Abstract
The colon differs regionally in local luminal environment, excretory function, and gene expression. Polycistronic microRNA (miR)-143 and miR-145 are downregulated early in colon cancer. We asked if these microRNAs (miRNAs) might be differentially expressed in the proximal vs. the distal colon, contributing to regional differences in protein expression. Primary transcripts and mature miR-143 and miR-145 were quantified by real-time PCR, putative targets were measured by Western blotting, and DNA methylation was assessed by sequencing bisulfite-treated DNA in proximal and distal normal colonic mucosa as well as colon cancers. Putative targets of these miRNAs were assessed following transfection with miR-143 or miR-145. Mean expression of mature miR-143 and miR-145 was 2.0-fold (P < 0.001) and 1.8-fold (P = 0.03) higher, respectively, in proximal than distal colon. DNA methylation or primary transcript expression of these miRNAs did not differ by location. In agreement with increased expression of miR-143 and miR-145 in proximal colon, predicted targets of these miRNAs, apoptosis inhibitor 5 (API5), ERK5, K-RAS, and insulin receptor substrate 1 (IRS-1), which are cell cycle and survival regulators, were expressed at a lower level in proximal than distal colon. Transfection of HCA-7 colon cancer cells with miR-145 downregulated IRS-1, and transfection of HT-29 colon cancer cells with miR-143 decreased K-RAS and ERK5 expression. In conclusion, miR-143 and miR-145 and the predicted target proteins API5, ERK5, K-RAS, and IRS-1 display regional differences in expression in the colon. We speculate that differences in these tumor suppressors might contribute to regional differences in normal colonic gene expression and modulate site-specific differences in malignant predisposition.
Keywords: miR-143, miR-145, colon, regional variation, proximal colon, distal colon, colon cancer
the proximal and the distal colon differ in many respects, including embryological origin, local luminal environment, excretory function, and gene expression. While colonic regional differences in the microbiome, fecal stream composition, transcription factor expression, and DNA promoter methylation are believed to contribute to these differences, microRNAs (miRNAs), a more recently described class of gene regulators, are also likely involved in differential gene expression.
miRNAs are small noncoding RNAs that regulate gene expression by binding to the 3′-untranslated region of mRNA, causing translational inhibition or degradation of mRNA (5). On the basis of intracellular and extracellular cues, miRNAs modulate gene expression to control cellular proliferation, differentiation, and apoptosis (3). miRNAs regulate normal intestinal cell function, and many miRNAs are dysregulated in carcinogenesis, acting as oncogenes when upregulated or unmasking tumor suppressor functions when downregulated (3, 26, 30). As such, expression profiles of miRNAs have been shown to be altered in many tumors, including colon cancer (13, 54, 57).
Most prior studies examining miRNAs in colonic tissue have not distinguished regional variation in miRNA expression, despite known differences in biological function and embryological origins of proximal and distal colon (33, 45, 46, 54). Previous genome-wide gene expression analyses have reported differential gene expression by location in normal colons (17, 38). Furthermore, tumors arising in proximal and distal colon differ in natural history, cell biology, mutation spectrum, and gene expression (2, 8, 9, 39). Because miRNAs regulate an estimated 60% of genes and miRNA profiles in tumors have been shown to differ between proximal and distal colon tumors, we speculated that proximal and distal colon might also differ in miRNA expression (15, 49). Such differences could elucidate region-specific mechanisms of cancer development.
Genes encoding polycistronic miR-143 (miR-143) and miR-145 on chromosome 5 are highly expressed in the colon and downregulated early in colon cancer (32). These miRNAs appear to drive a differentiated cell phenotype, inhibiting growth in transfected colon cancer cells (60). It seems likely, therefore, that these miRNAs play important roles in normal colonic biology and that their loss contributes causally to neoplastic progression. In this study, we demonstrate that these miRNAs are differentially expressed in proximal and distal colon. Increases in cellular proliferation and decreases in apoptosis have been described in colon cancer development. Therefore, we investigated regulation of these miRNAs, as well as their putative targets, which might control cell turnover and, thereby, contribute to malignant progression with loss of these miRNAs.
METHODS
Patients.
The study was approved by the Institutional Review Board at the University of Chicago (IRB 10-209-A). Informed consent was obtained from healthy individuals prior to colonoscopy as part of standard clinical care or from individuals with a colon cancer prior to surgical resection. Healthy individuals were included if they were >21 yr of age and had no history of colorectal cancer (CRC), family history of CRC in a first-degree relative, or history of advanced colonic neoplasia [large (>1 cm) adenoma, multiple (>3) adenomas, or high-grade dysplasia]. All samples from healthy individuals were from endoscopically and histologically normal colonic mucosa. Standard-sized colonic biopsies were obtained from the ascending colon and from the sigmoid colon, 20 cm proximal to the anus. Malignant tissue from colon cancers and adjacent normal mucosal tissue were obtained after surgical resection. Tissues were flash-frozen in liquid nitrogen and stored at −80°C until extraction of RNA, DNA, or protein. Because of limitations in biopsies and differences in methods of extraction, some comparisons involving RNA, DNA, or protein were performed on different patients. For any given comparisons between proximal and distal colon, DNA, RNA, or proteins were analyzed from the same patient.
Quantitative real-time PCR.
RNA extraction from mucosal biopsies was performed using the miRCURY RNA isolation kit (Exiqon, Vedbaek, Denmark) as previously described (41). RNA was extracted from cell lysates using the miRNeasy Mini Kit (Qiagen, Hilden, Germany). For mature miRNA analysis, cDNA was prepared from 100 ng of total RNA using specific primers and the MultiScribe reverse transcriptase kit (Life Technologies, Grand Island, NY). For primary miRNA analysis, cDNA was prepared from 100 ng of total RNA using random hexamers and the MultiScribe reverse transcriptase kit. TaqMan quantitative real-time reverse transcription-PCR (qPCR) was performed with TaqMan Fast Universal Master Mix (Life Technologies). Primers and probe for miR-143 (002249), miR-145 (002278), primary miR-143 (03303166_pri), primary miR-145 (03303169_pri), RNU48 (001006), and β-actin (catalog no. 4333762F) were provided by Life Technologies. PCR thermal profile included 45 cycles with a preamplification of 95°C for 10 min; 95°C for 15 s followed by 60°C for 60 s, 72°C for 1 s, and a final hold of 40°C for 30 s in a light cycler (model 480, Roche, Indianapolis, IN). PCR data were analyzed using the comparative threshold (ΔΔCT) method (27), and significance was calculated using Student's t-test.
Cell culture.
