Abstract
Bacterial or ingested food-derived short-chain fatty acids (SCFAs) are present in the duodenal lumen. Acetate, the most abundant SCFA in the foregut lumen, is absorbed immediately after ingestion, although the mechanism by which this absorption occurs is not fully understood. We investigated the distribution and function of candidate SCFA transporters in rat duodenum. The Na+-coupled monocarboxylate transporter-1 (SMCT1) was localized to the brush border, whereas the pH-dependent monocarboxylate transporter (MCT) 1 and MCT4 were localized to the duodenocyte basolateral membrane. In Ussing chambered duodenal mucosa, luminal acetate dose-dependently increased short-circuit current (Isc) in the presence of serosal bumetanide and indomethacin by a luminal Na+-dependent, ouabain-sensitive mechanism. The Isc response was inhibited dose-dependently by the SMCT1 nonsubstrate inhibitor ibuprofen, consistent with net electrogenic absorption of acetate via SMCT1. Other SCFAs and lactate also increased Isc. Furthermore, duodenal loop perfusion of acetate increased portal venous acetate concentration, inhibited by coperfusion of ibuprofen or a MCT inhibitor. Luminal acetate perfusion increased duodenal HCO3− secretion via capsaicin-sensitive afferent nerve activation and cyclooxygenase activity, consistent with absorption-mediated HCO3− secretion. These results suggest that absorption of luminal SCFA via SMCT1 and MCTs increases duodenal HCO3− secretion. In addition to SCFA sensing via free fatty acid receptors, the presence of rapid duodenal SCFA absorption may be important for the suppression of luminal bacterial colonization and implicated in the generation of functional dyspepsia due to bacterial overgrowth.
Keywords: acetate, monocarboxylate transporter, sodium-coupled monocarboxylate transporter, short-chain fatty acid
short-chain fatty acids (SCFAs) such as acetic (2-carbon), propionic (3-carbon), and butyric (4-carbon) acids are produced by the gut microbiota and also ingested in the human diet. Intestinal SCFA uptake is a transport function of importance to humankind in that it is the principal means by which domestic cattle obtain nourishment, enabling the production of dairy products and meat for human consumption (6). In the hindgut, luminal SCFAs increase colonic contractility and the secretion of Cl−, HCO3−, and mucus via neural and nonneural pathways (33, 45, 53, 58, 59). Moreover, SCFAs facilitate Na+ and Ca2+ absorption (51, 53). In the rat jejunum and ileum, intraluminal or jugular venous injection of SCFA increases transmural potential difference in vivo (55, 60). Nevertheless, foregut SCFA sensing and mucosal responses to luminal SCFAs have rarely been investigated, likely due to a perceived relative lack of bacterial colonization in proximal gut segments.
The duodenum possesses specialized chemosensing functions as part of an “early warning system” alerting the distal gut and neighboring organs to proximal conditions. We have previously demonstrated that mucosal defense mechanisms, including mucosal blood flow and mucus and HCO3− secretion, are activated by numerous luminal small molecules present after meal ingestion (reviewed in Ref. 3). Not only gastric acid, but also luminal amino acids, bile acids, and fatty acids activate defense mechanisms and the release of insulinotropic and intestinotrophic gut hormones (2, 5, 24, 56). The foregut mucosa encompasses several chemosensory mechanisms, in particular nutrient receptors expressed on enteroendocrine cells. The nutrient receptors include all known G protein-coupled receptor free fatty acid (FFA) receptors. The medium- and long-chain fatty acid receptors FFA1 (GPR40) and FFA4 (GPR120) are expressed in glucagon-like peptide (GLP-1 and -2)- and gastric inhibitory peptide (GIP)-producing enteroendocrine L and K cells (15, 20, 38). FFA2 (GPR43) and FFA3 (GPR41) are SCFA receptors; in colon, FFA2 is mostly expressed in L cells (27, 50), whereas duodenal serotonin (5-HT)-containing enterochromaffin cells express FFA2 (2, 26). FFA3 is expressed in GLP-1-, peptide YY-, cholecystokinin-, GIP-, and/or secretin-producing cells in the small intestine (2, 26, 36). Activation of enteroendocrine nutrient receptors is coupled to gut hormone release, which in turn has been implicated in the generation of functional gut symptoms and also in the pathogenesis of metabolic disorders (17).
Most recently, we reported that perfusion of the duodenal lumen with the selective FFA1 agonist GW-9508 and SCFAs elicited GLP-2 release with a consequent increased rate of HCO3− secretion, whereas the FFA2 agonist phenylacetamide-1 increased the rate of HCO3− secretion via 5-HT4 and muscarinic receptor activation independent of the GLP-2 pathway (2). These results suggest that FFA2 and FFA3 activate distinct secretory pathways in duodenum. Although acetate is an agonist for FFA2 and FFA3, the potency order differs among animal species. In humans, FFA2 has a higher affinity to acetate than does FFA3, but in mice FFA2 and FFA3 are activated equally by acetate (22). Therefore, luminal acetate may stimulate 5-HT- and GLP-2-mediated HCO3− secretion via FFA2 and FFA3. Indeed, in rat duodenum, luminal acetate increased the rate of HCO3− secretion followed by GLP-2 release and the activation of 5-HT4 and muscarinic receptors (2). Furthermore, the monocarboxylate transporter (MCT) inhibitor α-cyano-4-hydroxycinnamic acid (4-CHCA) partially inhibited the secretory response in vivo without altering GLP-2 release, indicating an MCT-dependent secretory pathway independent of L cell activation (2). These results raise a question as to whether MCTs mediate HCO3− secretion as an SCFA/HCO3− exchanger on the apical membrane, or whether MCTs facilitate a SCFA absorption mechanism in the duodenum.
The 13C-labeled acetate breath test is used clinically to measure the gastric emptying rate. Acetate is degraded to CO2 and detected in the breath within 10 min of ingestion (10), indicating rapid foregut acetate absorption. Although carrier-mediated SCFA transport was predicted by kinetic measurements in human and rat small intestine (30, 43, 44), the mechanism underlying intestinal acetate absorption is still obscure. The colonic mucosa is believed to absorb SCFAs largely via simple diffusion of the nonionic form and via SCFA−/HCO3− exchange, based on the observations that SCFA transport is facilitated by low luminal pH and related to luminal HCO3− accumulation (13, 42). Nevertheless, SCFAs are substrates of solute carrier proteins (SLCs), including the SLC16 isoforms MCT1−4 (35) and members of the SLC5 Na+-dependent monocarboxylate transporter (SMCT) family SMCT1 (SLC5A8) and -2 (SLC5A12) (reviewed in Ref. 18). SMCT1 is electrogenic due to its 1:2 or 1:3 stoichiometry with Na+ (34). SMCT2 neutrally transports SCFA with Na+ 1:1, whereas MCTs are either H+ cotransporters or HCO3− exchangers. The topography and segmental heterogeneity of transporter expression was reported in human and mouse intestine (19, 25, 49), but the precise expression pattern in the duodenum has not been previously reported in any animal species. In the present study, we investigated the localization and absorptive function of SMCT1 and MCTs in rat proximal duodenum.
METHODS
Animals.
Male Sprague-Dawley rats weighing 200–250 g (Harlan, San Diego, CA) were fed a pellet diet and water ad libitum. All studies were performed with approval of the Veterans Affairs Institutional Animal Care and Use Committee. Rats were fasted overnight with free access to water before the experiments.
Chemicals.
UK-5099 was obtained from Tocris Bioscience (Ellisville, MO). Sodium ibuprofen, sodium acetate, sodium butyrate, sodium propionate, sodium l-lactate, 4-CHCA, and other chemicals were purchased from Sigma Chemical (St. Louis, MO). Ibuprofen, acetate, butyrate, propionate, and l-lactate were dissolved in distilled water. UK-5099, amiloride, bumetanide, ouabain, and 4-CHCA were dissolved in dimethyl sulfoxide (DMSO). Indomethacin was dissolved in 100% ethanol.
Effect of acetate on duodenal HCO3− secretion in vivo.
