Abstract
ZIP14 is a zinc transport protein with high expression in the small intestine and liver. Zip14 is upregulated during endotoxemia and leads to increased liver zinc content and transient hypozinemia. Since body zinc status and inflammation are associated with changes in intestinal permeability, we hypothesized that ZIP14 may influence intestinal permeability. Wild-type (WT) and Zip14 knockout (KO) mice were used to determine ZIP14-associated intestinal zinc metabolism and effects on permeability. Fractionation of plasma membranes revealed that ZIP14 is localized to the basolateral membrane of enterocytes. Studies utilizing 65Zn administered by subcutaneous injection revealed greater zinc accumulation in the SI of Zip14 KO mice compared with WT mice. Isolation of endosomes confirmed the presence of ZIP14. Quantification of endosomal zinc concentration by FluoZin-3AM fluorescence demonstrated that zinc is trapped in endosomes of Zip14 KO mice. Intestinal permeability assessed both by plasma FITC-dextran following gavage and by serum endotoxin content was greater in Zip14 KO mice. Threonine phosphorylation of the tight junction protein occludin, which is necessary for tight junction assembly, was reduced in KO mice. Claudin 1 and 2, known to have an inverse relationship in regards to tight junction integrity, reflected impaired barrier function in KO jejunum. These data suggest involvement of ZIP14 in providing zinc for a regulatory role needed for maintenance of the intestinal barrier. In conclusion, ZIP14 is a basolaterally localized protein in enterocytes and is involved in endosomal trafficking of zinc and is necessary for proper maintenance of intestinal tight junctions.
Keywords: endotoxemia, zinc transporter, endosomes, intestinal permeability, mucosal defense
the gastrointestinal tract is responsible for both homeostatic control of zinc metabolism and zinc-dependent host barrier functions. Consequently, intestinal dysfunction as produced by inflammation influences zinc biology. Two families of metal ion transporters, comprised of a total of 24 individual members, the ZnT (SLC30A) and ZIP (SLC39A) proteins, are the major components of metabolic and functional pathways for zinc (16, 17). These metabolic pathways act as homeostatic determinants of zinc absorption from the gastrointestinal tract and potentiate the release of endogenous zinc, particularly pancreatic secretions (16). Both Zip and Znt transporters are regulated at the transcriptional and posttranslational level. Whereas some of the ZnT and Zip genes are regulated by dietary zinc intake, others respond to hormonal or cytokine signals.
We have investigated the responses of the Zip and ZnT genes to proinflammatory conditions. Focusing initially on the liver in response to a sterile abscess (turpentine administration) and lipopolysaccharide (LPS), we found that Znt5, Zip1, Zip6, Zip7, and Zip14 were upregulated by one or both treatments (21). Conversely, Zip2 and Zip8 were downregulated. Furthermore, the regulation of Zip14 in response to IL-6, IL-1β, and nitric oxide was shown to coincide with zinc accumulation by hepatocytes (18, 21). It was clearly established that ZIP14 expression had a positive influence on regeneration of the liver and that the transported zinc was responsible for the inhibition of protein tyrosine phosphatase 1B, which maintained c-Met phosphorylation and hepatocyte proliferation (2). Those findings support a signaling role for zinc as mediated by ZIP14.
At the transcript level, the murine duodenum and jejunum express more Zip14 mRNA than the liver (19). This difference in RNA abundance and regulation by proinflammatory stimuli suggests ZIP14 could have a role in maintaining barrier function for the gastrointestinal tract. Zinc-mediated alteration in intestinal permeability has been noted in several disease models, including diarrheal and Crohn's disease (29, 32). These diseases and overall health are known to be zinc responsive (7). The significance of these properties at the intestinal level includes the observation that the “Western diet” is proinflammatory (25) and that intestinal barrier dysfunction is zinc responsive and inflammation-related (32, 37, 39).
Reported here are experiments that describe the role of ZIP14 (Slc39a14) in the intestine. By using a Zip14 knockout (KO) mouse model, it was shown that ZIP14 is needed for systemic zinc uptake at the basolateral membrane of enterocytes. ZIP14 maintains the intestinal barrier via stabilization of occludin phosphorylation, a tight junction (TJ) protein. These findings provide insights into how systemic zinc maintains barrier function to limit the influences of endotoxin produced by enteric microbiota.
MATERIALS AND METHODS
Animal and treatments.
Development of the murine Zip14+/− genotype was described earlier (2, 3). A colony of Zip14+/− mice on the C57BL/6;129S5 background was maintained at the University of Florida to generate Zip1−/− KO and Zip14+/+ (wild type; WT) mice. Male mice were used as young adults (8–16 wk). All mice had free access to a standard commercial rodent diet (Harlan-Teklad 7912, Indianapolis, IN; containing 60 mg Zn/kg as ZnO) and tap water. A 12-h light-dark cycle was used. Mice were administered isoflurane (Baxter, Deerfield, IL) for anesthesia. Euthanasia was by cardiac puncture of anesthetized mice. Protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
LPS-induced endotoxemia.
Mice were given an injection of phosphate-buffered saline (PBS) alone or containing LPS (Escherichia coli serotype 055:B5: Sigma-Aldrich, St. Louis, MO) at 2 mg/kg body wt ip. The mice were killed 3, 6, or 18 h after the injection.
Cell culture.
Caco-2 cells, a human colorectal adenocarcinoma cell line, were purchased from American Type Culture (ATCC, Manassas, VA). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 20% fetal bovine serum, 2 mM glutamine, and penicillin/streptomycin (Sigma-Aldrich). Media was changed every 2 days. Experiments were performed 21 days postconfluence to ensure cells were fully differentiated.