HCT-116, HCA-7, and HT-29 colon cancer cells were cultured in McCoy's 5A modified medium. DLD-1 cells were cultured in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, and LoVo cells were cultured in Ham's F-12K medium. All experiments were conducted on preconfluent cells. HCA-7 cells were seeded on six-well plates and transfected on the following day with scrambled oligonucleotides (control) or 50 nM mimics of mature miR-145 (Life Technologies), and cells were harvested 48 h later [insulin receptor substrate 1 (IRS-1)]. HT-29 cells were seeded on six-well plates and either transfected for 2 consecutive days with control or 50 nM mimics of mature miR-143 (Life Technologies) and harvested 72 h later [apoptosis inhibitor 5 (API5) and ERK5] or transfected once with 50 nM negative control oligonucleotides or 50 nM mimics of mature miR-143 and harvested 72 h later (K-RAS).
DNA methyltransferase inhibitor studies.
LoVo, DLD-1, HT-29, and HCT-116 cells were seeded on six-well plates. After 24 h, the cells were treated with 0, 5, or 10 μM 5-aza-2′-deoxycytidine (5-AZA; Sigma-Aldrich, St. Louis, MO) for 24 h ×5. At 24 h after the last of a total of five treatments with 5-AZA, the cells were harvested, and miR-143 and miR-145 were measured by qPCR.
DNA sequencing of bisulfite-treated DNA.
DNA extraction was performed using the AllPrep DNA/RNA universal kit (Qiagen). Bisulfite treatment was carried out using the EpiTect bisulfite kit (Qiagen) according to the manufacturer's specifications. PCR was performed on bisulfite-treated DNA using primers designed with MethPrimer software. Primer sequences were as follows: TTGGGTGTTTAAATGGTAGGTTATAGPCR (forward) and TACACCTCAAACTAAAAAACAAAAC (reverse) for miR-143 and GTTATAGATGGGGTTGGATGTAGAA (forward) and CCTCAAAAACAATATTTCCAAAAAT (reverse) for miR-145. PCR was performed with 17.5 ng of bisulfite-converted DNA using an AmpliTaq Gold DNA polymerase (Life Technologies). The PCR thermal profile included activation at 95°C for 10 min, amplification for 45 cycles at 95°C for 30 s, 51°C to 47°C with 0.25°C decrease/cycle touchdown for 30 s, followed by 72°C for 60 s, a final extension of 62°C for 7 min, and a final hold at 37°C for 10 min in a light cycler (model 480, Roche). PCR products were purified using the QIAquick PCR purification kit (Qiagen). PCR products were subcloned into pCR4-TOPO vector using the TOPO TA cloning kit (Life Technologies). Chemically competent TOP10 Escherichia coli cells were transformed with the pCR4-TOPO vector via heat shock protocol. The bacteria were grown in the presence of ampicillin to select for those with the subcloned plasmid. Sequencing of cloned PCR products was carried out with the Applied Biosystems 3730XL DNA analyzer in the University of Chicago DNA sequencing facility. Analysis and representation of CpG dinucleotide methylation was carried out using BISMA (http://services.ibc.uni-stuttgart.de/BDPC/BISMA) (44).
Western blotting.
Protein extractions and Western blotting were performed as previously described (41). Primary antibodies included API5 (1:500 dilution; Sigma-Aldrich), CDK6 (1:1,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), cyclin D2 (1:500 dilution; Santa Cruz Biotechnology), E-cadherin (1:2,500 dilution; BD Biosystems, San Jose, CA), ERK5 (1:500 dilution; Cell Signaling Technology, Danvers, MA) IRS-1 (1:1,000 dilution; Cell Signaling Technology), and K-RAS (1:250 dilution; Santa Cruz Biotechnology). Blots were reprobed for β-actin (Sigma-Aldrich) to confirm comparable protein loading. Specific proteins were detected by xerography on X-OMAT AR film using an enhanced chemiluminescence system. Xerograms were digitized with an Epson flat-bed scanner and quantified using ImageJ v1.48 (National Institutes of Health) for data acquisition and analysis. Mean expression levels were compared by Student's t-test.
In situ hybridization.
In situ hybridization for miR-145 was performed using a 5′-end digoxigenin-labeled locked nucleic acid-modified miRCURY miR-145 detection probe or a scrambled probe as irrelevant control (Exiqon) as previously described (41). Sections were imaged using an epifluorescence microscope equipped with a charge-coupled device camera, and intensity values in appropriate wavelengths were recorded after images were locked at a single exposure for comparison.
RESULTS
Mature miR-143 and miR-145 are differentially expressed in proximal and distal colon.
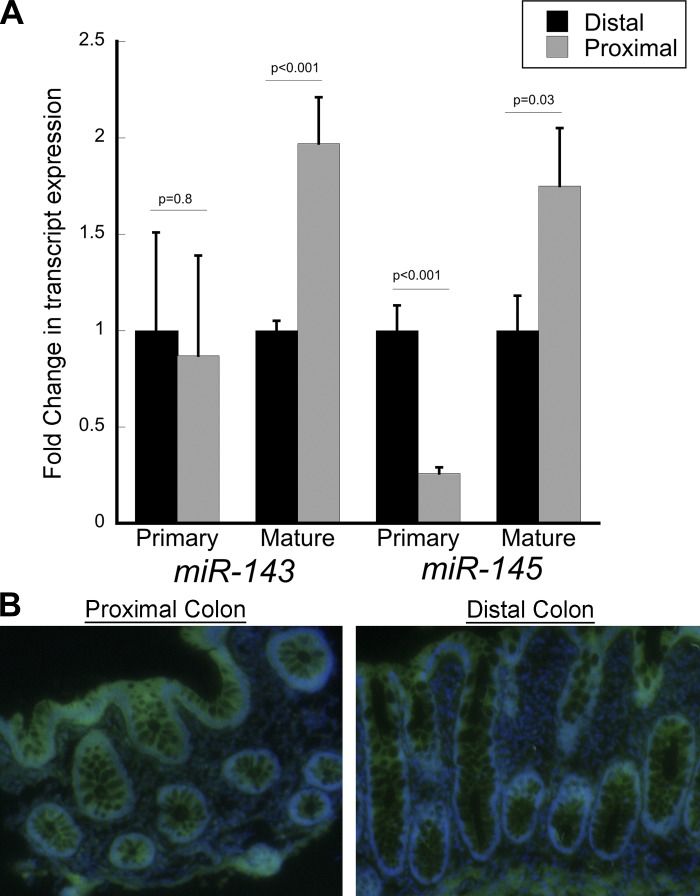
miR-143 and miR-145 expression levels were measured in proximal and distal colon in eight healthy individuals. The mean level of miR-143 was 2.0-fold higher in proximal than distal colon (P < 0.001), and miR-143 expression levels in all eight individuals were higher in proximal than distal colon. Similarly, the mean level of miR-145 expression was 1.8-fold higher in proximal than distal colon (P = 0.03; Fig. 1). In seven of eight individuals, miR-145 expression levels were higher in proximal than distal colon. In agreement with these real-time PCR findings, in situ hybridization of miR-145 was increased 1.8-fold in proximal compared with distal colonic mucosa (relative intensity: 30,000 for proximal colon and 17,000 for distal colon; Fig. 1).