Duodenal loops were prepared and perfused as previously described (4). In brief, under isoflurane anesthesia (2%), a 2-cm-length proximal duodenal loop was perfused with normal saline by using a peristaltic pump at 1 ml/min. The perfusate was bubbled with 100% O2 and was stirred and warmed at 37°C with a heating stirrer. The pH of the perfusate was kept constant at pH 7.0 with a pH stat (models PHM290 and ABU901; Radiometer Analytical, Lyon, France). To eliminate buffering by added compounds, which would affect the pH-stat titration volume, flow-through pH and CO2 electrodes (Lazar Research Laboratories, Los Angeles, CA) were connected to the perfusion loop enabling the simultaneous and continuous measurement of perfusate pH and CO2 concentrations ([CO2]). Because the input (perfusate) [CO2] is ≈0, the effluent [CO2] and pH were used to calculate the total CO2 output equivalent to the secreted HCO3−. To prevent contamination of the perfusate from bile or pancreatic juice, the pancreatobiliary duct was ligated just proximal to its insertion in the duodenal wall and was cannulated with a PE-10 tube to drain the juice. After stabilization with continuous perfusion of pH 7.0 saline for ∼30 min, the time (t) was set as t = 0. The duodenal loop was perfused with pH 7.0 saline from t = 0 min until t = 10 min (basal period). The perfusate was then changed to pH 7.0 Krebs buffer containing acetate (0.1 mM, pH adjusted to 7.0) from t = 10 min until t = 35 min (experiment period). At t = 10 min, the system was gently flushed so as to rapidly change the composition of the perfusate. Duodenal HCO3− secretion was expressed as total CO2 output (μmol·min−1·cm−1) calculated from the measured pH and [CO2] in the effluent solution as previously reported (4).
Some animals were deafferented with high-dose capsaicin pretreatment (125 mg/kg sc) 10–14 days before the experiments or were pretreated with indomethacin (5 mg/kg sc) to inhibit cyclooxygenase (COX) activity 1 h before the experiments as previously described (1).
Measurement of acetate concentration in portal venous blood.
The portal vein (PV) was cannulated using a 23-gauge needle attached with a PE-50 tube filled with heparin-containing saline as described previously (56). Two hundred microliters of venous samples were collected using a syringe with 1 μl EDTA at 0, 5, 10, and 30 min after luminal perfusion with acetate (10 mM) with or without the nonsubstrate SMCT1 inhibitor ibuprofen (1 mM) or the potent MCT inhibitor UK-5099 (1 μM) (11). At the end of the experiments, arterial blood was collected from the abdominal aorta as a reference. Each sample was immediately centrifuged at 5,000 g for 5 min, and plasma was stored at −80°C until measured. Acetate concentration was measured using the Acetate Colorimetric Assay Kit (BioVision, Milpitas, CA), according to the manufacturer's protocol.
Tissue preparation for Ussing chamber study.
Rats were anesthetized by isoflurane and killed by exsanguination. The mucosa-submucosa preparations were obtained from rat proximal duodenum, which is the segment between a point 0.5 cm distal to the pyloric ring and the insertion point of pancreatobiliary duct. The duodenal segments were opened along the mesenteric border, and the tunica muscularis was stripped with fine forceps under a stereomicroscope in ice-cold Krebs buffer containing 10 μM indomethacin. Two preparations were prepared from each segment by dividing each longitudinally, which were then mounted between two hemichambers with an aperture = 0.3 cm2 (Physiologic Instruments, San Diego, CA).
Short-circuit current measurement in Ussing chambers.
Chambers were bathed with serosal and luminal bathing solutions in a volume of 4 ml each, maintained at 37°C using a water-recirculating heating system. The serosal bathing solution contained (in mM) 120 NaCl, 4 KCl, 1.8 CaCl2, 25 NaHCO3, 10 HEPES (pH 7.4), 10 glucose, and 0.01 indomethacin, whereas luminal bathing solution contained 136 NaCl, 2.6 KCl, 1.8 CaCl2, 10 HEPES (pH 7.4), and 10 mannitol. The serosal bath was bubbled with 95% O2-5% CO2, and the luminal bath was bubbled with 100% O2. For the Na+-free solution, NaCl was replaced with N-methyl-d-glucamine (NMDG). For the HCO3−-free condition, NaHCO3 was replaced with NaCl, and acetazolamide (0.2 mM) was added to both serosal and luminal baths bubbled with 100% O2. The tissues were short-circuited by a voltage clamp (Physiologic Instruments) at zero potential difference with automatic compensation for solution resistance. Short-circuit current (Isc) was continuously measured with tissue conductance (Gt) determined every 20 s. An increase of Isc indicates luminal-to-serosal current flow, e.g., anion secretion or cation absorption. The current was recorded by the DataQ system (Physiologic Instruments). The tissues were stabilized for 30 min before the effects of SCFA and other drugs were investigated. DMSO <0.3% in the bathing solution did not affect the basal Isc.
Immunohistochemistry and real-time PCR.
Small pieces of rat intestine and submandibular glands were immersed overnight at 4°C in Zamboni's fixative containing 2% paraformaldehyde and 0.2% picric acid. The fixed tissues were washed in PBS (pH 7.4) containing 20% sucrose for cryoprotection and were frozen with optimal-cutting temperature compound (Sakura Finetek, Torrance, CA). Sections were cut at 8-μm thickness and stored at −20°C until use. After thawing, the sections were postfixed and permeabilized in cold acetone followed by 0.1% Triton X-100 in PBS. Nonspecific immunoreactions were blocked by preincubation with 1% BSA/PBS solution for 30 min at room temperature. Affinity-purified rabbit MCT1 and guinea pig MCT4 antibodies were raised against each COOH-terminal peptide of murine MCT sequence according to the method previously described (57). The selectivity and specificity of these antibodies were confirmed by Western blotting of human embryonic kidney (HEK293T) cells expressing murine MCT1, MCT2, or MCT4 and of the proximal duodenal mucosa from rats. MCT antibodies and the rabbit SMCT1 antiserum (25) were diluted with 1% BSA/PBS solution to a concentration of 1 μg/ml and reacted with the preblocked sections overnight at 4°C. After the sections were rinsed in PBS, fluorescence-conjugated secondary antibodies were reacted for 1 h at room temperature. The sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and observed by confocal laser microscope (LSM710; Carl Zeiss, Jena, Germany), and images were captured with a cooled charge-coupled device digital camera system (Axio Imager Z1; Carl Zeiss).
Expression of MCT1 and MCT4 in the tissues was also analyzed by real-time reverse transcription-PCR as previously described (5). The PCR primer sequences were rat MCT1: sense (5′-GCTGCTTCTGTTGTTGCGAA-3′) and antisense (5′-CGATGATGAGGATCACGCCA-3′), giving rise to a 298-bp PCR product; rat MCT4: sense (5′-acggcaggtttcataacagg-3′) and antisense (5′-AAACTTTCGTTGCGTCCAGC-3′), giving rise to a 313-bp PCR product; β-actin was used as an internal control. The expression level was presented as fold induction per 103 copies of β-actin by the ΔCt method.
Statistics.
Values are expressed as means ± SE, and n represents the number of rats. The significance of the difference between data of the control and experimental groups was determined by one-way or two-way ANOVA. The multiple comparisons were performed by Fischer's least-significant difference test or Dunnett's test. Differences were considered significant when P values <0.05.
RESULTS
Localization of SMCT and MCT proteins in rat proximal duodenum.