Intestinal permeability assay.
FITC-dextran 4000 (Sigma-Aldrich) at 40 mg/100 g body wt was administered by gavage (500 μl, to mice that were fasted overnight) 1 h before they were killed. The FITC-dextran content of the plasma (100 μl) was measured by spectrofluorometry at Ex/Em 488/520 nm. The FITC-dextran concentration was calculated from a standard curve. Alternatively, serum was prepared from mice that were not fasted. Endotoxin levels were measured by LAL chromogenic endotoxin qualitative assay (Thermo Scientific).
65Zn metabolism.
For transport and excretion of endogenous zinc, the mice were administered 65Zn (3 μCi; PerkinElmer, Waltham MA) by subcutaneous injection. Mice were killed after 3 h and the entire length of the small intestine and colon were excised and the 65Zn content was measured. Luminal contents were removed by perfusion with PBS/EDTA metal chelating buffer (10 mM EDTA, 10 mM HEPES, and 0.9% NaCl2) and an aliquot was measured for 65Zn. Values were normalized to the weight of the excised intestine. Gamma spectrometry was used to measure radioactivity, and values were expressed as percent of the total 65Zn administered.
RNA extraction and quantitative real-time PCR.
Intestinal mucosal scrapings were collected into RNAlater (Ambion, Carlsbad, CA), followed by homogenization in TRI Reagent (Ambion) for total RNA extraction. SYBR green Master Mix was used for quantitative PCR (qPCR) as described previously (2, 3). Amplification values were normalized to the expression of universal 18S or TATA binding protein mRNA.
Western analysis and polyclonal antibodies.
Flash-frozen mucosa was homogenized in 50 mM Tris buffer (pH 7.4) containing Triton X-100, 1 × HALT protease and phosphatase inhibitor cocktail (Thermo Scientific), and 1 mM PMSF. Cytoplasmic and membrane fractions were isolated by use of a subcellular protein fractionation kit (Thermo Scientific). Proteins were separated by 10% SDS-PAGE and then transferred to nitrocellulose membranes. Transfer was confirmed with Ponceau red staining. Western analysis used antibodies designed in-house that were prepared and used as previously described (20). Purchased antibodies were hZIP14 HPA016508, ACTB A2066 (Sigma-Aldrich), EEA1 3288P, phosphorylated threonine 8781S, RAB11 5589 (Cell Signaling, Danvers, MA), SGLT1 ab14686, CLDN1 ab15098, CLDN2 ab53032 (Abcam, Cambridge, MA), Na+-K+-ATPase sc-21712, OCLN sc-5562, and PKCζ sc-216 (Santa Cruz Biotechnology, Santa Cruz, CA).
Villus and crypt isolation.
Small intestines of WT mice were washed by perfusion with PBS and everted by use of a glass pipette. Two- to 3-mm segments of small intestine were placed in ice-cold Hanks' balanced salt solution (low Mg/Ca content). Villus/crypt fractions were isolated via repeated shaking and collection steps. Both alkaline phosphatase activity (measured via p-nitrophenyl phosphate, Sigma-Aldrich) and Western blotting confirmed villus/crypt isolation.
Endosome isolation.
Mucosa from the duodenum and jejunum were excised from WT and Zip14 KO mice and washed in ice-cold PBS. Crude endosomes were isolated from harvested mucosa by a previously described method (31). Crude endosomes were isolated from Caco-2 cells by the same method and were used to confirm results obtained with the mucosal preparation.
Endosomal intracellular labile zinc concentration.
Endosomes were sonicated in 200 μl PBS and 5 μM FluoZin-3AM (Invitrogen, Carlsbad, CA) was added. Samples were incubated at 37°C for 1 h and fluorescence was measured by spectrofluorometry at Ex/Em 495/516 nm.
Membrane isolation biotinylation assay.
Intestinal tissue excised from WT mice was washed with ice-cold PBS containing protease inhibitors, phosphatase inhibitors, and PMSF. The intestine was everted by use of a Pasteur pipette and incubated in PBS containing Sulfo-NHS-SS-Biotin (Thermo Scientific). Liberated cells were lysed with a nondenaturing lysis buffer. Streptavidin-conjugated Sepharose beads (Cell Signaling) were combined with the cell lysate from biotinylated intestinal segments and incubated overnight at 4°C with gentle agitation. Samples were then centrifuged at 14,000 g at 4°C and washed in nondenaturing lysis buffer. The pellet was suspended in SDS loading buffer and used for Western blotting (modified protocol from Ref. 24).
Immunofluorescence.
Tissue sections from the jejunum of WT and KO mice were flash frozen in optimal cutting temperature compound (VWR, Radnor, PA), embedded in paraffin, cut as 5-μm sections, and mounted. Sections were incubated with rabbit ZIP14 primary antibody, followed by addition of anti-rabbit IgG-Alexa 594 conjugate (Molecular Probes, Eugene, OR). Negative controls consisted of incubating the primary antibody with antigenic peptide before exposure to tissue (3). Nuclei counterstaining was performed with 4,6-diamidino-phenylindole (DAPI) (Invitrogen). Fluorescence was visualized by confocal microscopy.
Immunohistochemistry.
Sections of jejunum from WT and KO mice were fixed in 10% neural buffered formalin. The 5-μm sections were incubated with rabbit CLDN1 and CLDN2 followed by incubation with DAB chromagen (Vector, Burlingame, CA) for visualization by light microscopy.