Fig. 1.
Expression levels of mature and primary transcripts of microRNA (miR)-143 (miR-143) and miR-145 in distal and proximal colon. A: real-time PCR results showing mucosal transcript expression in proximal and distal colon. B: in situ hybridization for miR-145 with antisense-locked nucleic acid fluorescently labeled probes. 4′,6-Diamidino-2-phenylindole (blue) and fluorescein isothiocyanate (green) images were obtained separately from frozen biopsies and overlaid using the Olympus Cell P program. Relative microRNA (miRNA) intensities were 30,000 for proximal colon and 17,000 for distal colon. Each image is representative of 3 cases.
Impact of DNA methylation on miR-143 and miR-145 expression.
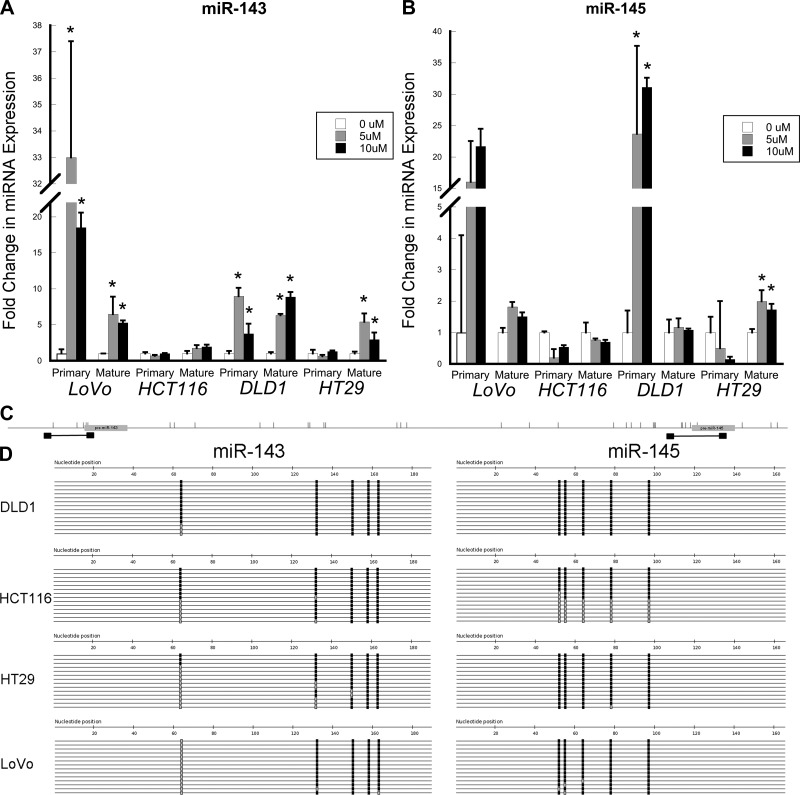
The DNA methyltransferase inhibitor 5-AZA results in net DNA demethylation. Expression levels of primary transcripts and mature miR-143 and miR-145 were measured in 5-AZA-treated LoVo, DLD-1, HCT-116, and HT-29 colon cancer cells. As shown in Fig. 2, for miR-143 there were significant 5-AZA-induced increases in LoVo and DLD-1 cells in primary miR-143 [33.0-fold (P = 0.02) and 18.5-fold (P = 0.004) with 5 and 10 μM 5-AZA, respectively, in LoVo cells and 8.9-fold (P = 0.002) and 3.7-fold (P = 0.04) with 5 and 10 μM 5-AZA, respectively, in DLD-1 cells] and mature miR-143 [6.5-fold (P = 0.04) and 5.3-fold (P < 0.001) with 5 and 10 μM 5-AZA, respectively, in LoVo cells and 6.3-fold (P < 0.001) and 9.0-fold (P < 0.001) with 5 and 10 μM 5-AZA, respectively, in DLD-1 cells]. There was no change in primary miR-143 following 5-AZA treatment in HT-29 cells, although there were significant increases in mature miR-143 [5.4-fold (P = 0.005) and 3.0-fold (P = 0.05) with 5 and 10 μM 5-AZA, respectively]. In contrast, there was no change in primary or mature miR-143 expression in HCT-116 cells (Fig. 2).
Fig. 2.
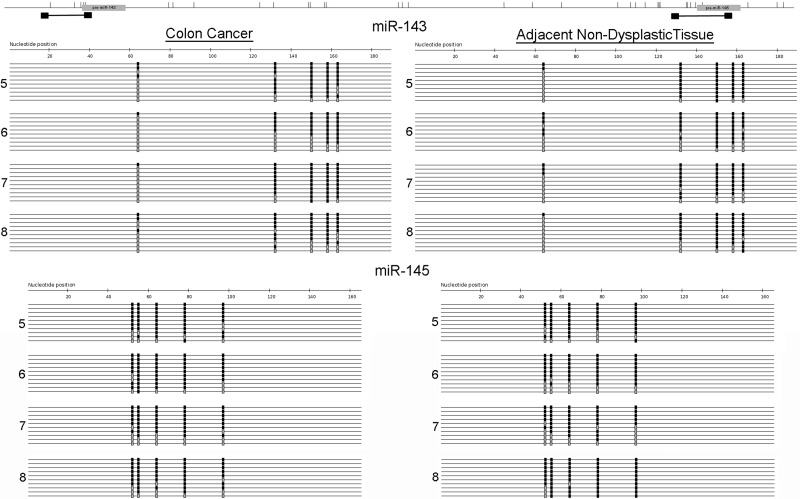
Primary and mature miR-143 and miR-145 expression and DNA methylation in colon cancer cells. A and B: real-time PCR measurement of expression of mature and primary miR-143 and miR-145 following 5 consecutive days of treatment with 0, 5, or 10 μM 5-aza-2′-deoxycytidine (5-AZA). C: primer positions (black boxes) relative to miRNAs (gray boxes) and CpG locations (vertical bars) for sequences of bisulfite-treated DNA around genes encoding miR-143 and miR-145. D: sequencing for the indicated loci in DLD-1, HCT-116, HT-29, and LoVo colon cancer cells after bisulfite conversion and PCR amplification. Each line represents a single clone. Black boxes, methylated CpG sites; gray boxes, unmethylated CpG sites.