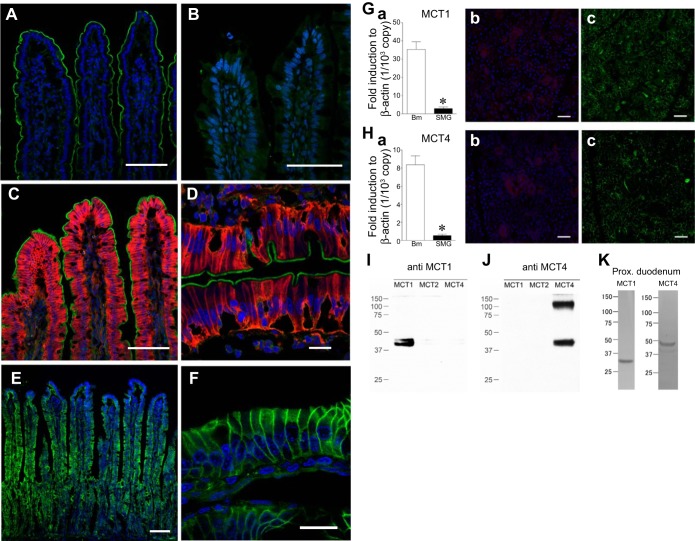
Immunoreactivity for SMCT1 was localized to the brush border of the enterocytes in the upper two-thirds of the villi in rat proximal duodenum (Fig. 1A). Consistent with a previous report (49), SMCT1 was not expressed in the midjejunum (Fig. 1B). MCT4 was localized to the basolateral membrane of epithelial cells lining the upper half of the villi (Fig. 1, C and D), whereas MCT1 was ubiquitously detected on the basolateral membrane of the enterocytes in villi and crypts (Fig. 1, E and F). In contrast, submandibular glands were faintly stained with MCT1 (Fig. 1Gb) and MCT4 (Fig. 1Hb), consistent with low mRNA expression (Fig. 1, Ga and Ha). Antibody selectivity for MCT1 and MCT4 was confirmed by Western blotting with HEK293T cells expressing each MCT homolog. No cross-reaction to other MCTs was observed (Fig. 1, I and J). Western blotting with proximal duodenal mucosa yielded a unique band for MCT1 and MCT4 (Fig. 1K).
Fig. 1.
Localization of Na+-coupled monocarboxylate transporter (SMCT1) and monocarboxylate transporter (MCT) in rat duodenum and midjejunum. A and B: SMCT1 immunoreactivity (green) was present in the brush border in the duodenum (A) but not in the midjejunum (B). C and D: distinct membrane expression of apical SMCT1 (green) with basolateral MCT4 (red) in the duodenum. MCT4 immunoreactivity was detected on the epithelial basolateral membrane in the upper half of villi. E and F: MCT1 immunoreactivity was detected on the epithelial basolateral membrane in villi and crypts in the duodenum. Bar = 100 (A–C and E) and 20 (D and F) μm. Counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue). G and H: negative control for MCT1 (G) and MCT4 (H) expression. mRNA expressions of MCT1 (Ga) and MCT4 (Ha) in the submandibular glands (SMG) were significantly lower than in the duodenal bulb mucosa (Bm). *P < 0.05 vs. Bm (n = 5). MCT1 (red, Gb) and MCT4 (red, Hb) were faintly stained in rat submandibular glands. The sections were counterstained with DAPI (blue) and phalloidin (green) for comparison (Gc and Hc). Bar = 50 μm. I and J: immunoblotting of HEK293T cells expressing murine MCT1, MCT2, or MCT4 with MCT1 (I) and MCT4 (J) antibodies. K: Western blotting of the scraped proximal duodenal mucosa for MCT1 and MCT4.
Effect of luminal acetate on Isc in rat proximal duodenum.
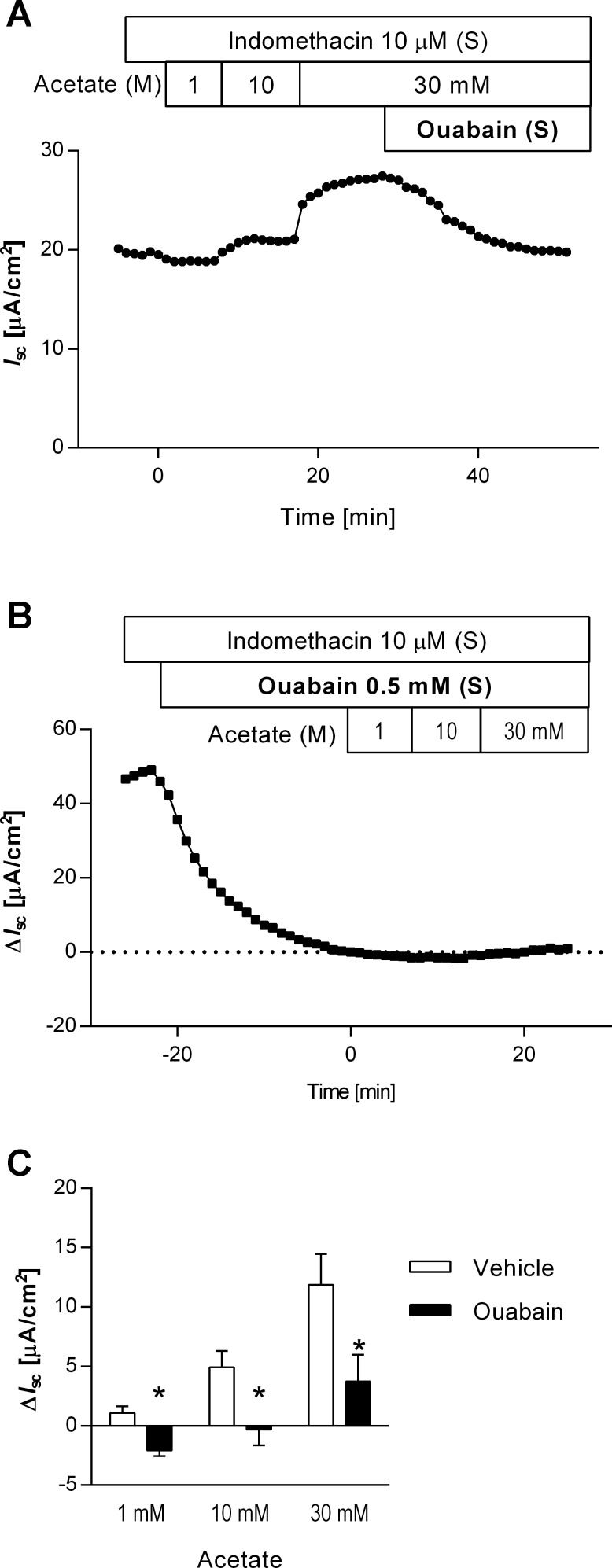
In Ussing chambered mucosa-submucosa preparations, the cumulative addition of acetate (pH 7.4, 1−30 mM) into the luminal bath increased Isc in a dose-dependent manner (Fig. 2, A and C). When 10 or 30 mM of acetate was added, Isc peaked 1–3 min after addition, sustained at an elevated value for >10 min, accompanied by an increase of Gt and potential difference. Subsequent addition of ouabain (0.5 mM) into the serosal bath decreased the acetate-evoked Isc to basal levels (Fig. 2A). Pretreatment with ouabain (0.5 mM) gradually decreased basal Isc by 51.8 ± 17.2 μA/cm2 (n = 4), stabilized within 25 min. Ouabain pretreatment significantly reduced the response to luminal acetate (Fig. 2, B and C). These results suggest that acetate-induced Isc response is dependent on basolateral Na+-K+-ATPase activity.
Fig. 2.
Acetate-induced increases in short-circuit current (Isc) and the effect of ouabain in Ussing chambered mucosa-submucosal preparation of rat duodenum. A: a representative Isc trace illustrated that cumulative application of acetate to the luminal bathing solution (M) increased and sustained Isc. The further addition of the Na+-K+-ATPase inhibitor ouabain to the serosal bath (S) gradually decreased the acetate-induced Isc to the basal level. B: a representative time course of ΔIsc illustrated that ouabain alone decreased the basal Isc and suppressed the response to luminal acetate. Ouabain was added to the serosal bathing solution 20 min before acetate application. The change of Isc (ΔIsc) was calculated as the difference between Isc measured just before the addition of acetate (time = 0) and peak Isc values. C: acetate-induced ΔIsc was significantly reduced by ouabain pretreatment; n = 4. *P < 0.05 vs. vehicle group.
Effect of bumetanide on acetate-induced Isc increases.
