Abstract
With an increase in urine flow there is a significant increase in shear stress against the renal epithelium including the inner medullary collecting duct, resulting in an increase in nitric oxide (NO) production. The mechanisms of the shear stress-mediated increases in NO are undetermined. Previous studies found that shear stress increases epithelial sodium channel (ENaC) open probability and endothelin (ET)-1 production in an ENaC-dependent mechanism in the collecting duct (CD). Given that ET-1 stimulates NO production in the CD, we hypothesized that shear stress-induced NO production is downstream of shear stress-induced ENaC activation and ET-1 production in a negative feedback loop. We determined that nitric oxide synthase 1 (NOS1) and NOS3 contribute to shear stress-mediated NO production in the CD, that is attenuated by low doses of the ENaC inhibitors amiloride and benzamil. Moreover, ETB receptor blockade significantly blunted the shear stress-mediated NO production. We further elucidated whether mice lacking NOS1 in the collecting duct (CDNOS1KO) have an impaired renal ET-1 system in the CD. Although urinary ET-1 production and inner medullary ET receptor expression were similar between flox control and CDNOS1KO mice, acute ET-1 treatment significantly reduced ENaC open probability in CDs from flox mice but not CDNOS1KO mice compared with basal. Basal ENaC activity in CDs was similar between the genotypes. We conclude that during acute shear stress across the CD, ENaC acts in a negative feedback loop to stimulate NO production in an ETB/NOS1-dependent manner resulting in a decrease in ENaC open probability and promoting natriuresis.
Keywords: collecting duct, epithelial sodium channel, nitric oxide, nitric oxide synthase
nitric oxide (NO) is a natriuretic and diuretic factor and is produced by the NO synthase (NOS) family of enzymes. In the kidney, medullary collecting ducts (MCDs) have the highest total NOS activity (37) and express NOS1 and NOS3 (23). NOS1 mRNA can undergo alternative splicing (3, 25). The mouse inner medullary collecting duct (IMCD) expresses predominantly the NOS1β splice variant (130 kDa) (10, 11, 13, 14). We recently developed a CD, principal cell-specific NOS1 knockout (CDNOS1KO) mouse that deleted all NOS1 splice variants from only the CD (10). When these mice are challenged with a high-sodium diet, they excrete less sodium and water and present with a salt-sensitive blood pressure phenotype (10). These data indicate that CD NOS1 is integral in fluid-electrolyte balance, and we hypothesized this is due to direct actions of NO on CD ion and water transport processes. However, the specific cellular mechanisms are yet to be elucidated.
With an increase in fluid flow across the tubular epithelium, there is an increase in shear stress. The CD, in particular the IMCD, can experience a 10-fold increase in shear during high-sodium/high-water consumption compared with normal-sodium/normal-water intake (6). An increase in shear stress causes a significant increase in IMCD NO production (6). Moreover, an increase in shear stress across the cortical CDs (CCD) leads to an increase in endothelin-1 (ET-1) mRNA (22). The IMCD is also the highest producer of ET-1 (17) and has high ET receptor expression (21). Studies have determined that ET-1 stimulates NO production in the CD via the ETB receptor (11, 34). Recently, it was determined that sodium delivery and shear stress across the CCD synergistically increase ET-1 production and this is mediated by epithelial sodium channel (ENaC) activity (27). ENaC is expressed in the apical membrane of the CD and it regulates sodium reabsorption. Previous work in the rabbit CD determined that shear stress increases ENaC open probability (Po) (24), suggesting that there is an increase in sodium reabsorption. Moreover, microcatheterization studies of the MCD determined that during extracellular fluid expansion by either water loading or potassium chloride loading led to a significant increase in sodium load delivery to the MCD and there is significant increase in sodium reabsorption that was linearly load dependent (7). From these studies, it was concluded that the MCD reabsorbs 80–96% of the NaCl delivered (7). However, if CD transport processes can be inhibited for example by atrial natriuretic peptides, there is a two- to threefold increase in load to the MCD but a 5- to 17-fold increase in natriuresis (7, 31). Thus, the CD is integral in regulating natriuresis through inhibition of CD sodium transport processes, and this is critical during times when the body needs to excrete excess sodium such as high dietary sodium intake. Interestingly, ET-1 inhibits ENaC Po (5). ENaC is necessary to initiate the cascade that stimulates ET-1 production in high shear conditions and ET-1 acts in a negative feedback manner to inhibit ENaC Po and promote natriuresis. We hypothesized that shear stress-induced increases in CD NO are also mediated via an ENaC-dependent mechanism (Fig. 1).