Similarly, primary miR-145 increased in LoVo and DLD-1 cells [16.1-fold (P = 0.2) and 21.6-fold (P = 0.06) with 5 and 10 μM 5-AZA, respectively, in LoVo cells and 23.6-fold (P = 0.01) and 31.1-fold (P = 0.002) with 5 and 10 μM 5-AZA, respectively, in DLD-1 cells]. However, expression of mature miR-145 did not change in these cell lines. In HCT-116 cells, treatment with 10 μM 5-AZA downregulated primary miR-145 expression (0.5-fold, P = 0.01) but did not affect mature miR-145. In HT-29 cells, there was no change in primary miR-145, although we noted a significant increase in mature miR-145 following 5-AZA treatment [2.0-fold (P = 0.02) and 1.7-fold (P = 0.02) with 5 and 10 μM 5-AZA, respectively; Fig. 2].
To further examine the impact of DNA methylation on expression levels of these miRNAs in colon cancer cells, we used direct bisulfite sequencing from these cell lines to assess methylation of the genes coding for miR-143 and miR-145 (Fig. 2). In all four cell lines, the overall percentage of DNA methylation was high (63–100%) at the CpG sites. Hence, differences in primary miRNA transcript expression levels following 5-AZA treatment were not directly explained by methylation differences in CpG sites in genes encoding miR-143 and miR-145.
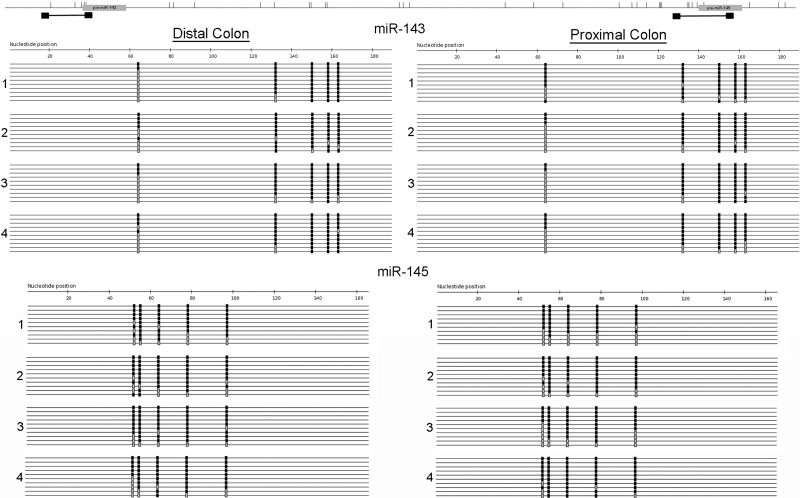
To investigate the impact of methylation on differences in miR-143 and miR-145 expression between normal proximal and distal colon, we performed sequencing of bisulfite-treated DNA on biopsies from proximal and distal colon in four subjects. As shown in Table 1, there were no differences in methylation between proximal and distal colon for miR-143 (78.7% vs. 81%, P = 0.39) or miR-145 (79.5% vs. 83.4%, P = 0.55). It is also important to note the high percentage of DNA methylation at the interrogated CpG sites in normal proximal and distal colon (Fig. 3).
Table 1.
Patient characteristics and percent methylation for each sample in methylation analysis of normal distal and proximal colon
| %Methylation |
|||||||
|---|---|---|---|---|---|---|---|
| miR-143 |
miR-145 |
||||||
| Subj No. | Age, yr | Sex | Race | Distal | Proximal | Distal | Proximal |
| 1 | 63 | M | Ca | 80 | 84 | 78.4 | 65.3 |
| 2 | 51 | M | AA | 81.3 | 73.3 | 86.3 | 95.8 |
| 3 | 65 | F | AA | 79 | 80 | 85 | 70.6 |
| 4 | 65 | F | AA | 83.7 | 80 | 84 | 86.3 |
| Mean | 81 | 78.7 | 83.4 | 79.5 | |||
| (P = 0.39) | (P = 0.55) | ||||||
Percent methylation is based on the number of CpG sites methylated of all CpG sites analyzed for all clones (range 10–23/sample). M, male; F, female; Ca, Caucasian; AA, African American.
Fig. 3.
Sequencing of bisulfite-treated DNA encoding miR-143 and miR-145 in proximal and distal colon. After bisulfite conversion and PCR amplification, DNA sequencing was performed in 4 subjects for the indicated loci in proximal and distal colon. Each line represents a single clone. Black boxes, methylated CpG sites; gray boxes, unmethylated CpG sites. Ten representative clones for each sample are included.
Expression of primary transcripts in proximal and distal colon.
As differences in expression of miR-143 and miR-145 could not be explained by differences in DNA methylation of genes encoding these miRNAs between proximal and distal colon, we sought to investigate if the regional differences in expression were more likely secondary to differences in miRNA transcription or processing. We measured primary transcripts of miR-143 and miR-145 by real-time PCR. As shown in Fig. 1, there were no differences in primary miR-143 expression between proximal and distal colon (P = 0.8). In contrast to mature miR-145 expression, which was higher in proximal colon, primary miR-145 was lower in proximal than distal colon (P < 0.001). These findings suggest that regional differences in mature miR-143 and miR-145 are likely secondary to regional differences in miRNA processing, rather than differences in transcription of these miRNAs.
Expression and DNA methylation of miR-143 and miR-145 in colon cancer.