Analytical Procedures.
Proximal sections of jejunum were removed and washed with PBS. Representative samples were used to measure zinc by atomic adsorption spectrophotometry and non-heme iron with the ferrozine method as previously described (3).
Statistics.
All statistical analyses were done by use of SigmaStat (Systat Software, Chicago, IL). For experiments comparing genotype, Student's t-test was used. Significance was considered to be a value P < 0.05.
RESULTS
Zip14 is highly expressed in the proximal intestine and is localized to the basolateral membrane of murine enterocytes.
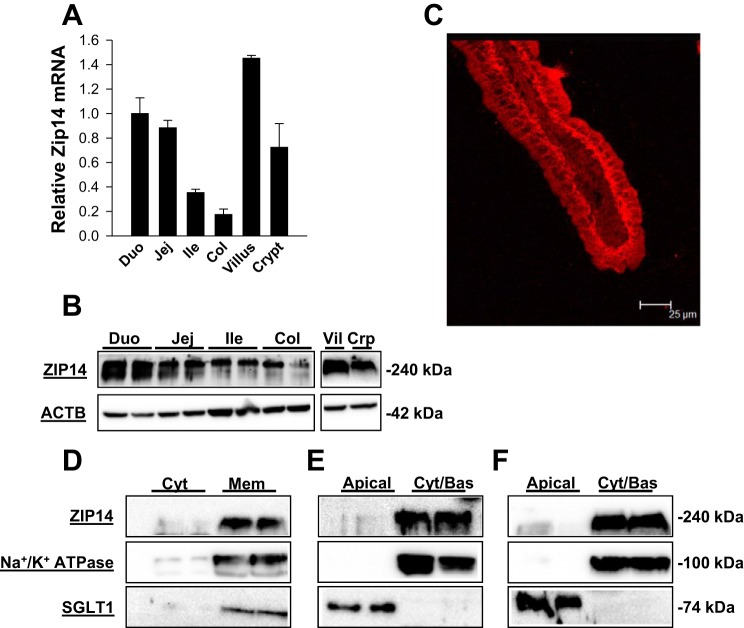
The steady-state abundance at both the transcript and protein level was highest in the duodenum and decreased along the gastrointestinal tract and displayed minimal expression in the colon (Fig. 1, A and B). The transcript and protein levels in the different regions were in very good agreement. Zip14 expression increased in abundance from the crypt to the villus. Enrichment of villus and crypt cells during fractionation was verified by use of alkaline phosphatase activity as the marker for enterocytes in the villar region (data not shown). Immunofluorescence of a duodenal villus showed a strong signal for ZIP14 along the basolateral membrane of intestinal epithelial cells (IEC) (Fig. 1C). Fractionation of cells into cytoplasmic and membrane compartments showed no presence of ZIP14 in the cytoplasm (Fig. 1D). Na+-K+-ATPase, a basolateral membrane marker, and SGLT1, an apical membrane marker, confirmed isolation of IEC membranes. Following biotinylation and separation of apical and basolateral fractions, the basolateral fraction revealed a strong enrichment of ZIP14 in both mouse small intestine (Fig. 1E) and Caco-2 cells (Fig. 1F). Clear alignment of ZIP14 with Na+-K+-ATPase confirmed a basolateral localization.
Fig. 1.
Zip14 is regionally expressed in mouse intestine and is localized to the basolateral membrane of enterocytes. The intestines of wild-type (WT) mice were separated into duodenum (Duo), jejunum (Jej), ileum (Ile), and colon (Col). In addition, villar (Vil) and crypt (Crp) epithelial cells from the entire intestine were separated by a mechanical method and collected by centrifugation. A: Zip14 mRNA was measured by quantitative PCR (qPCR) with values normalized to TATA binding protein (TBP). Data are expressed as means ± SD (n = 3 mice). B: cellular proteins were examined for ZIP14 expression by Western blot analysis with mZIP14 and β-actin (ACTB) as the loading control. C: immunofluorescence showing localization of ZIP13 (red) at the basolateral membrane of enterocytes of the jejunum of a WT mouse. The mZIP14 antibody as used in (B, D–F) was used to obtain this image. D: mucosa from jejunal tissue was collected from WT mice, and cytoplasmic (Cyt) and total membrane (Mem) fractions were isolated by use of NE-PER reagents. Proteins were analyzed by Western blot as above, plus Na+-K+-ATPase, and SGLT1 as positive controls for membrane localization. E: total small intestine from WT mice were subjected to apical surface biotinylation and purified by use of streptavidin-conjugated Sepharose beads. The pellet fraction (apical) and supernatant (basolateral/cytoplasmic; Cyt/Bas) were obtained and the proteins were analyzed as in D. SGLT1 served as the apical membrane marker and Na+-K+-ATPase served as a basolateral membrane marker. F: Caco-2 cells grown to 21-day postconfluence on Transwell plates were subjected to biotinylation in the upper well (apical compartment) and fractionated and analyzed as in E, except hZIP14 was used. Western blots are representative blots from multiple experiments.
Zip14 transports zinc into the intracellular compartment via endosomes.
