the extent of this effect varied with the AED prescribed. SV was associated with a decreased risk of both stroke and myocardial infarction (MI) compared with carbamazepine.6,7 This finding is consistent with HDAC inhibition reducing stroke risk, but data are only available from this one study, and other studies have suggested that SV treatment may adversely affect cardiovascular risk factor profile.8 For this reason, more data are required.
If HDAC9 is associated with stroke via atherosclerosis, one might expect there to be similar associations with other cardiovascular diseases related to atherosclerosis (i.e. MI). GWAS studies, however, have shown the association of the 7p21.1 variant with MI was weaker than that with large artery stroke.9 Therefore, it is possible that any protective effect of SV on ischaemic stroke may be greater than that seen with MI. We used a large database incorporating the electronic health records of family practices in the United Kingdom (UK) to determine the association of SV drug therapy with ischaemic stroke risk and MI in a cohort of epileptic patients. We compared any associations with those with other commonly used AEDs, which have been shown not to have HDAC inhibitory activity.10
METHODS
Data
A nested case–control study was implemented using data from the Clinical Practice Research Datalink (CPRD) (www.cprd.com). CPRD is the world's largest database of anonymous longitudinal patient records from primary care and collects data from over 600 UK general practices, covering over 10% of the UK population. Participating practices follow predefined protocols for recording and transferring of computerized clinical data to the research database. Data reaching a high enough standard for research are indicated as ‘up-to-standard’ (UTS).11 Information recorded in CPRD includes demographic information, clinical diagnoses, referral to secondary care, laboratory test results, all prescriptions issued, and hospital admission and discharge information. The database is broadly representative of the UK population in terms of sex, age and geography. The high quality of CPRD prescription and diagnosis information has been documented.12,13
The study population consisted of a cohort of epilepsy patients treated with at least one AED who were registered with 653 CPRD practices between 1 January 1992 and 31 January 2013. Data were extracted from the CPRD in February 2013. The cohort of epilepsy patients was identified using the Read diagnostic codes developed by Nicholas et al.14 Cases were all epilepsy patients with a first ever record of ischemic stroke during the study period. All cases had at least 24 months of record before the stroke incidence date. Incidence density sampling15 was used to identify up to six randomly selected controls for each case, matched for analysis time (study start date, within 2 years), age (year of birth, within 5 years) and sex, who did not have a stroke on or before the stroke index date for matched cases. All controls were epilepsy patients without a stroke who were alive and registered with the practice at the date of the first recorded diagnosis of stroke in their matched case. A different set of controls were selected for MI cases that were matched on the same variables as for stroke cases.
Exclusions
Patients were excluded if the latest of the start of UTS, registration date, study start date (1 January 1992), or the index date (date when a first epilepsy diagnosis was recorded) for epilepsy was after the stroke index date (date when a first stroke diagnosis was recorded). Individuals aged 30 years or younger at the time of an incident stroke were excluded because of the lower risk and differing aetiology in this age group. In line with study hypothesis, all patients diagnosed with hemorrhagic stroke were excluded from the analyses. Controls were excluded if their epilepsy index date or registration/UTS date was after the stroke index date for their matched case.
Outcomes
Read medical codes developed by Gulliford et al.16 were used to identify ischemic stroke events during the study period. Cases with haemorrhagic strokes were excluded but those with codes that did not distinguish between haemorrhage or infarction were included. To identify MI events, we used codes developed by Bhattarai et al.17 The date of first stroke or MI code is referred to as the stroke or MI index date. Index stroke and MI events within 2 years of the start date were excluded as prevalent cases.
Exposure
Sodium valproate treatment represented the primary exposure of interest for the present study. To assess the specificity of SV exposure, we also considered carbamazepine, phenobarbital and phenytoin treatments. These, with SV, are the most commonly prescribed AEDs in the UK. All three AEDs prescriptions after the study start date and before the stroke index date were included.
Four different exposure variables were created: ever treated, pre-stroke year treatment, cumulative number of prescriptions and duration of time on treatment. Every treatment exposure was constructed as a binary variable if any prescription was issued from the study start date to the index date for stroke (for controls, we used the matched case stroke index date). Pre-stroke year treatment was also constructed as a binary variable if any prescription was issued in the year prior to an incident stroke. Cumulative number of prescriptions was defined as the sum of all relevant AEDs prescriptions issued from study start date to the stroke index date. Time on treatment was calculated as total number of days from the study start date (mostly epilepsy index date) to the stroke index date for which the patients were issued any prescription for the relevant AED. This approach ensured a certain degree of overlap between SV use and epilepsy diagnosis. We considered consecutive prescriptions as being part of the same episode if the elapsed time between the end of one prescription and the issue of the next did not exceed 90 days.