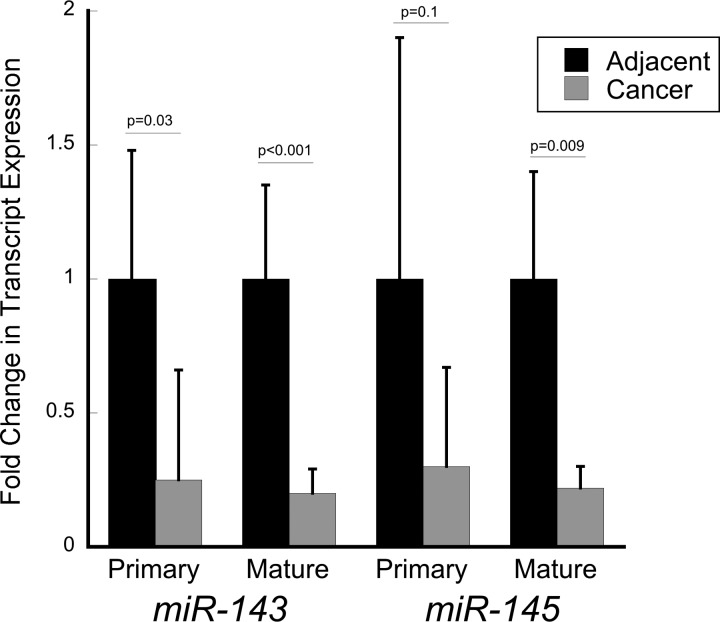
Because differences in mature miR-143 and miR-145 expression between normal proximal and distal colon are likely secondary to differences in processing of the primary transcript, we sought to determine whether expression differences between the mature miRNA and the primary transcript were similar in sporadic colon cancer. Primary miR-143 was downregulated 4.0-fold (P = 0.03) and primary miR-145 was downregulated 3.4-fold (P = 0.1) in colon cancer compared with adjacent tissue (n = 11). Similarly, mature miR-143 was downregulated 4.9-fold (P < 0.001) and miR-145 was downregulated 4.6-fold (P = 0.009) in colon cancer compared with adjacent tissue, suggesting that decreases in transcription probably contribute to downregulation of mature forms of these miRNAs in colon cancer (Fig. 4).
Fig. 4.
Expression levels of primary transcripts and mature miR-143 and miR-145 in colon cancers and adjacent normal-appearing colonic mucosa.
Bisulfite genomic sequencing was performed on four cancers and adjacent nondysplastic tissue. As shown in Table 2, no significant differences in methylation were observed in colon cancers compared with adjacent tissue for miR-143 (73.8% vs. 67.7%, P = 0.29) or miR-145 (81.2% vs. 79.7%, P = 0.79) (Fig. 5).
Table 2.
Patient characteristics and percent methylation for each sample in methylation analysis of colon cancer compared with adjacent tissue
| %Methylation |
||||||||
|---|---|---|---|---|---|---|---|---|
| miR-143 |
miR-145 |
|||||||
| Subj No. | Age, yr | Sex | Race | Location of Tumor | Cancer | Adjacent | Cancer | Adjacent |
| 5 | 64 | M | Ca | Left colon | 71.7 | 78.5 | 80.3 | 86 |
| 6 | 71 | F | AA | Left colon | 57.2 | 73.4 | 70.2 | 76.5 |
| 7 | 80 | M | U | Right colon | 76.3 | 69.8 | 83.3 | 69.5 |
| 8 | 74 | F | AA | Right colon | 65.5 | 73.3 | 84.8 | 92.7 |
| Mean | 67.7 | 73.8 | 79.7 | 81.2 | ||||
| (P = 0.29) | (P = 0.79) | |||||||
See Table 1 footnote for explanation of %methylation. U, unknown.
Fig. 5.
Sequencing of bisulfite-treated genes encoding miR-143 and miR-145 in primary colon cancers and adjacent nondysplastic tissue. After bisulfite conversion and PCR amplification, sequencing was performed for the indicated loci in proximal and distal colon in 4 subjects. Each line represents a single clone. Black boxes, methylated CpG sites; gray boxes, unmethylated CpG sites. Ten representative clones from each sample are included.
Expression of miR-143 and miR-145 target proteins in proximal and distal colon.
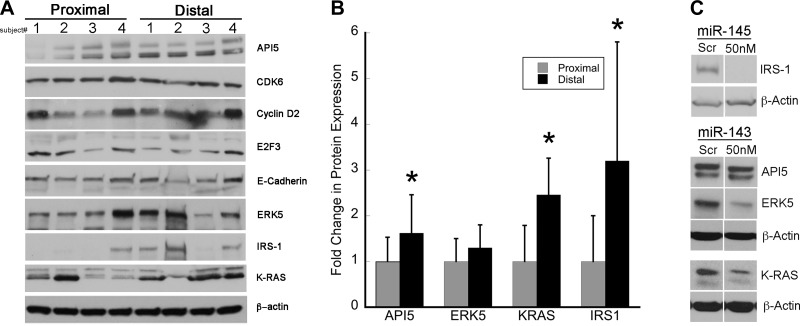
Since miR-143 and miR-145 are lost early in colon cancer development and premalignant lesions have increased proliferation and decreased apoptosis, we used TargetScan 6.2 (www.targetscan.org), microRNA.org (www.miRNA.org), and miRDB (www.mirdb.org) to examine putative targets of these miRNAs that might regulate cell cycle or survival. In a preliminary analysis in four patients, examination of putative target proteins identified four proteins (IRS-1, API5, ERK5, and K-RAS) with differential expression between proximal and distal colon. To confirm these results, expression of these four proteins was measured by Western blotting in proximal and distal colonic mucosal biopsies from an additional 8–11 patients. As shown in Fig. 6, these predicted targets demonstrate an inverse pattern of differential expression compared with miR-143 and miR-145, with higher expression levels in distal than proximal colon. Relative ratios of expression in distal colon to expression in proximal colon were 3.2 ± 2.6 for IRS-1 (P = 0.04), 2.5 ± 1.0 for K-RAS (P = 0.006), 1.6 ± 0.8 for API5 (0.05), and 1.3 ± 0.5 for ERK5 (P = 0.21). Comparison of expression of these proteins for each patient showed higher expression of IRS-1 in 6 of 8 patients, higher expression of K-RAS in 7 of 8 patients, higher expression of API5 in 9 of 11 patients, and higher expression of ERK5 in 8 of 11 patients in distal than proximal colon (Fig. 6).
Fig. 6.
Protein expression levels of targets of miR-143 and miR-145 in proximal and distal colon. A: representative Western blots of apoptosis inhibitor 5 (API5), cyclin-dependent kinase 6 (CDK6), cyclin D2, E2F3, E-cadherin, ERK5, insulin receptor substrate 1 (IRS-1), K-RAS, and β-actin in proximal and distal colon (n = 4). B: quantitative expression levels of API5, ERK5, K-RAS, and IRS-1 in proximal and distal colon for 8–11 subjects. C: Western blots of protein expression levels 48–72 h after transfection of 50 nM mimic of indicated mature miRNA or scrambled probe (Scr) into HCA-7 cells (IRS-1) or HT-29 cells (K-RAS, API5, and ERK5) (n = 3).
miR-143 and miR-145 transfection in HCA-7 and HT-29 colon cancer cells.
To further assess whether these regulators of cell cycle and survival are potential targets of miR-143 or miR-145, we transfected mimics of these miRNAs into HCA-7 cells (IRS-1) or HT-29 cells (K-RAS, API5, and ERK5) and examined their effects on these proteins. As shown in Fig. 6, transfected miR-145 or miR-143 downregulated IRS-1 (miR-145), K-RAS (miR-143), and ERK5 (miR-143), respectively. In contrast, no differences were seen in expression of API5 with transfection of miR-143 (Fig. 6).