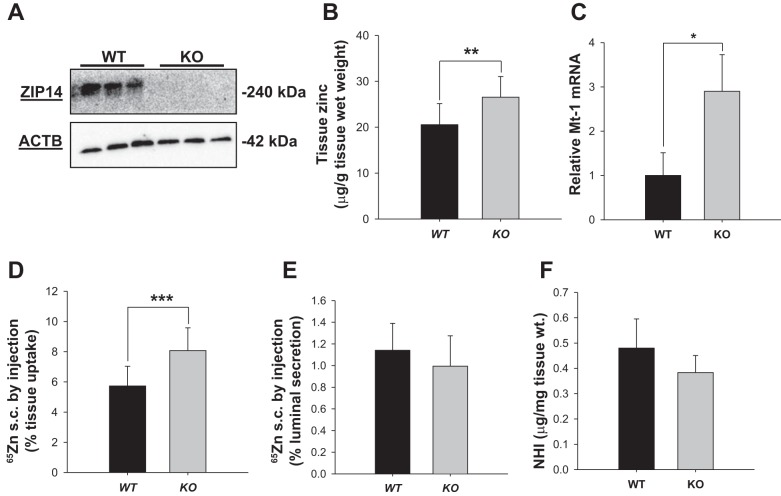
Western blot of proteins demonstrated the absence of ZIP14 in the jejunum of Zip14 KO mice (Fig. 2A). A qPCR screen of all known Zip and ZnT transporters in the jejunum showed that the only significant change between WT and KO mice was in Zip 2 and ZnT6 mRNAs. However, Western blots showed that neither gene produced a difference in abundance of these proteins between WT and KO mice (data not shown). A significant increase in total zinc content (P < 0.004) of the jejunum was present in the KO mice as shown by atomic absorption spectrophotometry (Fig. 2B). In support of the increase of cellular zinc is the increase in Mt-1 mRNA (P < 0.008), a zinc-responsive gene in the KO mice (Fig. 2C). To understand the transport process that could lead to intestinal zinc accumulation in the KO mice, 65Zn was administered by subcutaneous injection. A significant 40% increase (P < 0.001) of the 65Zn dose was retained in intestinal tissue of the KO mice (Fig. 2D); however, there was no change in the percent of the 65Zn dose secreted into the lumen in either genotype (Fig. 2E). Of note is that the nonheme iron content of the jejunum was not different between the two genotypes (Fig. 2F).
Fig. 2.
Zip14 knockout (KO) mice display increased intestinal zinc concentrations via accumulation of systemic zinc. A: intestinal mucosa was obtained from the jejunum of WT and Zip14 KO mice. Abundance of ZIP14 was analyzed by Western blot with mZIP14 with ACTB as the loading control. B: the zinc concentration of jejunal mucosa was measured by absorption spectrophotometry. C: abundance of Mt-1 mRNA in jejunal total RNA. D and E: WT and Zip14 KO mice were fasted overnight then each mouse received an subcutaneous injection of 3 μCi of 65Zn. The mice were killed 3 h later. The 65Zn content of intestinal tissue and amount of 65Zn secreted into the lumen were measured. 65Zn was measured and the percentage of jejunal 65Zn uptake was calculated as percentage of the total 65Zn in the dose administered. F: nonheme iron concentration of jejunal mucosa was determined colorimetrically. Data are expressed as means ± SD (n = 5) (*P < 0.05; **P < 0.01; ***P < 0.001).
To explain the accumulation of zinc in the jejunal tissue of the KO mice with no change in zinc secretion into the lumen, experiments were directed toward looking for trapped intracellular zinc. Crude endosomes were isolated from total protein lysate via a sucrose gradient protocol. ZIP14 was found associated with endosomes isolated from both murine jejunum and Caco-2 cells (Fig. 3, A and B). Endosomal isolation was confirmed with EEA1 and RAB11, markers of early and late endosomes, respectively. Further confirmation of the endosomal fraction was provided by GPR39, an apical membrane marker that does not undergo endocytic recycling (Fig. 3A) (11, 12). Endosomal membrane localization of ZIP14 was also demonstrated by using a comparable membrane fraction from Caco-2 cells (Fig. 3B). The labile zinc content of intestinal endosomes in KO mice was found to be fivefold greater than endosomes from WT mice upon incubation with the membrane-permeable zinc chelating fluorophore FluoZin-3 AM (Fig. 3C). Next we determined how changes in zinc trafficking would alter tissue function.
Fig. 3.
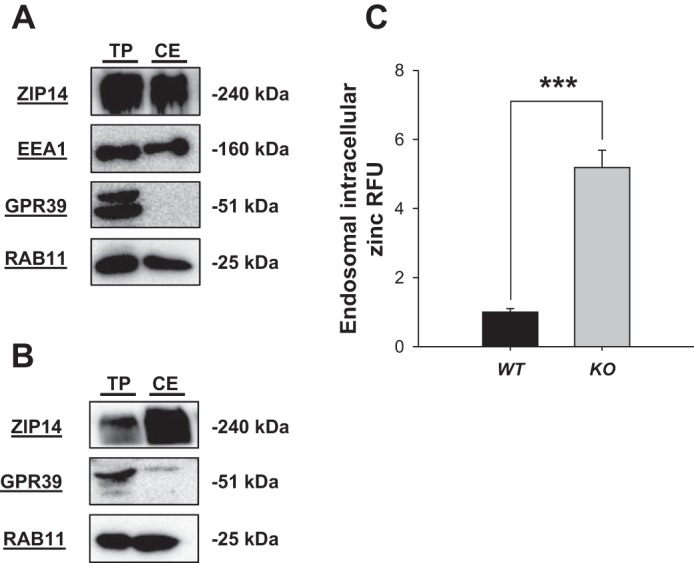
Intestinal cell endosomes accumulate zinc with deletion of Zip14. Crude endosomal (CE) fractions were isolated by sucrose gradient centrifugation. A: endosomes were isolated from jejunal mucosa of WT and KO mice. Proteins from CE and total jejunal proteins (TP) were analyzed by Western blot with mZIP14. RAB11 and EEA1 were used as endosomal markers and GPR39, a plasma membrane marker, served as a negative control. B: endosomes from Caco-2 cells were isolated and analyzed as in A. RFU, relative fluorescence units. C: endosomes from WT and KO mice, isolated as in A, were incubated with the intracellular zinc-fluorophore FluoZin-3AM to detect labile zinc. Fluorescence was measured after 1 h. Data are expressed as relative fluorescence and are expressed as means ± SD (n = 3 separate preparations) (***P < 0.001).