Confounders
The selection of the covariates was informed by those factors identified in the literature as being associated both with epilepsy and stroke.6,18 These included body mass index ((BMI); <18.5, 18.5–25, >25–<30, 30–<35 and >35 kg/m2), smoking (never, ex, current), blood pressure ((BP); diastolic BP <90 and systolic BP <140(normal), diastolic BP 90–94 or systolic BP 140–159 (borderline), and diastolic BP ≥95 or systolic BP ≥160 mmHg(hypertension)), total cholesterol (0 to <4, 4 to <5, 5 to <6, 6 to 15 mmol/l), and a series of binary variables: coronary heart disease (CHD), psychiatric illness, lipid-lowering drugs (statins), antihypertensive drugs, antipsychotic drugs, and type 2 diabetes drugs. For each confounder, the value closer to the study baseline (start date) was considered.
Statistical analysis
The study start date was represented by the later of the start of the participant's record in CPRD, 1 January 1992, or the diagnosis date for epilepsy. The follow-up ended at the earliest of the study outcome index date (i.e. stroke date diagnosis), date of death, 31 January 2013, or the end of the CPRD record. Conditional logistic regression analysis was employed to estimate odds ratio and 95% confidence intervals (CI) for the association between AED's with ischemic stroke and MI. Separate estimation models were performed for ischemic stroke and MI. Also, separate models were estimated for ever treated, pre-stroke year, cumulative number of prescriptions and time on AED treatment. The reference group for the exposure variables was represented by other AED prescriptions but the prescription of interest. For instance, the reference category for SV prescription analysis was no SV prescription. All analyses were adjusted for study covariates including BP, BMI, cholesterol, smoking, CHD, psychiatric illness, antihypertensive drugs, lipid-lowering drugs, antipsychotic drugs and type 2 diabetes drugs. Data for confounders were not available for all patients, and we used missing indicator variables to address missing data. Specifically, patients with missing data on a covariate were included in analysis as a separate category. This approach prevents the loss of power and allows an insight into whether missing data is informative.19 When data may be missing not at random, as in the present data, multiple imputation may also give biassed estimates.20 Therefore, a missing indicator variable was used to explore whether patients with missing data were at greater risk of study outcome, which was not the case. Therefore, a missing indicator variable was used to explore whether patients with missing data were at greater risk of study outcome, which was not the case. Sensitivity analyses were conducted to investigate for possible survival bias effects by matching cases and controls on time from epilepsy diagnosis to study start date. Following Rothman21 and Greenland,22 the analyses did not adjust for multiple comparisons. Data were analysed using stata version 12.
RESULTS
Figure 1 illustrates the selection of study participants. After excluding ineligible patients and matching procedure, there were 2002 cases and 13 098 controls. For MI analyses, 1153 cases and 7109 controls were included in the analyses. Baseline characteristics of the stroke cases and controls are in Table 1. Baseline characteristics for MI patients presented similar patterns to the stroke patients, with higher rates of antihypertensive (75%) and lipid-lowering drugs (61%). There was a declining trend in recording of SV drug prescription from 1992 to late 2000s (see supplementary material).
Figure 1.