DISCUSSION
While downregulation of miR-143 and miR-145 has been reported in colon cancer, this is the first report describing regional variation of these miRNAs in normal colon (1, 32, 51). Interestingly, ratios of abundance of predicted targets, API-5, ERK5, IRS-1, and K-RAS, in distal colon to that in proximal colon showed an inverse relationship to the concentrations of these miRNAs. Although in this report proliferation and antiapoptotic signals were increased in distal colon compared with proximal colon, previous studies have been inconsistent with respect to regional variation in proliferation rates in normal colon (4, 24, 25, 28, 40, 56). Since homeostatic regulation is complex, however, other mechanisms are undoubtedly also involved in controlling proliferation and apoptosis.
In agreement with other studies in which increased DNA methylation was shown to contribute to downregulation of miR-143 and miR-145 in other cell types, we used 5-AZA to demonstrate that DNA methylation likely contributes to downregulation of these miRNAs in colon cancer cells (14, 53). Our analysis, however, did not demonstrate significant differences in DNA methylation in genes coding for these miRNAs in colon cancers compared with adjacent tissue or in colon cancer cell lines in which expression in the primary transcript of miR-143 and miR-145 was increased following 5-AZA treatment compared with those in which expression was not changed. The finding that both miRNAs increased following 5-AZA treatment in colon cancer cells, despite the absence of differences detected in DNA methylation in colon cancers compared with adjacent tissues, suggests that DNA methylations controlling miRNA expression are occurring at loci remote from those we investigated or in a single cell type that could not be detected given the cellular heterogeneity in human colonic biopsies.
Previous analyses demonstrated differences in DNA methylation between proximal and distal colon (21, 22). Despite the finding that 5-AZA upregulated expression of miR-143 and miR-145 in some colon cancer cell lines, we did not identify changes in methylation of these miRNAs between proximal and distal colon. In fact, the discordance between mature miRNAs and primary transcript expression of these miRNAs in proximal and distal colon suggests that regional differences in expression of mature miR-143 and miR-145 are secondary to differences in miRNA processing. In contrast, the mature and primary transcripts were downregulated in sporadic colon cancer compared with adjacent nondysplastic tissue. These findings suggest that transcriptional dysregulation is contributing to the downregulation of these miRNAs in colon cancer. As mature miR-143 and miR-145 were more significantly downregulated than the primary transcripts in our analysis of colon cancers, however, we hypothesize that alterations in miRNA processing are also contributing to decreases in mature miRNAs in colon cancer.
Several potential targets of miR-143 and miR-145 have been suggested in silico and further supported in cell culture studies (18, 55, 58, 59). In this study, we examined API5, ERK5, IRS-1, and K-RAS, which are reported targets of miR-143 or miR-145 and causally implicated in colon cancer progression. API5 is an antiapoptotic protein that suppresses E2F-dependent apoptosis, an important regulator of tumor growth in colon cancer (11, 34). Upregulation of API5 has not been reported in colon cancer but appears to play an important tumor-promoting role in other cancers (23, 52). ERK5 is a member of the mitogen-activated protein kinase family, which is important in cellular proliferation, including mediation of epidermal growth factor-induced proliferation (20, 35). IRS-1 is involved in cellular proliferation and survival and is upregulated in multiple cancers, including colon cancer. IRS-1 is required for efficient neoplastic transformation induced by β-catenin (43). K-RAS is an upstream activator of ERKs, important transducers of proliferation (19, 47). In the present study, these proteins displayed regional variations consistent with differences in miR-143 and miR-145 expression. In agreement with other studies, we showed that transfection of miR-143 downregulated ERK5, as well as K-RAS, and transfection of miR-145 downregulated IRS-1 in CRC cells (12, 48). We did not observe differences in API5 expression after transfection of miR-143 in HT-29 cells. In a previous analysis, we did demonstrate such a downregulation of API5 with miR-143 transfection in HCT-116 cells, another colon cancer cell line. It is possible that this discrepancy could be related to cell-specific differences, such as alternative splicing, which might alter the 3′-untranslated region of API5.
There are several potential limitations of this study. 1) While we demonstrated significant differences between proximal and distal colon in a relatively small cohort of normal individuals, larger studies are needed to confirm these differences. 2) The regional variation in miRNAs and proteins identified in this study cannot be extrapolated to other miRNAs and proteins that may be regulated by different mechanisms. 3) Despite demonstrating an association between specific miRNAs and proteins in vivo and a potential causal effect in cell culture, we cannot conclude that there is a direct causal relationship in vivo, where miRNA-mRNA interactions are more complex. 4) The methylation analysis may have been limited by the number of patients studied, the heterogeneous cell type sampled in human colonic tissues, and known variation in total methylation among individuals. Although we controlled for interindividual variability in tissue methylation by comparing regional DNA methylation in the same subject, the degree of methylation may be related to other factors such as age and sex.
Distal colon tumors are more prevalent than proximal colon cancers (7). In contrast, proximal colon cancers present with larger tumor size, higher grade, and worse outcomes than distal colon tumors (7, 16, 31, 50). These findings likely are driven in part by biological differences between normal proximal and distal colon. Indeed, molecular markers of colon cancer are known to vary by colon location (2, 6, 9, 10, 29, 36, 37). These observations may result from different embryological origins of proximal vs. distal colon, as well as regional differences in the colonic microbiome and differences in constituents of the fecal stream, such as secondary bile acids and short-chain fatty acids, and potential carcinogen concentrations in the aqueous phase. Because the miRNAs and proteins examined in this study are potentially implicated in colonic carcinogenesis, their differential expression might contribute to differences in the pathobiology of proximal vs. distal colon cancers and have implications for CRC prevention or treatment.
In conclusion, we have demonstrated differential expression of miR-143 and miR-145 and API5, ERK5, IRS-1, and K-RAS, proteins that are putative targets of these mRNAs, in normal proximal and distal colon. These differences may contribute to regional differences in cell turnover in normal colon and site-specific predilections in colonic malignant transformation. Examination of these miRNAs, as well as their putative target transcripts and proteins, within tumors of proximal and distal colon is needed to further explore this hypothesis. Given the differential miRNA expression pattern we observed in proximal vs. distal colon, location should be taken into account in future studies that examine miRNAs in the colon.