Intestinal permeability is altered with loss of Zip14.
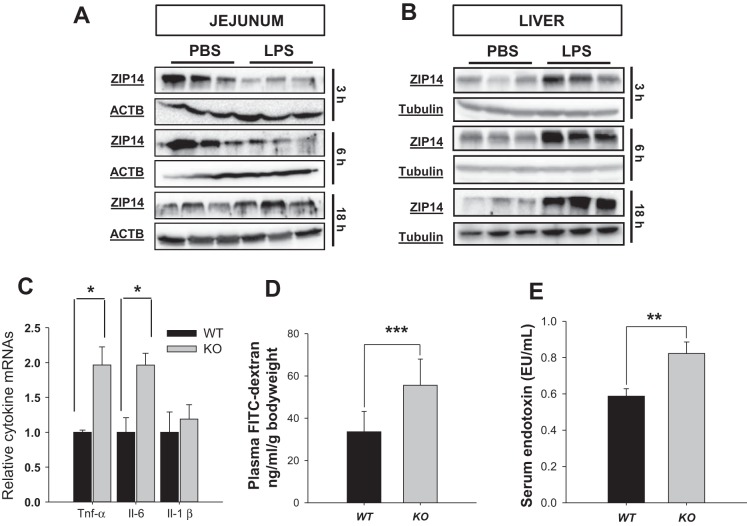
Previous reports from our laboratory have reported that ZIP14 acted as a proinflammatory mediator within the liver. Therefore we sought to determine the effects of LPS administration on ZIP14 within the intestine. The response of the jejunum to LPS was evaluated at three time points: 3, 6, and 18 h. Protein levels of ZIP14 decreased following LPS at the 3- and 6-h time points (Fig. 4A). Interestingly, this was opposite from the liver response, which had elevated ZIP14 expression at all measured time points (Fig. 4B) as reported previously (2, 3, 20). Nevertheless induced inflammation has an impact on ZIP14-related effects within the jejunum. Basal levels of cytokine mRNA expression were measured. KO jejunum was found to have significantly higher (P < 0.05) transcript levels of TNF-α and IL-6 (Fig. 4C). Inflammation has been reported to cause an increase in TJ permeability (23). Hence, we next sought to establish whether ZIP14 influences barrier permeability. Using FITC-dextran as a marker of intestinal permeability, we observed a significant increase (P < 0.001) in permeability in the KO mice (Fig. 4D). Increased levels of serum endotoxin in KO mice confirmed (P < 0.005) the effects of increased permeability (Fig. 4E).
Fig. 4.
Intestinal permeability increases with the loss of ZIP14 (KO). A and B: WT mice were injected with LPS (2 mg/kg ip) and were killed 3, 6, and 18 h later. Jejunal and liver tissues were obtained. Intestine and liver total lysates from WT mice were analyzed by Western blot with ACTB (jejunum) or tubulin (liver) as the loading control. C: relative cytokine expression in WT and KO jejunum. The qPCR data were normalized to TBP. Means are expressed as means ± SD (n = 6 mice). D: WT and KO mice were fasted overnight. The mice were administered FITC-dextran by gavage 1 h before being killed. Plasma was prepared and fluorescence was measured. Data were normalized to body weight and expressed as means ± SD (n = 10 mice). E: WT and KO mice were traditionally housed for 4 wk after weaning. Serum was prepared after the mice were killed and endotoxin levels were measured via a spectrophotometric assay. Data are expressed as means ± SD (n = 5 mice) (*P < 0.05; **P < 0.005; ***P < 0.001).
TJ proteins are differently expressed in intestines of WT and Zip14 KO mice.