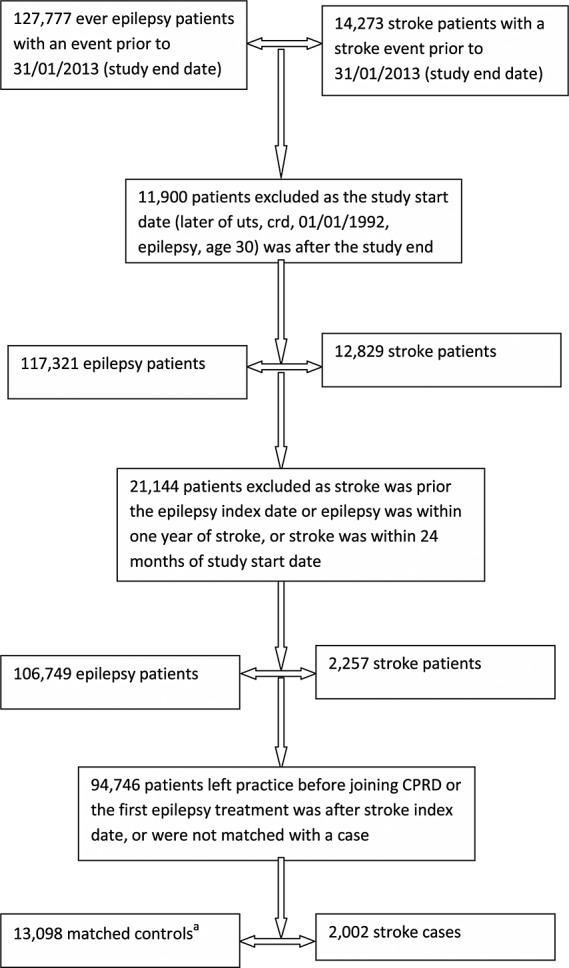
Flow diagram for the selection of nested case–control patients. aSome patients could be controls for more than one case. Abbreviations: UTS, up-to-standard; CRD, current registration date; CPRD, Clinical Research Practice Datalink. Event refers to the date of a first ischemic stroke diagnosis
Table 1.
Characteristics of participants at baseline (column %) unless otherwise specified
| Stroke cases | Controls | |
|---|---|---|
| N = 2002 | N = 13 098 | |
| Female | 1006 (50) | 6604 (50) |
| Age (Mean(SD)) | 65 (15) | 65 (15) |
| Blood pressure (mm/Hg) | ||
| Diastolic <90|Systolic <140 | 606 (30) | 5345 (42) |
| Diastolic ≥90|Systolic ≥140 | 501 (25) | 3987 (30) |
| Diastolic ≥95|Systolic ≥160 | 452 (23) | 2809 (21) |
| Missing | 443 (22) | 957 (7) |
| Total cholesterol (mmol/l) | ||
| 0–3.99 | 74 (4) | 670 (5) |
| 4–4.99 | 193 (10) | 1852 (14) |
| 5–5.99 | 277 (14) | 2718 (21) |
| 6–15 | 335 (16) | 3264 (25) |
| Missing | 1123 (56) | 4594 (35) |
| Body mass index (kg/m2) | ||
| <18.5 | 45 (2) | 279 (2) |
| 18.5–24.99 | 336 (17) | 33 353 (26) |
| 25–29.99 | 351 (18) | 3395 (26) |
| 30–34.99 | 169 (8) | 1511 (11) |
| 35–70 | 92 (5) | 642 (5) |
| Missing | 1009 (50) | 3915 (30) |
| Smoking | ||
| No | 359 (18) | 2217 (17) |
| Ex-smoker | 572 (28) | 6360 (49) |
| Current smoker | 260 (13) | 2633 (20) |
| Missing | 811 (41) | 1888 (14) |
| Antihypertensive drugs | 1153 (58) | 8338 (64) |
| Lipid-lowering drugs | 520 (26) | 4644 (35) |
| Antipsychotic drugs | 439 (22) | 3746 (29) |
| Type II diabetes drug therapy | 167 (8) | 1078 (8) |
| Coronary heart disease | 228 (11) | 2047 (16) |
| Diabetes mellitus | 194 (10) | 1497 (11) |
| Psychiatric illness | 371 (19) | 3228 (25) |
Table 2 shows that about a third (34%) of stroke cases and controls were ever prescribed SV during the study period. A higher proportion of stroke cases (28%) were prescribed SV in the year prior to an incident ischemic stroke compared with controls (24%). Mean number of years on SV therapy amongst stroke cases was 0.66 years in the lowest quarter of time on treatment, 3.24 in the second quarter, 7.61 in the third quarter and 14.56 in the highest quarter.