GRANTS
This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grant K08 DK-09015 (J. Pekow), the Crohn's and Colitis Foundation of America (J. Pekow), and National Institutes of Health Digestive Diseases Research Core Center Grant P30 DK-42086 and Grant 1R01 CA-164124 (M. Bissonnette).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P., L.J.J., and M.B. developed the concept and designed the research; J.P., K.M., U.D., F.B., R.M., J.L., C.C., and X.C. performed the experiments; J.P., K.M., U.D., F.B., and M.B. analyzed the data; J.P., K.M., U.D., L.J.J., and M.B. interpreted the results of the experiments; J.P. prepared the figures; J.P. drafted the manuscript; J.P., K.M., L.J.J., and M.B. edited and revised the manuscript; J.P., K.M., U.D., F.B., R.M., J.L., C.C., X.C., L.J.J., and M.B. approved the final version of the manuscript.
REFERENCES
- 1.Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol Rep 16: 845–850, 2006. [PubMed] [Google Scholar]
- 2.Akkiprik M, Ataizi-Celikel C, Dusunceli F, Sonmez O, Gulluoglu BM, Sav A, Ozer A. Clinical significance of p53, K-ras and DCC gene alterations in the stage I–II colorectal cancers. J Gastrointest Liver Dis 16: 11–17, 2007. [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Anti M, Armuzzi A, Morini S, Iascone E, Pignataro G, Coco C, Lorenzetti R, Paolucci M, Covino M, Gasbarrini A, Vecchio F, Gasbarrini G. Severe imbalance of cell proliferation and apoptosis in the left colon and in the rectosigmoid tract in subjects with a history of large adenomas. Gut 48: 238–246, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281–297, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bendardaf R, Buhmeida A, Hilska M, Laato M, Syrjanen S, Syrjanen K, Collan Y, Pyrhonen S. VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival. Anticancer Res 28: 3865–3870, 2008. [PubMed] [Google Scholar]
- 7.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, Colon/Rectum Carcinomas Study Group. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 53: 57–64, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Bertucci F, Salas S, Eysteries S, Nasser V, Finetti P, Ginestier C, Charafe-Jauffret E, Loriod B, Bachelart L, Montfort J, Victorero G, Viret F, Ollendorff V, Fert V, Giovaninni M, Delpero JR, Nguyen C, Viens P, Monges G, Birnbaum D, Houlgatte R. Gene expression profiling of colon cancer by DNA microarrays and correlation with histoclinical parameters. Oncogene 23: 1377–1391, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, Laurberg S, Laiho P, Aaltonen LA, Orntoft TF. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut 54: 374–384, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleeker WA, Hayes VM, Karrenbeld A, Hofstra RM, Hermans J, Buys CC, Plukker JT. Impact of KRAS and TP53 mutations on survival in patients with left- and right-sided Dukes' C colon cancer. Am J Gastroenterol 95: 2953–2957, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Bramis J, Zacharatos P, Papaconstantinou I, Kotsinas A, Sigala F, Korkolis DP, Nikiteas N, Pazaiti A, Kittas C, Bastounis E, Gorgoulis VG. E2F-1 transcription factor immunoexpression is inversely associated with tumor growth in colon adenocarcinomas. Anticancer Res 24: 3041–3047, 2004. [PubMed] [Google Scholar]
- 12.Chen X, Guo X, Zhang H, Xiang Y, Chen J, Yin Y, Cai X, Wang K, Wang G, Ba Y, Zhu L, Wang J, Yang R, Zhang Y, Ren Z, Zen K, Zhang J, Zhang CY. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene 28: 1385–1392, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, Raymond CK, Roberts BS, Juhl H, Kinzler KW, Vogelstein B, Velculescu VE. The colorectal microRNAome. Proc Natl Acad Sci USA 103: 3687–3692, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou L, Zheng D, Li J, Li Y, Gao L, Wang L, Yu L. Methylation-mediated repression of microRNA-143 enhances MLL-AF4 oncogene expression. Oncogene 31: 507–517, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gervaz P, Cerottini JP, Bouzourene H, Hahnloser D, Doan CL, Benhattar J, Chaubert P, Secic M, Gillet M, Carethers JM. Comparison of microsatellite instability and chromosomal instability in predicting survival of patients with T3N0 colorectal cancer. Surgery 131: 190–197, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Glebov OK, Rodriguez LM, Nakahara K, Jenkins J, Cliatt J, Humbyrd CJ, DeNobile J, Soballe P, Simon R, Wright G, Lynch P, Patterson S, Lynch H, Gallinger S, Buchbinder A, Gordon G, Hawk E, Kirsch IR. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev 12: 755–762, 2003. [PubMed] [Google Scholar]
- 18.Gregersen LH, Jacobsen AB, Frankel LB, Wen J, Krogh A, Lund AH. MicroRNA-145 targets YES and STAT1 in colon cancer cells. PLos One 5: e8836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaiswal BS, Janakiraman V, Kljavin NM, Eastham-Anderson J, Cupp JE, Liang Y, Davis DP, Hoeflich KP, Seshagiri S. Combined targeting of BRAF and CRAF or BRAF and PI3K effector pathways is required for efficacy in NRAS mutant tumors. PLos One 4: e5717, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395: 713–716, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Kaz AM, Wong CJ, Dzieciatkowski S, Luo Y, Schoen RE, Grady WM. Patterns of DNA methylation in the normal colon vary by anatomical location, gender, and age. Epigenetics 9: 492–502, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koestler DC, Li J, Baron JA, Tsongalis GJ, Butterly LF, Goodrich M, Lesseur C, Karagas MR, Marsit CJ, Moore JH, Andrew AS, Srivastava A. Distinct patterns of DNA methylation in conventional adenomas involving the right and left colon. Mod Pathol 27: 145–155, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krejci P, Pejchalova K, Rosenbloom BE, Rosenfelt FP, Tran EL, Laurell H, Wilcox WR. The antiapoptotic protein Api5 and its partner, high molecular weight FGF2, are up-regulated in B cell chronic lymphoid leukemia. J Leukoc Biol 82: 1363–1364, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Kubben FJ, Peeters-Haesevoets A, Engels LG, Baeten CG, Schutte B, Arends JW, Stockbrugger RW, Blijham GH. Proliferating cell nuclear antigen (PCNA): a new marker to study human colonic cell proliferation. Gut 35: 530–535, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu LU, Holt PR, Krivosheyev V, Moss SF. Human right and left colon differ in epithelial cell apoptosis and in expression of Bak, a pro-apoptotic Bcl-2 homologue. Gut 45: 45–50, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Mao SY, Zhu WY. Impact of tiny miRNAs on cancers. World J Gastroenterol 13: 497–502, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Ma QY, Williamson KE, Rowlands BJ. Variability of cell proliferation in the proximal and distal colon of normal rats and rats with dimethylhydrazine induced carcinogenesis. World J Gastroenterol 8: 847–852, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marx A, Simon P, Simon R, Mirlacher M, Izbicki JR, Yekebas E, Kaifi JT, Terracciano L, Sauter G. AMACR expression in colorectal cancer is associated with left-sided tumor localization. Virchows Arch 453: 243–248, 2008. [DOI] [PubMed] [Google Scholar]