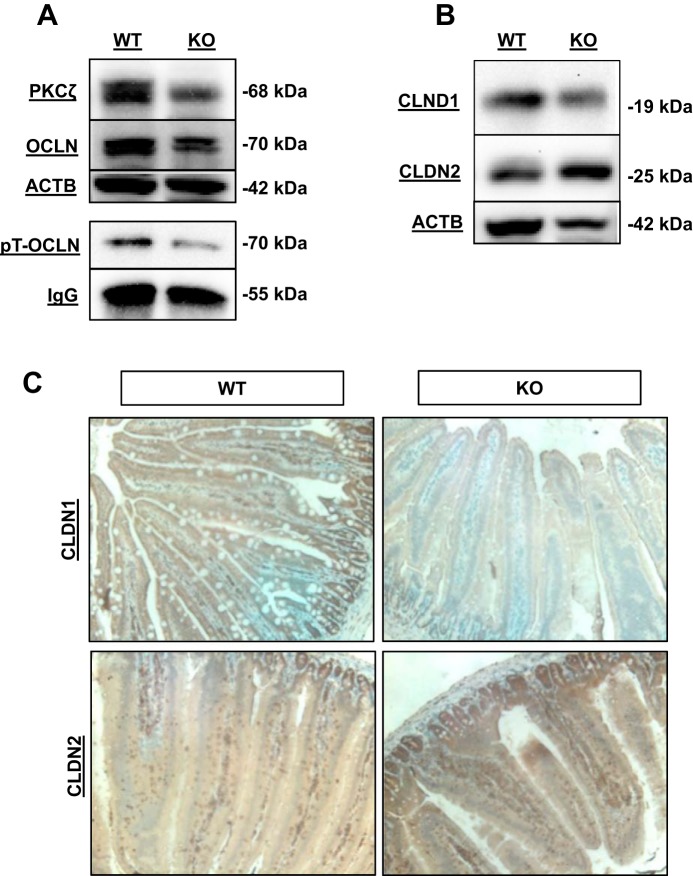
An initial screening of TJ mRNAs revealed no major difference between WT or KO genotypes (data not shown). However, protein abundance of PKCζ, a key regulator of TJ proteins, was reduced in KO jejunal tissue (Fig. 5A). Since the TJ protein OCLN is phosphorylated when it participates in barrier function, we investigated the potential that deletion of Zip14 influences OCLN phosphorylation. Immunoprecipitation with anti-phospho-threonine antibody was performed and phosphorylated OCLN was examined by Western blot. KO mice showed a marked reduction in the amount of threonine-phosphorylated OCLN (Fig. 5A). In addition, Western analysis showed a marked reduction in CLDN1 and increase in CLDN2 in the KO mice (Fig. 5B). Immunohistochemistry of CLDN1 and CLDN2 proteins is consistent with the observed change in expression detected by Western analysis (Fig. 5C).
Fig. 5.
ZIP14 deletion (KO) produces differential expression of specific tight junction proteins. Jejunal tissue from WT and Zip14 KO mice was used. A: Western blot analysis of PKCζ, OCLN, and proteins with ACTB as the loading control along with analysis of immunoprecipitated phospho-threonine OCLN (pT-OCLN) with IgG as the loading control (n = 3 mice per treatment). B: CLDN1 and CLDN2 Western blot with ACTB as the loading control (n = 3 mice per treatment). C: representative immunohistochemistry of intestinal CLDN1 and CLDN2 from WT and KO mice.
DISCUSSION
Dietary zinc uptake along the intestinal tract in animal models is highest in the proximal small intestine and lowest toward the colon (34). It has been well established that ZIP4 is a major apically localized transporter responsible for zinc uptake by enterocytes (5, 20). ZnT1 is a basolateral transporter in enterocytes (23). Together, these transporters account for a substantial amount of dietary zinc taken up by the enterocytes and transferred into the plasma pool. In our present study, we have shown that ZIP14 has a distribution pattern consistent with the intestinal zinc utilization, as well as functional roles in mucosal health.
To conclusively establish ZIP14 localization, we used a recently validated method of apical and basolateral fractionation (24) and confirmed those results by immunofluorescence. Prior to the identification of ZIP14 to the basolateral membrane in this report, the only ZIP transporter known to be at the basolateral membrane of enterocytes was ZIP5 (8, 35). From the limited data available, ZIP5 expression along the basolateral membrane increases from media containing high zinc concentrations in cell culture models, suggesting that ZIP5 may be responsible for increased accumulation of endogenous zinc in enterocytes during high-zinc conditions. Hence, ZIP5 may function to clear excess systemic zinc through the intestine and the intestinal lumen (8, 35). In contrast, ZIP14 may facilitate an intestinal function of zinc, since it already has a high level of transcript expression in the intestine and is unresponsive to dietary zinc conditions (9, 20, 21).
When Zip14 was deleted to examine basal zinc homeostasis, the zinc concentration of intestinal tissue of KO mice was higher. Altered expression of zinc transporters highly expressed in the intestine, particularly ZIP4, ZIP5, or ZnT1 (data not shown), were not influenced in this null genotype. Greater intestinal 65Zn accumulation following a subcutaneous injection in the KO mice with no change in 65Zn contents in the lumen suggests that there is dysregulation of intestinal zinc processing in the KO mice leading to retention of labile zinc, initially in endosomes. Utilizing a Zip14 overexpression system in HepG2 cells, Zhao et al. (38) observed a 50% colocalization of FLAG-tagged Zip14 with early endosome antigen and lysosome-associated membrane protein 1. However, that study did not examine whether the endosomes contained any zinc. Through the novel use of the zinc fluorophore FluoZin-3AM, we identified that there is an endosomal fraction in the intestine that contains labile zinc and the labile zinc pool increases with Zip14 deletion. Use of this technique is limited in that truly quantitative measures of this cellular pool of total labile zinc cannot be made, since the total cellular endosome content cannot be quantitatively measured. Also, it is possible that trapped endosomal zinc is just one mechanism by which excess zinc is accumulated in the enterocyte. Elevated Mt-1 mRNA as observed in the KO mice is likely the result of increased free zinc in the enterocyte, which activates the Mt promoter via binding of the MTF1 transcription factor. Since intestinal non-heme iron concentrations are not different between the WT and KO mice, these endosomes are not likely to contain different amounts of iron. The presence of ZIP14 in an endosomal fraction suggests that release of endosomal zinc could affect regulatory mechanisms of signaling pathways associated with the immune response (10, 28). Of particular relevance is that ZIP14, in multiple tissues, is tightly coupled with the inflammatory response caused by endotoxins. We established Zip14 as an LPS-responsive gene in the liver, muscle, pancreas, and white adipose tissue where increased expression leads to elevated tissue zinc and hypozincemia (3, 18, 21). Therefore, the lack of increased intestinal Zip14 mRNA and ZIP14 protein abundance following acute LPS administration was surprising. A unique characteristic of the intestinal epithelium, which contrasts with those other tissues, is that it is constantly exposed to resident microbiota. Toll-like receptors TLR-2 and TLR-4 are active in the intestine in the presence of commensal bacteria as well as pathogenic bacteria (26). The major ligand for TLR-4, LPS (22) produces a downregulation of the Tlr-4 gene in IEC (33). Therefore with the constant exposure to this environment, mechanisms involved in intestinal Zip14 may be fully activated and do not respond to acute LPS administration.