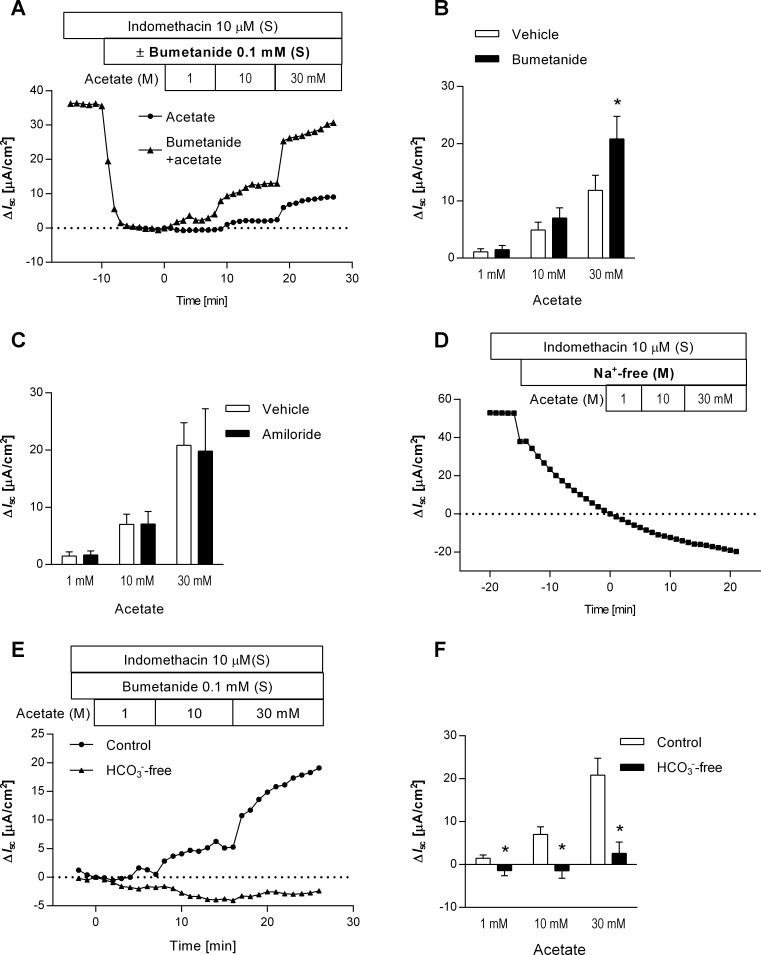
The Na+-K+-2Cl− cotransporter (NKCC) 1 inhibitor bumetanide was used to investigate the ionic components of the acetate-induced increase in Isc. Serosal application of bumetanide (0.1 mM) decreased basal Isc by 27.0 ± 6.0 μA/cm2 (n = 5), stabilized within 5 min, indicating that basolateral NKCC1 is involved in electrogenic Cl− secretion, contributing to basal Isc in rat proximal duodenum. The presence of bumetanide exaggerated the dose-dependent Isc response to the luminal application of acetate (Fig. 3, A and B). To minimize the confounding effect of NKCC1-dependent ion transport on the acetate-induced Isc response, the succeeding experiments were performed in the presence of bumetanide.
Fig. 3.
Ionic characterization of luminal acetate-induced Isc change. A and B: effect of bumetanide on the response to luminal acetate. Representative time courses of ΔIsc (A) illustrated the acetate-induced Isc change in the presence (triangles) or absence (circles) of the Na+-K+-2Cl− cotransporter (NKCC1) inhibitor bumetanide. Bumetanide was applied to the serosal bath (S) 10 min before the stepwise addition of acetate. ΔIsc values were determined as the difference from Isc just before the acetate application (time = 0) to peak Isc values. Acetate-induced ΔIsc was significantly increased by bumetanide treatment (B); n = 5. *P < 0.05 vs. vehicle groups. C: effect of amiloride on acetate-induced Isc increases. No significant difference was detected between vehicle and amiloride-treated group in acetate-induced ΔIsc; n = 3−5. D: effect of Na+ depletion on the response to luminal acetate. A representative time course of ΔIsc illustrated that replacement of luminal bathing solution with a Na+-free buffer decreased basal Isc and that luminal acetate had no effect on Isc in the absence of luminal Na+. E and F: effect of serosal HCO3− depletion on acetate-induced Isc increases. Representative time courses (E) illustrated luminal acetate-induced ΔIsc in the presence (circles) or absence (triangles) of serosal HCO3−. HCO3−-free conditions significantly reduced the response to luminal acetate (F); n = 5. *P < 0.05 vs. the control groups.
Effect of amiloride on acetate-induced Isc increases.
Amiloride (0.1 mM) was used to examine the involvement of epithelial Na+ channels in the response to luminal acetate. In the presence of bumetanide, luminal application of amiloride had no effect on basal Isc, indicating negligible electrogenic Na+ absorption in the duodenum. Five minutes after amiloride application, acetate was cumulatively added to the luminal bath. Pretreatment with amiloride did not change acetate-induced increases in Isc (Fig. 3C), suggesting that SCFA-related Isc is not due to electrogenic Na+ channel activity such as ENaC.
Effect of Na+ or HCO3− depletion on acetate-induced Isc increases.
Replacement of luminal Na+ with NMDG gradually decreased basal Isc and abolished the response to luminal acetate (1−30 mM) (Fig. 3D), suggesting that acetate-induced Isc response is luminal Na+-dependent.
Removing HCO3− from the serosal solution did not affect basal Isc, indicating that electrogenic HCO3− transport contributes minimally to the generation of the basal Isc in rat proximal duodenum. The absence of serosal HCO3−, however, significantly reduced acetate-induced increases in Isc in the presence of bumetanide (Fig. 3, E and F), suggesting that the acetate-generated Isc requires serosal HCO3−.
Effect of monocarboxylate transport inhibitors on acetate-induced Isc increases.
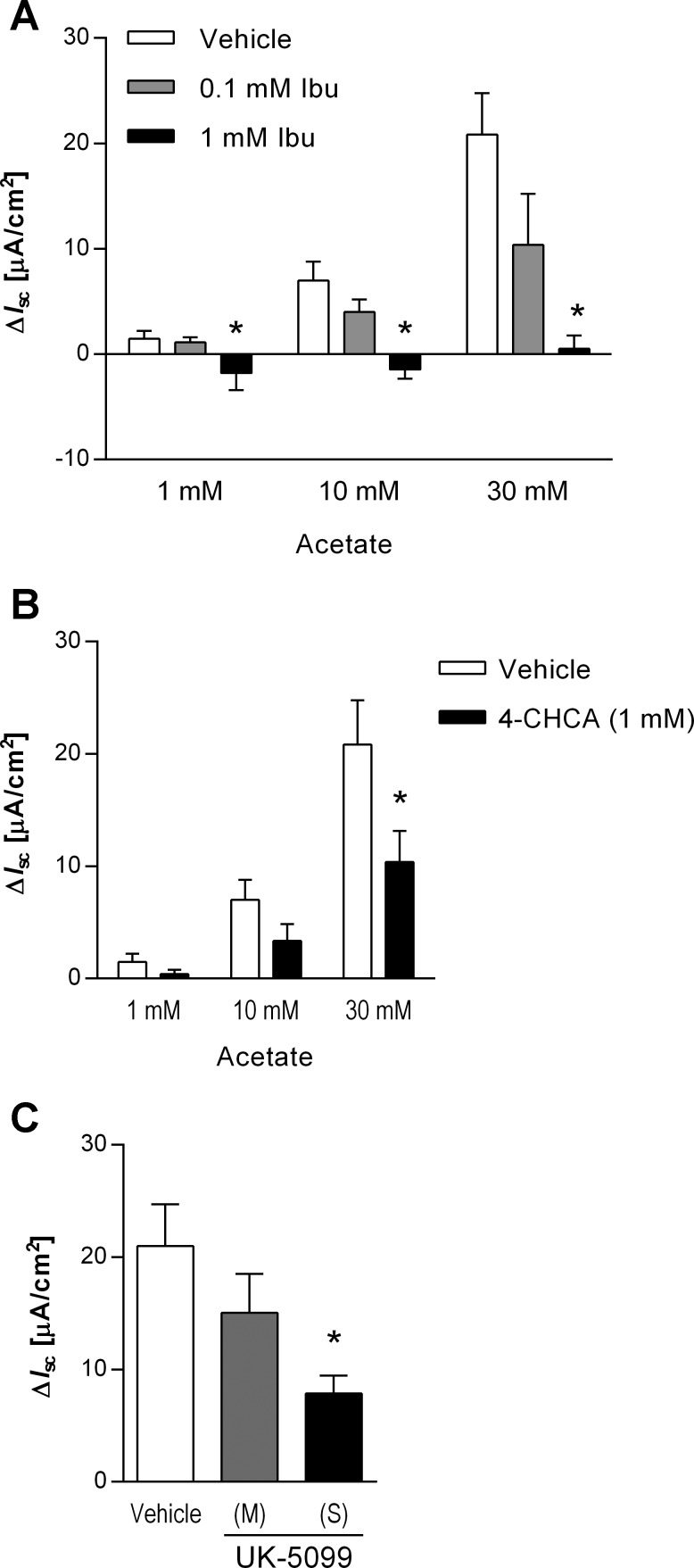
A nonsubstrate SMCT1 inhibitor, ibuprofen (14), was used to examine the contribution of SMCT1 to the acetate-induced Isc response in rat duodenum. Luminal application of ibuprofen (0.1 or 1 mM) following bumetanide had no effect on basal Isc. Acetate-induced Isc was dose-dependently reduced by the addition of ibuprofen (Fig. 4A). The absence of a direct toxic or injurious effect of ibuprofen was supported by the lack of effect of ibuprofen on the response to serosally applied carbachol (10 μM), which transiently increased Isc in the presence of bumetanide and acetate to the same extent with (52.0 ± 10.7 μA/cm2) or without (43.8 ± 13.5 μA/cm2) 1 mM ibuprofen (P > 0.05).