Descriptive statistics and conditional logistic regression results for the association of sodium valproate with ischemic stroke
| Case | Control | Unadjusted model | Fully adjusted model | |||
|---|---|---|---|---|---|---|
| (N = 2,002) | (N = 13 098) | OR† (95% CI) | p-value | OR† (95% CI) | p-value | |
| Ever prescribed SV | 681 (34) | 4407 (34) | 1.03 (0.93,1.14) | 0.555 | 1.01 (0.91,1.12) | 0.875 |
| Pre-stroke year SV treatment | 555 (28) | 3,106 (24) | 1.27 (1.14,1.41) | 0.001 | 1.22 (1.09,1.38) | 0.001 |
| Number of SV prescriptions | ||||||
| None | 1321 (66) | 8691 (66) | Reference | Reference | ||
| Lowest quarter | 227 (11) | 1075 (8) | 1.47 (1.26,1.72) | 0.001 | 1.22 (1.02,1.45) | 0.025 |
| Second quarter | 198 (10) | 1062 (8) | 1.28 (1.09,1.59) | 0.003 | 1.21 (1.02,1.45) | 0.033 |
| Third quarter | 166 (8) | 1100 (9) | 0.99 (0.83,1.18) | 0.924 | 1.00 (0.83,1.21) | 0.972 |
| Highest quarter | 90 (5) | 1170 (9) | 0.49 (0.39,0.61) | <0.001 | 0.59 (0.46,0.74) <0.001 | <0.001 |
| Time on SV prescriptions | ||||||
| None | 1321 (66) | 8915 (68) | Reference | Reference | ||
| Lowest quarter | 256 (13) | 962 (7) | 1.97 (1.68,2.29) | <0.001 | 1.62 (1.37,1.92) | <0.001 |
| Second quarter | 194 (10) | 1023 (8) | 1.35 (1.14,1.60) | 0.001 | 1.28 (1.07,1.54) | 0.007 |
| Third quarter | 146 (7) | 1068 (8) | 0.92 (0.76,1.11) | 0.373 | 0.95 (0.78,1.15) | 0.584 |
| Highest quarter | 85 (4) | 1130 (9) | 0.48 (0.38,0.60) | 0.001 | 0.57 (0.44,0.72) | <0.001 |
Figures are numbers and percentages (brackets).
OR, odds ratio; SV, sodium valproate.
The analyses are adjusted for sex, age, BP, cholesterol, BMI, smoking, AHT, statins, antipsychotic drugs, type 2 diabetes drugs, diabetes mellitus, CHD and psychiatric illness.
There was no association between being ever treated with SV and ischemic stroke (OR = 1.01, 95% CI: 0.91–1.12, p = 0.875). In contrast, a record of SV prescribing in the year before stroke was associated with higher odds ratio of ischemic stroke (OR = 1.22, 95% CI: 1.09–1.38, p < 0.001). A dose response relationship was revealed for duration of SV therapy with the lowest quarter of duration being associated with increased stroke risk (OR = 1.62, 95% CI: 1.37–1.92, p < 0.001 and the highest quarter of duration associated with reduced stroke risk (OR = 0.57, 95% CI: 0.44–0.72, p < 0.001).
The results for the MI outcome (Table 3) revealed that MI cases (28%) had a lower proportion of ever SV prescriptions relative to their controls (34%). Being ever on SV therapy was associated with lower risk of incident MI (OR = 0.78, 95% CI: 0.67–0.90, p < 0.001) compared with other AED therapy. Pre-MI year record of SV prescription was not associated with incident MI (OR = 0.88, 95% CI: 0.75–1.04, p = 0.142). The results for number of and duration of SV prescriptions showed similar patterns to those for the ischemic stroke outcome.
Descriptive statistics and conditional logistic regression results for the association of sodium valproate with myocardial infarction
| Case† | Control | Unadjusted model | Fully adjusted model | |||
|---|---|---|---|---|---|---|
| (N = 1,153) | (N = 7,109) | OR‡ (95% CI) | p-value | OR‡ (95% CI) | p-value | |
| Ever prescribed SV | 327 (28) | 2400 (34) | 0.77 (0.66,0.89) | 0.001 | 0.78 (0.67,0.90) | 0.001 |
| Pre-stroke year treatment | 251 (22) | 1696 (24) | 0.86 (0.72,1.02) | 0.086 | 0.88 (0.75,1.04) | 0.142 |
| Number of SV prescriptions | ||||||
| None | 826 (72) | 4709 (66) | Reference | Reference | ||
| Lowest quarter | 113 (10) | 570 (8) | 1.03 (0.82,1.31) | 0.783 | 1.08 (0.85,1.38) | 0.534 |
| Second quarter | 108 (9) | 587 (8) | 0.98 (0.77,1.24) | 0.846 | 1.04 (0.82,1.32) | 0.730 |
| Third quarter | 73 (6) | 597 (9) | 0.61 (0.46,0.81) | 0.001 | 0.71 (0.53,0.91) | 0.009 |
| Highest quarter | 33 (3) | 646 (9) | 0.28 (0.19,0.41) | 0.001 | 0.31 (0.21,0.45) | <0.001 |
| Time on SV prescriptions | ||||||
| None | 826 (72) | 4846 (68) | Reference | Reference | ||
| Lowest quarter | 122 (10) | 526 (7) | 1.25 (0.99,1.57) | 0.065 | 1.32 (1.04,1.66) | 0.021 |
| Second quarter | 100 (9) | 547 (8) | 1.10 (0.87,1.40) | 0.427 | 1.15 (0.90,1.47) | 0.277 |
| Third quarter | 75 (6) | 573 (8) | 0.61 (0.45,0.81) | 0.001 | 0.76 (0.58,0.99) | 0.044 |
| Highest quarter | 30 (3) | 617 (9) | 0.26 (0.17,0.39) | 0.001 | 0.29 (0.20,0.44) | <0.001 |
SV, sodium valproate; OR, odds ratio.