Fig. 1.
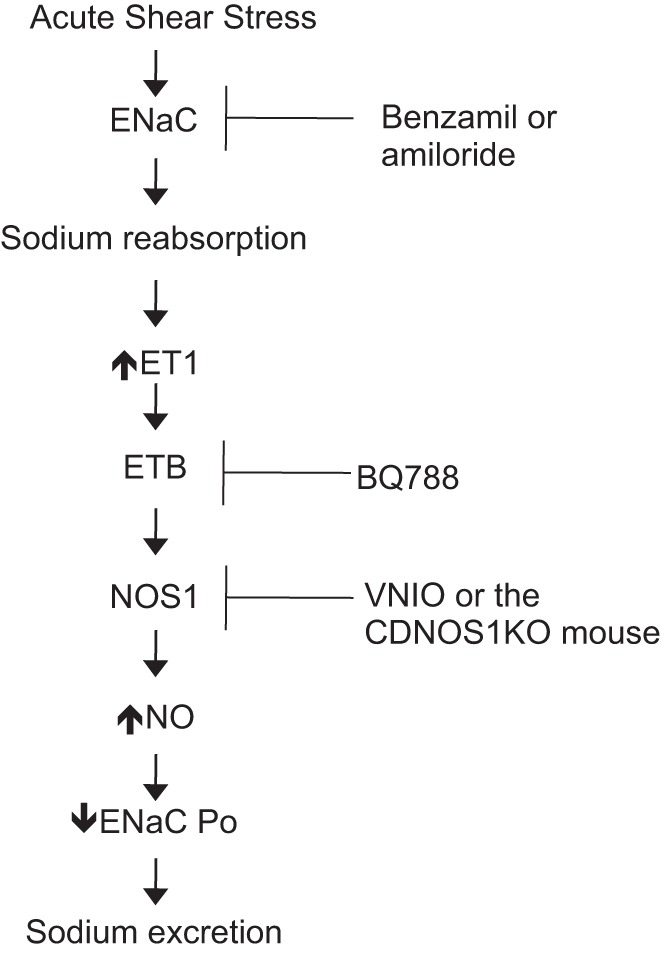
Hypothetical scheme for shear stress-mediated nitric oxide (NO) production in the collecting duct (CD) with inhibitors and mouse model used in the study to test the hypothesis. ENaC, epithelial sodium channel; ET1, endothelin-1; Po, open probability; VNIO, vinyl-l-NIO hydrochloride.
In CD ETB receptor knockout (CDETBKO) mice, which display a salt-sensitive blood pressure phenotype, ENaC activity was enhanced compared with littermate control mice (4). Moreover, acute ET-1 stimulation resulted in a decrease in ENaC Po in CCD from control mice, but CDETBKO mice were unresponsive to the treatment (4). These data indicate that ET-1 activation of the ETB receptor leads to inhibition of ENaC activity. Given the interactions between the ET-1/ETB pathway and the NOS1/NO pathway in the CD, we further hypothesized that CDNOS1KO mice will lack the ET-1-mediated inhibition of ENaC.
The aims of this study were to elucidate the mechanisms of the shear stress-mediated NO response, and the role of CD NOS1 in modulating the CD ET system and ENaC activation. We determined that CD NOS1 is integral in the shear-mediated NO response and is stimulated via an ENaC- and ETB-dependent mechanism. Moreover, mice lacking CD NOS1 have an intact renal ET-1 and ET receptor system but an impaired ENaC response to acute ET-1 stimulation, suggesting that CD NOS1 is critical for the ET-1-dependent decrease in ENaC Po.
MATERIALS AND METHODS
Shear stress.