Fig. 4.
Effect of monocarboxylate transport inhibitors on acetate-induced Isc increases. Luminal acetate-induced ΔIsc was measured in the presence or absence of the SMCT1 inhibitor ibuprofen (Ibu, A), the MCT inhibitors α-cyano-4-hydroxycinnamic acid (4-CHCA, B), or UK-5099 (C). Ibu was added into the luminal whereas 4-CHCA was added into the serosal bathing solution. UK-5099 followed by 30 mM acetate was added to the luminal (M) or serosal (S) bath. Pretreatment with 1 mM ibuprofen, 4-CHCA, or serosal UK-5099 significantly decreased the response to luminal acetate; n = 3−5. *P < 0.05 vs. the vehicle group.
Addition of the MCT inhibitor 4-CHCA (1 mM) to the serosal bath did not change basal Isc, whereas 4-CHCA significantly reduced the Isc response to 30 mM acetate (Fig. 4B). The potent and specific MCT inhibitor UK-5099 (10 μM) added to the serosal bath significantly reduced the Isc response to 30 mM acetate, whereas luminal treatment had no significant effect (Fig. 4C).
Effect of luminal SMCT1 substrates on Isc in rat proximal duodenum.
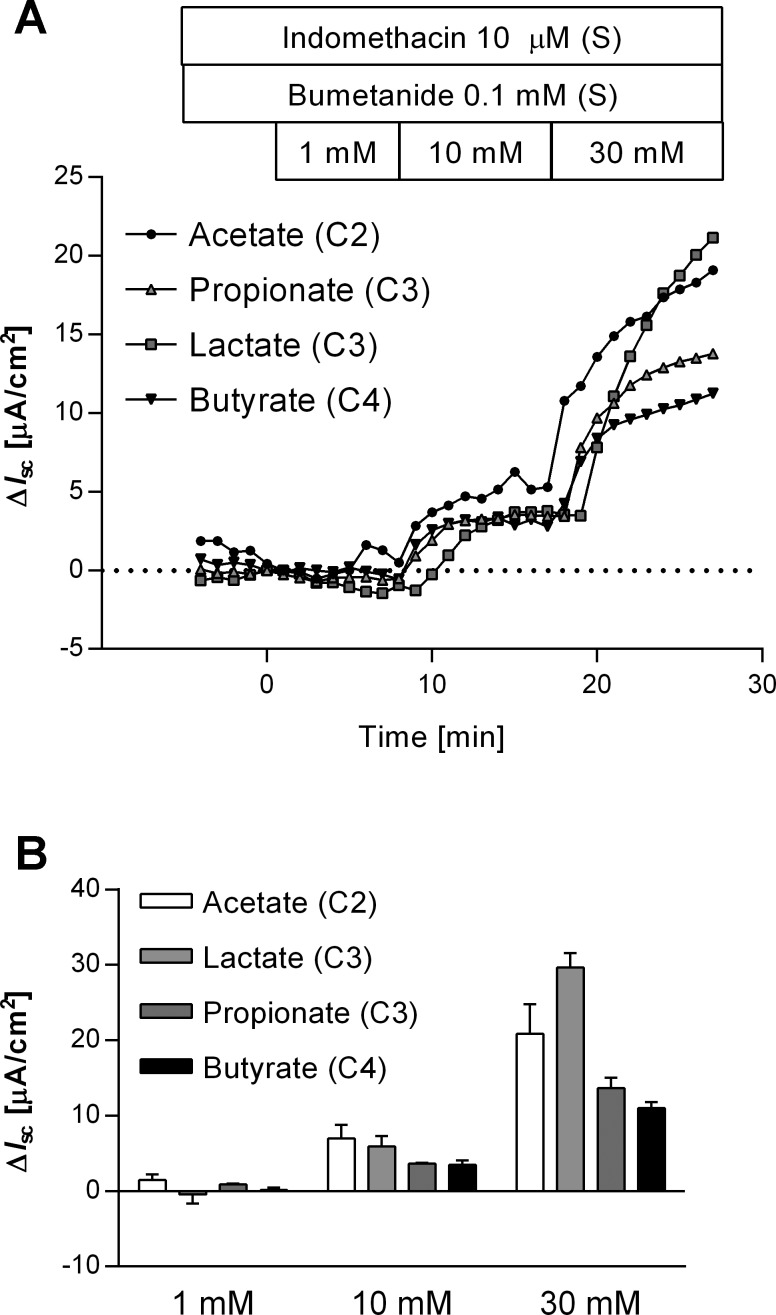
To confirm the functional expression of SMCT1, we investigated the effects of established SMCT1 substrates: l-lactate, propionate, and butyrate. In the presence of bumetanide, the stepwise application of these substrates (pH 7.4, 1−30 mM) increased Isc dose-dependently, similar to the response to acetate (Fig. 5, A and B).
Fig. 5.
Effect of SMCT1 substrates on Isc. A: representative time courses illustrated luminal SMCT1 substrate-induced ΔIsc. SMCT1 substrates acetate, propionate, lactate, or butyrate were cumulatively added into the luminal bath. B: SMCT1 substrate-induced ΔIsc was dose-dependently increased; n = 3−5.