Because ZIP14 expression is high in the intestine relative to other tissues, we examined the role it may play in supplying zinc for maintenance of barrier function. Two in vivo models of intestinal permeability used in this study established that the KO mice have a twofold greater permeability. It is important to note that these changes in permeability are indicators of both small molecule permeability, FITC-dextran (4 kDa), and large molecule permeability, LPS (<10 kDa). An increase in both markers indicates a global defect in permeability produced by a lack of ZIP14, but produced by more than one mechanism. There have been a number of proposed mediators of permeability in the literature, including TJ regulation, produced via inflammation (15, 27). We focused on the role of zinc on TJ protein expression and assembly. Zinc has been shown to directly alter permeability owing to modifications to TJ proteins (37, 39), and they were our initial candidates for investigation.
A key regulator of TJ protein assembly is the PKC family (6). PKCζ is capable of phosphorylating multiple threonine residues of the integral TJ protein OCLN (14). Threonine phosphorylation is necessary for the appropriate assembly of the TJ complex. Immunoprecipitation of phosphorylated threonine and Western blot with OCLN antibody showed that KO mice had decreased OCLN abundance, which was correlated with a reduced abundance of phosphorylated OCLN. This observation suggests that there are transcriptional and posttranslational regulatory mechanisms for TJ proteins controlled by ZIP14. It is possible that loss of ZIP14 can play a direct role on OCLN phosphorylation. This could occur through altered PRKC activity or through inhibition by PP2A, as OCLN threonine phosphorylation can be directly inhibited by the activity of PP2A (30). Zinc is capable of inactivating PP2A through a src-dependent pathway (36). Loss of ZIP could lead to constitutively elevated PP2A activity, OCLN dephosphorylation and impaired assembly of OCLN at the TJ. It needs to be clarified in further research whether regulation of the PP2A activity in this phosphatase via ZIP14 activity is involved in the regulation of both TJ proteins.
CLDN1 is a positive regulator of paracellular permeability by which increased protein abundance along the TJ decreases permeability (13). By contrast, CLDN2 is a negative regulator of TJ permeability and an increase is associated with greater permeability (1). Within this study, we have found that both of these integral TJ proteins are altered by ZIP14 expression in a manner that is consistent with increased permeability. The increased flux of FITC-dextran could be accounted for by the alteration in these two TJ proteins, since claudins are known to mediate small-pore intestinal permeability (4). These data provide a basis for further experiments.
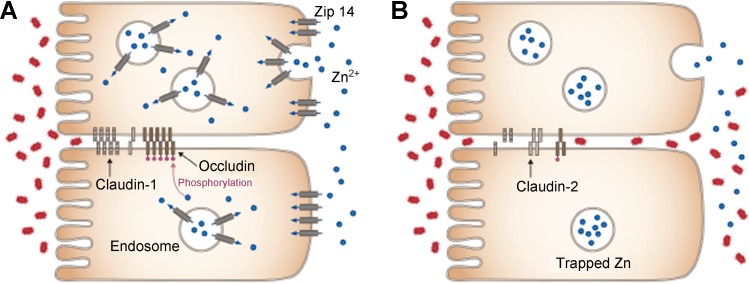
In summary, data from the experiments reported here have established that ZIP14 is most abundantly expressed in the villar enterocytes of the proximal small intestine. ZIP14 is localized to the basolateral membrane of the enterocyte (Fig. 6A). Zip14 KO mice have greater intestinal zinc content through enhanced accumulation in endosomes of enterocytes (Fig. 6B). The KO mice present with a phenotype that includes reduced intestinal barrier function. The KO mice exhibited an increase in CLDN2 and a decrease in CLDN1, along with impaired phosphorylation of OCLN (Fig. 6B). These findings suggest that zinc trapped in endosomes of mice lacking ZIP14 leads to decreased OCLN phosphorylation, which results in decreased barrier function and increased paracellular movement of endotoxins and enteric microflora into the systemic circulation. Taken together, these results suggest that decreased availability of systemic zinc to enterocytes, as would occur in dietary zinc deficiency, could relate to the diarrheal disease that has been shown to be responsive to zinc therapy (7, 29) and provide a target (i.e., ZIP14 expression) for intestinal diseases that influence permeability (32).
Fig. 6.
Proposed model for the ZIP14-mediated influence on TJ function and intestinal permeability. ZIP14 is localized to the basolateral membrane and transports endogenous zinc (blue) into enterocytes. A: in WT mice after endocytosis, ZIP14 functions to release vesicular zinc into the cytoplasm where it acts to maintain OCLN phosphorylation. B: in ZIP14 KO mice, zinc is accumulated in vesicles, and there is loss of a gain in CLDN2 and OCLN phosphorylation at the TJ. Changes in these proteins could contribute to loss of TJ barrier function and lead to increased paracellular movement of enteric endotoxins (red) into systemic circulation. Blue represents Zn2+ and red represents enteric endotoxin.