These figures are based on a subset of CHD codes developed by Bhattarai et al.17
The analyses are adjusted for sex, age, BP, cholesterol, BMI, smoking, AHT, statins, antipsychotic drugs, type 2 diabetes drugs, diabetes mellitus, CHD and psychiatric illness.
The results of sensitivity analyses (Table 4) indicated that every treatment with phenytoin was associated with lower odds ratio of ischemic stroke (OR = 0.80, 95% CI: 0.72–0.89, p < 0.001). Conversely, pre-stroke year carbamazepine therapy was associated with increased odds ratio of ischemic stroke (OR = 1.52, 95% CI: 1.35–1.70, p < 0.001) compared with other AED therapy. Likewise, sensitivity analyses for MI as outcome indicated a positive association between pre-MI year carbamazepine and phenytoin therapy with an incident MI event. The results for the duration on other AED prescriptions showed similar patterns to those for SV. Also, sensitivity analyses that adjusted for time from epilepsy diagnosis to study entry provided similar results.
Table 4.
Conditional logistic regression results for the association of other antiepileptic drugs with ischemic stroke and myocardial infarction
| SV | Phenytoin | Carbamazepine | Phenobarbital | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Ischemic stroke | ||||
| Ever prescribed | 1.01 (0.91,1.12) | 0.80 (0.72,0.89) | 1.07 (0.97,1.19) | 0.63 (0.53,0.74) |
| Pre-stroke year treatment | 1.22 (1.09,1.38) | 0.99 (0.88,1.11) | 1.52 (1.35,1.70) | 0.69 (0.57,0.83) |
| Duration on treatment | ||||
| Lowest quarter | 1.62 (1.37,1.92) | 1.45 (1.24,1.70) | 1.61 (1.37,1.90) | 0.92 (0.70,1.21) |
| Highest quarter | 0.57 (0.44,0.72) | 0.35 (0.30,0.47) | 0.65 (0.52,0.83) | 0.16 (0.09,0.31) |
| Myocardial infarction | ||||
| Ever prescribed | 0.78 (0.67,0.90) | 1.00 (0.87,1.15) | 1.03 (0.88 to 1.17) | 0.68 (0.54,0.85) |
| Pre-MI year treatment | 0.88 (0.75,1.04) | 1.27 (1.09,1.48) | 1.34 (1.15 to 1.55) | 0.72 (0.56,0.93) |
| Duration on treatment | ||||
| Lowest quarter | 1.32 (1.04,1.66) | 1.45 (1.17,1.78) | 1.54 (1.24,1.90) | 1.31 (0.93,1.84) |
| Highest quarter | 0.29 (0.20,0.44) | 0.37 (0.25,0.53) | 0.63 (0.46,0.86) | 0.22 (0.11,0.46) |
Bold figures imply significant results at 0.05 or lower level. The analyses are adjusted for sex, age, BP, cholesterol, BMI, smoking, AHT, statins, antipsychotic drugs, type 2 diabetes drugs, diabetes mellitus, CHD, and psychiatric illness.
SV, sodium valproate; OR, odds ratio; CI, confidence interval; MI, myocardial infarction.