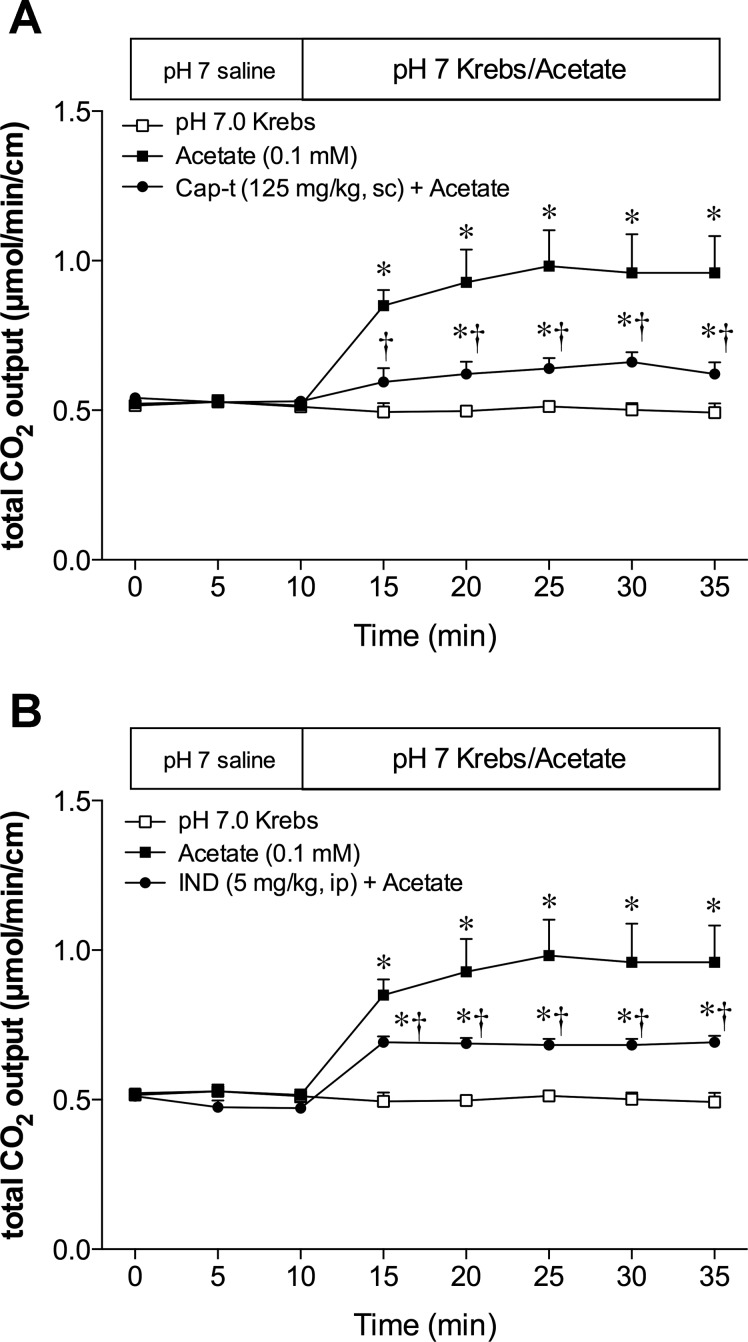
Effect of capsaicin or indomethacin treatment on acetate-induced HCO3− secretion in vivo.
Acetate itself is absorbed in relation to HCO3− secretion in the large intestine (53) and in the duodenum (2). Because mucosal HCO3− secretion in the proximal duodenum is mediated by the activation of capsaicin-sensitive afferent nerves and COX activity (28), we examined the effect of acetate in capsaicin-denervated or in indomethacin-treated rats. Deafferentation or indomethacin pretreatment inhibited acetate-induced HCO3− secretion (Fig. 6, A and B). These results support our hypothesis that acetate-induced HCO3− secretion is partially mediated by an absorptive pathway in which absorbed acetate stimulates capsaicin-sensitive afferent nerves and increases COX activity.
Fig. 6.
Effect of deafferentation or cyclooxygenase (COX) inhibition on acetate-induced HCO3− secretion in vivo. The duodenal loop was perfused with acetate (0.1 mM) under capsaicin (Cap)-treated (A) or indomethacin (IND)-treated (B) conditions. Cap or IND treatment significantly decreased the acetate-induced HCO3− secretion; n = 6. *P < 0.05 vs. the pH 7.0 Krebs group. *†P < 0.05 vs. the acetate group.
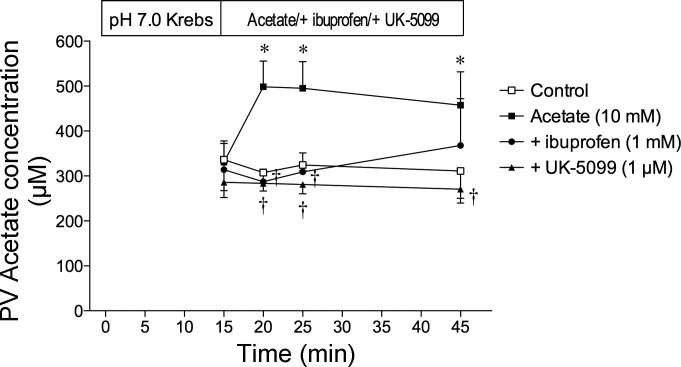
PV acetate concentrations during duodenal perfusion with acetate.
To confirm the presence of transmucosal acetate transport, PV plasma acetate concentrations were measured before and after luminal perfusion of acetate with or without an SCFA transporter inhibitor. Acetate concentrations in the arterial blood at the end of experiments were similar to those in PV before the perfusion of acetate (data not shown). Stable acetate concentrations were observed in the PV during luminal perfusion of pH 7.0 Krebs as a control group (Fig. 7). PV acetate concentration in the fasted rats was ∼600 μM, as determined with gas chromatography (23), similar to our results. Luminal perfusion of acetate (10 mM) markedly increased PV acetate concentrations at 5 min after starting the perfusion, remaining elevated for 30 min. Coperfusion of the nonsubstrate SMCT1 inhibitor ibuprofen (1 mM) or the specific MCT inhibitor UK-5099 (1 μM) with acetate abolished the increase of PV acetate concentrations, indicating that acetate absorption by the duodenal mucosa was mediated by SMCT1 and MCT.
Fig. 7.
Portal vein (PV) acetate concentration during luminal perfusion of acetate in vivo. The duodenal loop was perfused with acetate (10 mM) from time (t) = 15 min. PV blood was collected before (t = 15 min) and 5, 10, and 30 min after the perfusion of acetate with or without ibuprofen (1 mM) or UK-5099 (1 μM); n = 6. *P < 0.05 vs. control group. †P < 0.05 vs. acetate group.
DISCUSSION
We have demonstrated the functional expression of SMCT1 on the enterocyte brush border and MCTs on the basolateral membrane, which account for acetate absorption in the proximal duodenum. Furthermore, we have shown that luminal acetate stimulates duodenal HCO3− secretion in vivo partially via activation of capsaicin-sensitive afferent nerves and the COX-dependent pathways. We recently reported that luminal acetate increased duodenal HCO3− secretion through FFAs expressed on enteroendocrine cells followed by GLP-2 release and the stimulation of 5-HT-dependent neural pathways and, additionally, through an MCT-dependent pathway (2). These results indicate that luminal acetate activates multiple mucosal responses, including gut hormone release, prostaglandin (PG) production, and afferent nerve activation, mediated by endocrine FFAs and by SMCT1 and MCTs expressed in the duodenal enterocytes. These findings underscore the importance of SCFAs in duodenal chemosensing and the regulation of mucosal defenses.
In Ussing chambered duodenal mucosa, luminal acetate increased luminal Na+-dependent and ouabain-sensitive Isc, consistent with the presence of a Na+-dependent electrogenic SCFA transport mechanism. Immunoreactivity for SMCT1 in the duodenal brush border further supports the functional expression of SMCT1 in duodenal enterocytes. SMCT1 is a unique MCT whose electrogenicity is attributable to its asymmetric transport properties; 2–3 Na+:1 monocarboxylate (34). Acetate-induced Isc was enhanced by NKCC1 inhibition but was not affected by ENaC inhibition, indicating that acetate stimulates ENaC-independent, electrogenic Na+ absorption rather than anion secretion. Because NKCC1 provides intracellular Cl− for electrogenic anion secretion, the decrease of basal Isc due to the application of the NKCC1 inhibitor bumetanide indicates that Cl− secretion contributes to the measured Isc. Na+ uptake from the serosal solution into the epithelial cells via NKCC1 increases intracellular Na+ concentration, decreasing the transmembrane Na+ gradient, therefore reducing the Isc signal attributable to SMCT1 activity. An acetate-induced Isc was observed at substrate concentrations >1 mM, consistent with the Km calculated for human SMCT1 (2.46 mM) for acetate transport in an oöcyte expression system (34). Although the lack of a selective inhibitor of SMCT1 impairs the study of SMCT1 function in vivo, the nonsubstrate SMCT1 inhibitor ibuprofen (1 mM) inhibited acetate-induced Isc increases in vitro in the presence of indomethacin, consistent with the inhibition of SCFA uptake by ibuprofen in SMCT1-expressing oöcytes (14), independent of the COX inhibitory effects of ibuprofen. These findings suggest that apical SMCT1 electrogenically cotransports luminal acetate and Na+ into duodenal epithelial cells.