- 30.McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology 139: 1654–1664, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol 15: 2388–2394, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael MZ, O'Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 1: 882–891, 2003. [PubMed] [Google Scholar]
- 33.Minoo P, Zlobec I, Peterson M, Terracciano L, Lugli A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int J Oncol 37: 707–718, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Morris EJ, Michaud WA, Ji JY, Moon NS, Rocco JW, Dyson NJ. Functional identification of Api5 as a suppressor of E2F-dependent apoptosis in vivo. PLos Genet 2: e196, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulloy R, Salinas S, Philips A, Hipskind RA. Activation of cyclin D1 expression by the ERK5 cascade. Oncogene 22: 5387–5398, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Nehls O, Okech T, Hsieh CJ, Enzinger T, Sarbia M, Borchard F, Gruenagel HH, Gaco V, Hass HG, Arkenau HT, Hartmann JT, Porschen R, Gregor M, Klump B. Studies on p53, BAX and Bcl-2 protein expression and microsatellite instability in stage III (UICC) colon cancer treated by adjuvant chemotherapy: major prognostic impact of proapoptotic BAX. Br J Cancer 96: 1409–1418, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilbert M, Planck M, Fernebro E, Borg A, Johnson A. Microsatellite instability is rare in rectal carcinomas and signifies hereditary cancer. Eur J Cancer 35: 942–945, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Noble CL, Abbas AR, Cornelius J, Lees CW, Ho GT, Toy K, Modrusan Z, Pal N, Zhong F, Chalasani S, Clark H, Arnott ID, Penman ID, Satsangi J, Diehl L. Regional variation in gene expression in the healthy colon is dysregulated in ulcerative colitis. Gut 57: 1398–1405, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Papagiorgis P, Oikonomakis I, Karapanagiotou I, Wexner SD, Nikiteas N. The impact of tumor location on the histopathologic expression of colorectal cancer. J BUON 11: 317–321, 2006. [PubMed] [Google Scholar]
- 40.Patchett SE, Alstead EM, Saunders BP, Hodgson SV, Farthing MJ. Regional proliferative patterns in the colon of patients at risk for hereditary nonpolyposis colorectal cancer. Dis Colon Rectum 40: 168–171, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Pekow JR, Dougherty U, Mustafi R, Zhu H, Kocherginsky M, Rubin DT, Hanauer SB, Hart J, Chang EB, Fichera A, Joseph LJ, Bissonnette M. miR-143 and miR-145 are downregulated in ulcerative colitis: putative regulators of inflammation and protooncogenes. Inflamm Bowel Dis 18: 94–100, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramocki NM, Wilkins HR, Magness ST, Simmons JG, Scull BP, Lee GH, McNaughton KK, Lund PK. Insulin receptor substrate-1 deficiency promotes apoptosis in the putative intestinal crypt stem cell region, limits Apcmin/+ tumors, and regulates Sox9. Endocrinology 149: 261–267, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohde C, Zhang Y, Reinhardt R, Jeltsch A. BISMA—fast and accurate bisulfite sequencing data analysis of individual clones from unique and repetitive sequences. BMC Bioinformatics 11: 230, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjot L, Wiuf C, Sorensen FJ, Kruhoffer M, Laurberg S, Kauppinen S, Orntoft TF, Andersen CL. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res 68: 6416–6424, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz KJ, Hey S, Schinwald A, Wohlschlaeger J, Baba HA, Worm K, Schmid KW. Differential expression of microRNA 181b and microRNA 21 in hyperplastic polyps and sessile serrated adenomas of the colon. Virchows Arch 455: 49–54, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leopold WR, Saltiel AR. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med 5: 810–816, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, deAngelis T, Baserga R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem 282: 32582–32590, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Slattery ML, Wolff E, Hoffman MD, Pellatt DF, Milash B, Wolff RK. MicroRNAs and colon and rectal cancer: differential expression by tumor location and subtype. Genes Chromosomes Cancer 50: 196–206, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snaebjornsson P, Jonasson L, Jonsson T, Moller PH, Theodors A, Jonasson JG. Colon cancer in Iceland—a nationwide comparative study on various pathology parameters with respect to right and left tumor location and patients age. Int J Cancer 127: 2645–2653, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, Muranyi A, Hirst M, Hogge D, Marra M, Wells RA, Buckstein R, Lam W, Humphries RK, Karsan A. Identification of miR-145 and miR-146a as mediators of the 5q− syndrome phenotype. Nat Med 16: 49–58, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Su DM, Zhang Q, Wang X, He P, Zhu YJ, Zhao J, Rennert OM, Su YA. Two types of human malignant melanoma cell lines revealed by expression patterns of mitochondrial and survival-apoptosis genes: implications for malignant melanoma therapy. Mol Cancer Ther 8: 1292–1304, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, Tanaka Y, Dahiya R. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis 32: 772–778, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103: 2257–2261, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang CJ, Zhou ZG, Wang L, Yang L, Zhou B, Gu J, Chen HY, Sun XF. Clinicopathological significance of microRNA-31, -143 and -145 expression in colorectal cancer. Dis Markers 26: 27–34, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Will OC, Leedham SJ, Elia G, Phillips RK, Clark SK, Tomlinson IP. Location in the large bowel influences the APC mutations observed in FAP adenomas. Fam Cancer 9: 389–393, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2α. Gastroenterology 135: 1624–1635, 2008. [DOI] [PubMed] [Google Scholar]
- 58.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 137: 647–658, 2009. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Chaerkady R, Kandasamy K, Huang TC, Selvan LD, Dwivedi SB, Kent OA, Mendell JT, Pandey A. Identifying targets of miR-143 using a SILAC-based proteomic approach. Mol Biosyst 6: 1873–1882, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu HM, Dougherty U, Robinson VL, Mustafi R, Pekow J, Kupfer SS, Li YC, Hart J, Goss KH, Fichera A, Joseph L, Bissonnette M. EGFR signals down-regulate tumor suppressors miR-143 and miR-145 in Western diet-promoted murine colon cancer: role of G1 regulators. Mol Cancer Res 9: 960–975, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]