DISCUSSION
In a large case control study of epilepsy treated patients, SV therapy was associated with lower risk of MI incidence but not ischemic stroke. There was some evidence of an exposure–response relationship between SV prescribing and risk of an incident ischemic stroke. Specifically, epilepsy patients who were in the highest quarter of number of SV prescriptions or drug therapy duration showed lower risk of ischemic stroke relative to those not on SV. In contrast, epilepsy patients in the lowest quarter of number of SV prescriptions or the lowest quarter of SV drug therapy duration appeared to be at increased risk of an ischemic stroke. Similar patterns were observed with respect to the MI outcome. Sensitivity analyses using carbamazepine, phenytoin and phenobarbital therapy showed a similar pattern of association between duration of therapy and lower stroke risk. The non-specific patents observed when assessing the risk of ischemic stroke by duration of exposure suggests the possibility of survivor bias
The genetic association of the HDAC9 variant is limited to large artery stroke and therefore any effect of SV on stroke incidence would be expected to be limited to this subgroup, which comprises about 20% of all ischaemic strokes. The present study data did not allow for such detailed subtyping of ischaemic stroke cases, and this might be expected to reduce the strength of any association between SV therapy and all ischaemic stroke. Because most cases of MI relate to large artery atherosclerosis, there may be less dilution of any association with MI, and this would be consistent with our results.
Any drug protecting against large artery stroke could act at a number of stages from prevention of atherosclerosis to stabilising the unstable carotid plaque. The association of the HDAC9 variant with carotid intima-media thickness and asymptomatic plaque3 suggests that any effect of SV would be at this earlier stage; therefore prolonged therapy might be expected to be required to reduce atherosclerosis progression. Thus, any preventative influence of SV therapy on ischemic stroke would be noticed with longer exposure to SV therapy. This may account for the observed association of longer exposure to SV therapy with lower incidence of ischemic stroke, but no association with short exposure.
Methodological biases are also plausible explanations for the study findings. The high risk of ischemic stroke observed with short time on SV therapy could be explained by confounding by indication. In this study, mean age at epilepsy diagnosis for the lowest quarter of SV therapy duration was 64 years compared with 42 years in the highest quarter. In older adults with late onset epilepsy, SV is generally the first choice of therapy.23 Thus, short time SV therapy exposure may be associated with older age at epilepsy diagnosis,24 and as late onset seizures increase the risk of ischemic stroke,18 this may explain the apparent higher risk of ischemic stroke associated with short time exposure to SV therapy. The low risk of ischemic stroke associated with longer exposure to SV therapy may be accounted for by survival bias (i.e. only the healthiest patients survived long enough to reach the longest durations of SV exposure). Sensitivity analyses that adjusted for time from epilepsy diagnosis to study entry suggested that survival bias was unlikely to be the sole explanation for the findings, and other factors not collected in the study data (i.e. epilepsy aetiology) need consideration. Furthermore, both phenytoin and phenobarbital drug therapies appeared to be associated with lower risk of ischemic stroke, suggesting that this observation might result from a generic survivor bias24 rather than from exposure to a specific AED class.
Strengths and limitations
A major strength of the present study was the use of a primary care database with documented validity on the recording epilepsy diagnosis, epilepsy pharmacology and stroke diagnosis. Several limitations are also important to note. The present analysis was unable to take into account aetiology or severity of patients' epilepsy as GP records rarely contain coded data describing the frequency, aetiology or duration of seizures. Another problem with pharmacoepidemiology studies is that it is not easy to separate the unique effects of any particular drug because polypharmacy is common especially in older patients. This applies to the present study where over a third of patients were prescribed two or more AEDs during the study period. It is possible that number and duration of SV use mixed the time on drug with drug withdrawal effect. The consistent association across different AED's and across both stroke and MI suggests however that withdrawal effects are unlikely to explain the observed associations. The study was restricted to epilepsy diagnosed patients who were treated, also the proportion of excluded patients is likely to be minimal.25 The subtype of stroke is not well captured by CPRD data not allowing association with specific stroke subtypes to be determined. The study included only incidence stroke and MI events; however, it cannot exclude the possibility that undiagnosed stroke events may have triggered epilepsy in a minority of patients. Additionally, selection bias may have led to overestimation or underestimation of the actual SV effects. The study design allowed the opportunity to explore the possibility of control-selection bias by comparing the distribution of SV in cases and controls before and after the development of the case–control study. The distribution of the SV therapy between cases and controls was similar before and after the selection of the nested case–control study, with controls having a slightly higher drop in percentage treated with SV therapy compared to cases (7%) suggesting that the study estimates would be minimally affected. Recall bias is unlikely considering that all drug prescriptions are recorded on the computer at the time of drug prescribing. Unmeasured confounding may also explain some of the inconsistency in the results. If residual confounding explained our results, then we would have expected to observe similar associations across all drugs, and for both MI and stroke, which was not the case in general. A nested case–control design was used because of the evidence for its superior computational efficiency to survival analysis when exploring rare diseases (such as stroke in the context of epilepsy) and that the two analyses produce similar results.26 Nested case–control design is also a standard technique when evaluating the impact of drug treatment on survival.27,28 When analysing primary care electronic health record data, such as CPRD, baseline measurements are drawn from clinically recorded data, which may not be recorded in a similar way across all patients. This is a common occurrence in most observational studies using electronic health records data for pharmacoepidemiological studies.