SMCT1 is highly expressed in mouse distal ileum and large intestinal epithelial cells, where luminal SCFA concentration can exceed 100 mM (47). The localization of SMCT1 to the apical membrane of colonic epithelial cells in the midcrypt region (25) supports SMCT1-mediated absorption of luminal SCFAs. In perfused rat colon, acetate absorption was partially Na+-dependent (53). Nevertheless, SMCT1-dependent SCFA absorption has not been confirmed with broken preparations such as isolated colonocytes or brush-border membrane vesicles (31, 52). Because colonic surface cells lack SMCT1 expression (25), isolated surface colonocytes or brush-border membrane vesicles may not be good model systems for the study of SMCT1 function.
MCT1 and MCT4 were expressed in the basolateral membrane of duodenal epithelial cells, consistent with MCT1 and MCT4 localization on the basolateral membrane of epithelial cells in human, mouse, and rat large intestine (19, 25). In contrast, MCT1 expression on the enterocyte apical membrane is reported in human colon (19) and substrate-exposed rat colon (8). Luminal application of the nonspecific MCT inhibitor 4-CHCA has no effect on transmucosal SCFA transport in Ussing chambered mouse cecum (29), indicating that it is unlikely that MCTs function on the apical membrane. In rat duodenum, 4-CHCA partially inhibited acetate-induced HCO3− secretion in vivo (2) and inhibited electrogenic acetate absorption in Ussing chamber measurements, suggesting that acetate exits the enterocyte via basolateral MCTs. Moreover, serosal application of the potent and specific MCT inhibitor UK-5099 decreased electrogenic acetate absorption, supporting the basolateral localization of MCTs. Furthermore, PV acetate concentration was rapidly increased by duodenal perfusion with acetate in an ibuprofen- and UK-5099-inhibitable manner, indicating that rapid monocarboxylate absorption is mediated by SMCT1 and MCTs in rat duodenum.
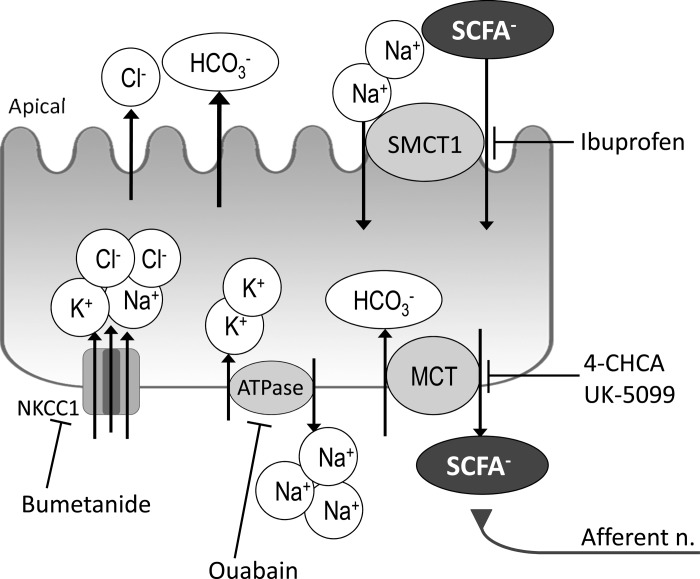
We have also reported in vivo experiments that luminal acetate-induced HCO3− secretion was mediated by activation of capsaicin-sensitive afferent nerves. Because luminal acetate was rapidly absorbed and MCT inhibitor reduced acetate-induced HCO3− secretion, our results suggest that the absorbed acetate stimulates subepithelial afferent nerves. Capsaicin-sensitive chemosensory vagal and spinal afferent nerves, which innervate the foregut, actuate gastrointestinal functions through interaction with intrinsic secretomotor neurons and PG production (40, 41, 48, 54). The rat proximal duodenum is densely innervated by branched vagal afferent nerves, extending nearly to the enterocyte basolateral membrane (7, 39). Our preliminary study showed that FFAs were expressed in rat nodose and dorsal root ganglia, the origins of extrinsic afferent nerves (26). These findings support our hypothesis that luminal acetate is transported across the villous epithelium into the subepithelial space, where activation of FFAs expressed on capsaicin-sensitive afferent nerves may occur. Taking these observations together, the primary function of SCFAs absorbed by the duodenum may be chemosensory rather than nutritional. A schema of duodenal SCFA absorption as part of a physiological absorptive, chemosensing, and signaling system is depicted in Fig. 8.
Fig. 8.
Schematic summary of ion transport and SCFA absorption mechanism of epithelial cells in rat duodenum. Luminal SCFAs are electrogenically cotransported with Na+ via the apical SMCT1, inhibited by ibuprofen in a bumetanide-facilitated, ouabain-sensitive manner. The SCFAs transported into the duodenocytes are exchanged with HCO3− via basolateral MCTs, inhibited by 4-CHCA or UK-5099. The SCFAs extruded across the enterocyte basolateral membrane activate subepithelial capsaicin-sensitive afferent nerves, stimulating HCO3− secretion. ATPase, Na+-K+-ATPase.
Among SCFAs, acetic acid is frequently ingested in the diet, since the common condiment vinegar is 4–7% acetic acid by volume (∼0.7−1.2 M). Oral commensal bacteria are another source of acetate; after overnight fasting, 4–6 mM acetate can be measured in human saliva and 0.4 mM in duodenal juice (9, 21), suggesting that substantial acetate concentrations are regularly present in the duodenal lumen, although the concentrations of the other major SCFAs propionate and butyrate are relatively low (0.05 and 0.01 mM, respectively) (21). Plasma acetate, due to its conversion to acetyl-CoA followed by rapid oxidation, is called a “ready-to-use fuel” (46). In human clinical studies, the supplementation of acetic acid to a carbohydrate-rich diet improves glycemic control (12, 37), not only due to slowing gastric emptying via gut hormone release, but also possibly due to slowing Na+-dependent glucose absorption by Na+-dependent acetate transport via SMCT.
If SCFAs are not ingested in the diet, their presence in the gut lumen is indicative of bacterial activity. Therefore, luminal SCFA sensing may be important for the maintenance of symbiosis between host and gut microbiota. Human amniotic fluid contains organic acids, including acetate and lactate at concentration 6.9 and 9.7 mM, respectively (32). In infants, feces contain significant amounts of acetate and lactate, but rarely propionate and butyrate, produced by bacterial fermentation from oligosaccharides contained in breast milk (16). These reports suggest that the human intestine is exposed to monocarboxylates throughout life and even prenatally, further supporting the key functions of duodenal SCFA sensing and absorption mechanisms. Because SCFAs stimulate hormone release from enteroendocrine cells and absorbed SCFAs activate afferent nerves, SCFA sensing and absorption mechanisms in the duodenum may also provide a logical basis for the pathogenesis of functional dyspepsia syndromes in the presence of intestinal bacterial overgrowth.
GRANTS
This study was supported by a Grant-in-Aid for the Japan Society for the Promotion of Science Fellows to I. Kaji (no. 24-5317), a Department of Veterans Affairs Merit Review Award from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01-DK-54221 to J. Kaunitz), and the animal core of the NIDDK (P30-DK-0413 to J. E. Rozengurt).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.K. and Y.A. conception and design of research; I.K., M.W., and Y.A. performed experiments; I.K. and Y.A. analyzed data; I.K., T.I., and Y.A. interpreted results of experiments; I.K. prepared figures; I.K. drafted manuscript; I.K., T.I., M.W., P.H.G., E.E., J.D.K., and Y.A. edited and revised manuscript; I.K., T.I., M.W., P.H.G., E.E., J.D.K., and Y.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bea Palileo for assistance in preparing the article.