GRANTS
This research was supported by National Institute of Diabetes, Digestive and Kidney Diseases Grant RO1 DK94244 and from Boston Family Endowment Funds of the University of Florida to R. J. Cousins and a Davis Graduate Nutrition Enhancement Award to G. J. Guthrie.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.J.G., T.B.A., and R.J.C. conception and design of research; G.J.G., T.B.A., C.T., A.B.M., S.-M.C., and R.J.C. performed experiments; G.J.G. and R.J.C. analyzed data; G.J.G. and R.J.C. interpreted results of experiments; G.J.G., C.T., and R.J.C. prepared figures; G.J.G., C.T., and R.J.C. drafted manuscript; G.J.G., T.B.A., C.T., A.B.M., S.-M.C., and R.J.C. edited and revised manuscript; G.J.G., T.B.A., C.T., S.-M.C., and R.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge helpful discussions with Drs. James F. Collins and Bobbi Langkamp-Henkin.
Present address of G. J. Guthrie: USDA/ARS Children's Nutrition Research Center, Baylor College of Medicine, Houston, TX.
REFERENCES
- 1.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Aydemir TB, Sitren HS, Cousins RJ. The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice. Gastroenterology 142: 1536–1546.e5, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beker Aydemir T, Chang SM, Guthrie GJ, Maki AB, Ryu MS, Karabiyik A, Cousins RJ. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia). PloS One 7: e48679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 283: C142–C147, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem 279: 49082–49090, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther 316: 1–7, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Fischer-Walker C, Lamberti L, Roth D, Black RE. Zinc and infectious diseases. In: Zinc in Human Health, edited by Rink L. Amsterdam: IOS, 2011, p. xiii. [Google Scholar]
- 8.Geiser J, De Lisle RC, Andrews GK. The zinc transporter Zip5 (Slc39a5) regulates intestinal zinc excretion and protects the pancreas against zinc toxicity. PloS One 8: e82149, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol 73: 1413–1423, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr 29: 133–152, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Holliday ND, Holst B, Rodionova EA, Schwartz TW, Cox HM. Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol Endocrinol 21: 3100–3112, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem 279: 53806–53817, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Inai T, Kobayashi J, Shibata Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol 78: 849–855, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Jain S, Suzuki T, Seth A, Samak G, Rao R. Protein kinase Czeta phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem J 437: 289–299, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John LJ, Fromm M, Schulzke JD. Epithelial barriers in intestinal inflammation. Antioxid Redox Signal 15: 1255–1270, 2011. [DOI] [PubMed] [Google Scholar]
- 16.King JC, Cousins R. Zinc. In: Modern Nutrition in Health and Disease, edited by Ziegler T, Ross AC, Caballero B, Tucker K, and Cousins R. Washington, DC: Lippincott Williams & Wilkins, 2012, p. 189–205. [Google Scholar]
- 17.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29: 153–176, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Lichten LA, Liuzzi JP, Cousins RJ. Interleukin-1β contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am J Physiol Gastrointest Liver Physiol 296: G860–G867, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103: 13612–13617, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA 101: 14355–14360, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 102: 6843–6848, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 42: 145–151, 2008. [DOI] [PubMed] [Google Scholar]
- 23.McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci USA 95: 4841–4846, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, Spears JW, Thiele DJ. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem 285: 32385–32392, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142: 1100–1101.e2, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Ranaldi G, Ferruzza S, Canali R, Leoni G, Zalewski PD, Sambuy Y, Perozzi G, Murgia C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFalpha. J Nutr Biochem 24: 967–976, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol 28: 1–4, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Roy SK, Behrens RH, Haider R, Akramuzzaman SM, Mahalanabis D, Wahed MA, Tomkins AM. Impact of zinc supplementation in intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J Pediatr Gastroenterol Nutr 15: 289–296, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem 282: 11487–11498, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Stasyk T, Schiefermeier N, Skvortsov S, Zwierzina H, Peranen J, Bonn GK, Huber LA. Identification of endosomal epidermal growth factor receptor signaling targets by functional organelle proteomics. Mol Cell Proteomics 6: 908–922, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Sturniolo GC, Di Leo V, Ferronato A, D'Odorico A, D'Inca R. Zinc supplementation tightens “leaky gut” in Crohn's disease. Inflamm Bowel Dis 7: 94–98, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Sugi Y, Hosono A, Kaminogawa S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol 183: 6522–6529, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Vancampen DR, Mitchell EA. Absorption of Cu-64, Zn-65, Mo-99, and Fe-59 from ligated segments of the rat gastrointestinal tract. J Nutr 86: 120–124, 1965. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Kim BE, Petris MJ, Eide DJ. The mammalian Zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J Biol Chem 279: 51433–51441, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Xiong Y, Jing XP, Zhou XW, Wang XL, Yang Y, Sun XY, Qiu M, Cao FY, Lu YM, Liu R, Wang JZ. Zinc induces protein phosphatase 2A inactivation and tau hyperphosphorylation through Src dependent PP2A (tyrosine 307) phosphorylation. Neurobiol Aging 34: 745–756, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr 102: 687–693, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J Biol Chem 285: 32141–32150, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol 298: G625–G633, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]