Comparison with previous studies
A previous Danish study7 found SV treated epilepsy patients had reduced risk of MI, which our findings support. In a linked study, Olesen et al.6 found reduced risk of both stroke and MI associated with SV therapy compared with carbamazepine. Present study findings are consistent with respect to MI but not ischemic stroke. Methodological and sampling differences between the two studies may explain some of these differences. For instance, the reference group in our study was all other AEDs rather than carbamazepine. Present study analyses accounted for additional confounders including smoking and BMI, the latter known to influence the choice of AED therapy and a documented risk factor for atherosclerosis and stroke.12,29 Present study definition of ischemic stroke was restricted to patients aged 30 years and over as the aetiology of stroke might differ in younger individuals. By contrast, Olesen et al. included all epilepsy patients over 10 years.
Implications for practice and research
Study findings indicated that quantifying SV therapy as present or absent can lead to different assumptions about its association with ischemic stroke compared to when considering patterns of exposure. By considering the nature (i.e. duration and cumulative number) of SV drug therapy it was possible to identify a monotonous exposure–response relationship between SV drug therapy and ischemic stroke or MI. This association was not specific to SV drug therapy suggesting the possibility that the findings may be explained by methodological artefacts common to most observational studies. The postulated inhibitory role of SV against ischemic stroke was made in relation to large vessel thrombosis, and studies employing a more specific definition of ischemic stroke (i.e. large or small artery) are necessary to validate the present study findings. The reduced risk of MI associated with SV drug therapy might suggest that SV drug therapy may have a role in MI prevention although this proposition needs confirmation in clinical trials. Future studies stratifying patients by their stroke risk score could be employed to estimate whether the inhibitory role of SV may be stronger in certain population groups.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
KEY POINTS
Recently, HDAC9 genetic variant has been associated with ischaemic stroke in a large GWAS.
SV is a non-specific inhibitor of HDAC9 raising the possibility for HDAC inhibition reducing stroke risk.
Present findings suggest that exposure to SV therapy is inconsistently associated with ischemic stroke.
SV drug therapy may have a role in MI prevention although this proposition needs confirmation in clinical trials.
ETHICS STATEMENT
The authors state that no ethical approval was needed.
Acknowledgments
Hugh Markus is supported by a National Institute for Health Research (NIHR) Senior Investigator award and the NIHR Biomedical Research Centre at Cambridge. Alex Dregan, Charles Wolfe and Martin Gulliford are supported by the NIHR Biomedical Research Centre at Guy's and St Thomas' National Health Service Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the National Institute of Health Research or the Department of Health.
Funded by a British Heart Foundation Project Grant (Ref CRM:0006031).
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web site.