REFERENCES
- 1.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol Gastrointest Liver Physiol 277: G268–G274, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, Watanabe M, Guth PH, Engel E, Kuwahara A, Kaunitz JD. Short-chain fatty acid sensing in rat duodenum. J Physiol (Lond) In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiba Y, Kaunitz JD. Duodenal luminal chemosensing; Acid, ATP, and nutrients. Curr Pharm Des 20: 2760–2765, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Akiba Y, Mizumori M, Guth PH, Engel E, Kaunitz JD. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol 293: G1223–G1233, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Akiba Y, Watanabe C, Mizumori M, Kaunitz JD. Luminal l-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol 297: G781–G791, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 70: 567–590, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud HR, Kressel M, Raybould HE, Neuhuber WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 191: 203–212, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Borthakur A, Priyamvada S, Kumar A, Natarajan AA, Gill RK, Alrefai WA, Dudeja PK. A novel nutrient sensing mechanism underlies substrate-induced regulation of monocarboxylate transporter-1. Am J Physiol Gastrointest Liver Physiol 303: G1126–G1133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botta GA, Radin L, Costa A, Schito G, Blasi G. Gas-liquid chromatography of the gingival fluid as an aid in periodontal diagnosis. J Periodontal Res 20: 450–457, 1985. [DOI] [PubMed] [Google Scholar]
- 10.Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, Hor G, Caspary WF. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology 108: 1048–1055, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Brailsford MA, Thompson AG, Kaderbhai N, Beechey RB. Pyruvate metabolism in castor-bean mitochondria. Biochem J 239: 355–361, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brighenti F, Castellani G, Benini L, Casiraghi MC, Leopardi E, Crovetti R, Testolin G. Effect of neutralized and native vinegar on blood glucose and acetate responses to a mixed meal in healthy subjects. Eur J Clin Nutr 49: 242–247, 1995. [PubMed] [Google Scholar]
- 13.Charney AN, Micic L, Egnor RW. Nonionic diffusion of short-chain fatty acids across rat colon. Am J Physiol Gastrointest Liver Physiol 274: G518–G524, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah JF, Markowitz SD, Romero MF, Lapointe JY. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol 557: 719–731, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57: 2280–2287, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards CA, Parrett AM, Balmer SE, Wharton BA. Faecal short chain fatty acids in breast-fed and formula-fed babies. Acta Paediatr 83: 459–462, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Feinle-Bisset C, Vozzo R, Horowitz M, Talley NJ. Diet, food intake, and disturbed physiology in the pathogenesis of symptoms in functional dyspepsia. Am J Gastroenterol 99: 170–181, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, Prasad PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 10: 193–199, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol 289: C846–C852, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Hoverstad T, Bjorneklett A, Midtvedt T, Fausa O, Bohmer T. Short-chain fatty acids in the proximal gastrointestinal tract of healthy subjects. Scand J Gastroenterol 19: 1053–1058, 1984. [PubMed] [Google Scholar]
- 22.Hudson BD, Tikhonova IG, Pandey SK, Ulven T, Milligan G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. J Biol Chem 287: 41195–41209, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Illman RJ, Topping DL, Trimble RP. Effects of food restriction and starvation-refeeding on volatile fatty acid concentrations in the rat. J Nutr 116: 1694–1700, 1986. [DOI] [PubMed] [Google Scholar]
- 24.Inoue T, Wang JH, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD, Akiba Y. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol 303: G810–G816, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwanaga T, Takebe K, Kato I, Karaki S, Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed Res 27: 243–254, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Kaji I, Akiba Y, Kaunitz JD, Karaki Si, Kuwahara A. Differential expression of short-chain fatty acid receptor FFA2 and FFA3 in foregut (Abstract). Gastroenterology 142: S494, 2012. [Google Scholar]
- 27.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res 324: 353–360, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kaunitz JD, Akiba Y. Acid-sensing protective mechanisms of duodenum. J Physiol Pharmacol 54: 19–26, 2003. [PubMed] [Google Scholar]
- 29.Kawamata K, Hayashi H, Suzuki Y. Propionate absorption associated with bicarbonate secretion in vitro in the mouse cecum. Pflügers Arch 454: 253–262, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Lamers JM. Some characteristics of monocarboxylic acid transfer across the cell membrane of epithelial cells from rat small intestine. Biochim Biophys Acta 413: 465–476, 1975. [PubMed] [Google Scholar]
- 31.Mascolo N, Rajendran VM, Binder HJ. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology 101: 331–338, 1991. [DOI] [PubMed] [Google Scholar]
- 32.McGowan PE, Reglinski J, Wilson R, Walker JJ, Wisdoms S, McKillop JH. Quantitative 1H-NMR analysis of amniotic fluid. J Pharm Biomed Anal 11: 629–632, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil 17: 585–594, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J Biol Chem 279: 13293–13296, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse γ-hydroxybutyric acid. AAPS J 10: 311–321, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Seier-Poulsen S, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154: 3552–3564, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Ostman E, Granfeldt Y, Persson L, Bjorck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr 59: 983–988, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52: 289–298, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powley TL, Spaulding RA, Haglof SA. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. J Comp Neurol 519: 644–660, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol 284: G367–G372, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Raybould HE, Holzer H. Dual capsaicin-sensitive afferent pathways mediate inhibition of gastric emptying in rat induced by intestinal carbohydrate. Neurosci Lett 141: 236–238, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology 78: 1500–1507, 1980. [PubMed] [Google Scholar]
- 43.Schmitt MG Jr, Soergel KH, Wood CM. Absorption of short chain fatty acids from the human jejunum. Gastroenterology 70: 211–215, 1976. [PubMed] [Google Scholar]
- 44.Schmitt MG Jr, Soergel KH, Wood CM, Steff JJ. Absorption of short-chain fatty acids from the human ileum. Am J Dig Dis 22: 340–347, 1977. [DOI] [PubMed] [Google Scholar]
- 45.Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp Biochem Physiol A Mol Integr Physiol 125: 525–531, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Skutches CL, Holroyde CP, Myers RN, Paul P, Reichard GA. Plasma acetate turnover and oxidation. J Clin Invest 64: 708–713, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takebe K, Nio J, Morimatsu M, Karaki S, Kuwahara A, Kato I, Iwanaga T. Histochemical demonstration of a Na+-coupled transporter for short-chain fatty acids (slc5a8) in the intestine and kidney of the mouse. Biomed Res 26: 213–221, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi K, Matsumoto J, Ueshima K, Okabe S. Role of capsaicin-sensitive afferent neurons in alkaline secretory response to luminal acid in the rat duodenum. Gastroenterology 101: 954–961, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Teramae H, Yoshikawa T, Inoue R, Ushida K, Takebe K, Nio-Kobayashi J, Iwanaga T. The cellular expression of SMCT2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomed Res 31: 239–249, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol 209: 309–335, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Trinidad TP, Wolever TM, Thompson LU. Effect of acetate and propionate on calcium absorption from the rectum and distal colon of humans. Am J Clin Nutr 63: 574–578, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Umesaki Y, Yajima T, Tohyama K, Mutai M. Characterization of acetate uptake by the colonic epithelial cells of the rat. Pflügers Arch 388: 205–209, 1980. [DOI] [PubMed] [Google Scholar]
- 53.Umesaki Y, Yajima T, Yokokura T, Mutai M. Effect of organic acid absorption on bicarbonate transport in rat colon. Pflügers Arch 379: 43–47, 1979. [DOI] [PubMed] [Google Scholar]
- 54.Vanner S, MacNaughton WK. Capsaicin-sensitive afferent nerves activate submucosal secretomotor neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 269: G203–G209, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Wall MJ, Declusin RJ, Soergel KH, Baker RD. The effect of short chain fatty acids on transmural electrical potential across rat small intestine in vivo. Biochim Biophys Acta 433: 654–661, 1976. [DOI] [PubMed] [Google Scholar]
- 56.Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther 339: 464–473, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci 10: 478–487, 1998. [DOI] [PubMed] [Google Scholar]
- 58.Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. J Physiol 368: 667–678, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yajima T, Inoue R, Matsumoto M, Yajima M. Non-neuronal release of ACh plays a key role in secretory response to luminal propionate in rat colon. J Physiol 589: 953–962, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yajima T, Kojima K, Tohyama K, Mutai M. Effect of short - chain fatty acids on electrical activity of the small intestinal mucosa of rat. Life Sci 28: 983–989, 1981. [DOI] [PubMed] [Google Scholar]