REFERENCES
- International Stroke Genetics C; Wellcome Trust Case Control C. Bellenguez C, et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Gen. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traylor M, Farrall M, Holliday EG, et al. Genetic risk factors for ischaemic stroke and its subtypes (the metastroke collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus HS, Makela KM, Bevan S, et al. Evidence HDAC9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke. 2013;44:1220–1225. doi: 10.1161/STROKEAHA.111.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L, Sun Y, Han X, Xu CC, Tang YP, Dong Q. Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain Res. 2011;1396:60–68. doi: 10.1016/j.brainres.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Bowes AJ, Khan MI, Shi Y, Robertson L, Werstuck GH. Valproate attenuates accelerated atherosclerosis in hyperglycemic apoe-deficient mice: evidence in support of a role for endoplasmic reticulum stress and glycogen synthase kinase-3 in lesion development and hepatic steatosis. Am J Pathol. 2009;174:330–342. doi: 10.2353/ajpath.2009.080385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen JB, Abildstrom SZ, Erdal J, et al. Effects of epilepsy and selected antiepileptic drugs on risk of myocardial infarction, stroke, and death in patients with or without previous stroke: a nationwide cohort study. Pharmacoepidem Dr S. 2011;20:964–971. doi: 10.1002/pds.2186. [DOI] [PubMed] [Google Scholar]
- Olesen JB, Hansen PR, Abildstrom SZ, Andersson C, Weeke P, Schmiegelow M, et al. Valproate attenuates the risk of myocardial infarction in patients with epilepsy: a nationwide cohort study. Pharmacoepidem Dr S. 2011;20:146–153. doi: 10.1002/pds.2073. [DOI] [PubMed] [Google Scholar]
- Pylvanen V, Knip M, Pakarinen AJ, et al. Fasting serum insulin and lipid levels in men with epilepsy. Neurology. 2003;60:571–574. doi: 10.1212/01.wnl.0000048209.07526.86. [DOI] [PubMed] [Google Scholar]
- Consortium CAD, Deloukas P, Kanoni S, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Gen. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol. 2004;24:8467–8476. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollowell J. The general practice research database: quality of morbidity data. Pop Trends. 1997;87:36–40. [PubMed] [Google Scholar]
- Dregan A, Toschke MA, Wolfe CD, et al. Utility of electronic patient records in primary care for stroke secondary prevention trials. BMC Public Health. 2011;11:86–94. doi: 10.1186/1471-2458-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschke AM, Gulliford MC, Wolfe CD, Rudd AG, Heuschmann PU. Antihypertensive treatment after first stroke in primary care: results from the general practitioner research database. J Hypertens. 2011;29:154–160. doi: 10.1097/HJH.0b013e32833f3897. [DOI] [PubMed] [Google Scholar]
- Nicholas JM, Ridsdale L, Richardson MP, Ashworth M, Gulliford MC. Trends in antiepileptic drug utilisation in UK primary care 1993–2008: cohort study using the general practice research database. Seizure. 2012;21:466–470. doi: 10.1016/j.seizure.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Richardson DB. An incidence density sampling program for nested case–control analyses. Occup Env Med. 2004;61:e59–e63. doi: 10.1136/oem.2004.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliford MC, Charlton J, Rudd A, Wolfe CD, Toschke AM. Declining 1-year case-fatality of stroke and increasing coverage of vascular risk management: population-based cohort study. J Neurol Neurosur Ps. 2010;81:416–422. doi: 10.1136/jnnp.2009.193136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai N, Charlton J, Rudisill C, Gulliford MC. Coding, recording and incidence of different forms of coronary heart disease in primary care. PLoS One. 2012;7:e29776–e29781. doi: 10.1371/journal.pone.0029776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P, Shorvon S, Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet. 2004;363:1184–1186. doi: 10.1016/S0140-6736(04)15946-1. [DOI] [PubMed] [Google Scholar]
- Huberman M, Langholz B. Application of the missing-indicator method in matched case–control studies with incomplete data. Am J Epidemiol. 1999;150:1340–1345. doi: 10.1093/oxfordjournals.aje.a009966. [DOI] [PubMed] [Google Scholar]
- Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338 doi: 10.1136/bmj.b2393. b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Greenland S. Multiple comparisons and association selection in general epidemiology. Int J Epidemiol. 2008;37:430–434. doi: 10.1093/ije/dyn064. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol. 2009;8:1019–1030. doi: 10.1016/S1474-4422(09)70240-6. [DOI] [PubMed] [Google Scholar]
- Moran NF, Poole K, Bell G, et al. Epilepsy in the United Kingdom: seizure frequency and severity, anti-epileptic drug utilization and impact on life in 1652 people with epilepsy. Seizure. 2004;13:425–433. doi: 10.1016/j.seizure.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Giuliani G, Terziani S, Senigaglia AR, Luccioni G, Foschi N, Maffei C. Epilepsy in an Italian community as assessed by a survey for prescriptions of antiepileptic drugs: epidemiology and patterns of care. Acta Neurol Scandinav. 1992;85:23–31. doi: 10.1111/j.1600-0404.1992.tb03991.x. [DOI] [PubMed] [Google Scholar]
- Essebag V, Platt RW, Abrahamowicz M, Pilote L. Comparison of nested case–control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005;5:5–10. doi: 10.1186/1471-2288-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case–control analysis. BMJ. 2005;330:1366–1369. doi: 10.1136/bmj.330.7504.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case–control study. Lancet. 2005;365:475–481. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45(Suppl 6):13–18. doi: 10.1111/j.0013-9580.2004.455003.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