Once confluent in a 100-mm cell culture dish, the mouse IMCD segment 3 cells (mIMCD-3; ATCC, Manasseas, VA) were split 1/10 and seeded on nitric acid (30% in water)-etched glass slides (3“ × 1” premium glass slides, Fisher Scientific, Waltham, MA) in 100-mm cell culture dishes. Cells were grown to confluency and serum starved overnight before experimentation. The rectangular flow apparatus was purchased from Glycotech (Gaithersburg, MD) and connected via Tygon tubing (Cole ParmerVernon Hills, IL) to an Ismatec pump (Glattbrugg, Switzerland). To generate shear stress, Hank's buffered salt solution (HBSS) with final concentrations of 250 μM l-arginine and 20 U/ml superoxide dismutase was pumped to produce a “low” shear of 3 dyn/cm2 or “high” shear of 30 dyn/cm2 for 1 h. These shear stresses were based on our previous study that determined this high shear maximally stimulated NO production and is similar to the calculated shear stress the IMCD experiences in vivo during high urine flow (6). Samples were taken from the reservoir at 15-min intervals or after 1 h of shear. For static (control) conditions, the remainder of the cells in the 100-mm plate was used.
Cells were pretreated for 30 min with vehicle (0.1% DMSO), 100 nM 1400W dihydrochloride to selectively inhibit NOS2 (Cayman Chemical, Ann Arbor, MI), 1 μM vinyl-l-NIO hydrochloride (VNIO) to selectively inhibit NOS1 (Cayman Chemical), or 1 mM l-NG-nitroarginine methyl ester (l-NAME) to inhibit all NOS isoforms. Then, the HBSS was removed and replaced with fresh HBSS + inhibitors for the 1-h collection period. Based on the percent inhibition, we calculated the percent contribution of each NOS isoform to the shear stress-induced nitrite production.
To block the endothelin A receptor, BQ123 (dissolved in DMSO, final concentration in study 0.1%) was given at 1 μM 30 min before and during experimentation. To inhibit the endothelin B receptor, BQ788 (in DMSO) was given 30 min before experimentation at 1 μM, which we previously determined to maximally inhibit ET-1-mediated stimulation of IMCD nitrite production (11). To inhibit ENaC, cells were pretreated for 30 min with either 1 μM amiloride or 0.2 μM benzamil (initially dissolved in water). Cells were subjected to shear stress for 1 h and nitrite was measured using HPLC as previously described (10, 11, 13).
Animals.
All animal use and welfare adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of Georgia Regents University, University of Alabama at Birmingham, and the University of Texas Health Science Center at San Antonio. CDNOS1KO and flox control mice were bred at Georgia Regents University and the University of Alabama at Birmingham as previously described (10). Male CDNOS1KO and flox littermate control mice were maintained with standard chow {0.3% [Na+]; Teklad 8604. Harlan, Madison, WI}. At University of Texas Health Science Center at San Antonio, mice were maintained on standard chow (0.32% [Na+]; Teklad 7912, Harlan). For all mouse studies, only male mice, ages 12–16 wk of age, with average masses of 26.5 ± 0.93 g for flox control and 26.9 ± 2.0 g for the CDNOS1KO mice were utilized. For collection of urine, mice were housed individually in metabolic cages as previously described (10).
Isolated, split-open CD preparation.
Isolation of CDs suitable for electrophysiology has been described (4, 5). ENaC activity in principal cells of murine CDs was quantified in cell-attached patches of the apical membrane made under voltage-clamp conditions (−Vp = −60 mV) using standard procedures (4, 5). As recording of the current tracings continued, 20 nM ET-1 was applied to the preparation, and the number of channels (n) and Po were determined as described in detail elsewhere (4, 5).
Urinary ET-1 excretion.
ET-1 was measured in neat urine samples by radioimmunoassay (Peninsula Laboratories, San Carlos, CA) and excretion was expressed as picograms per day.
Endothelin receptor binding.
As previously described (18, 19, 35), receptor binding curves were generated to determine binding of ETA and ETB receptors in the IMCD membrane preparations of IM from flox and CDNOS1KO mice.
Quantitative real-time PCR.
Total RNA was extracted from inner medullas or cortical samples of Flox and CDNOS1KO mice using Tri-Reagent following the manufacturer's instructions (Sigma). RNA (5 μg) was reverse transcribed using Invitrogen's superscript III RT-Kit and relative quantitative expression of EDN1, EDNRA, EDNRB, endothelin-converting enzyme-1 (ECE1), and EDN3 were determined using Biorad's (Carlsbad, CA) SoAdvanced Universal SYBR Green Supermix and commercially available primers from Qiagen (Valencia, CA).
Statistical analysis.
Data are reported as means ± SE. To determine the effect of shear stress on nitrite production, a repeated-measures, two-factor ANOVA (time and shear) was performed. For all other shear stress experiments, two-factor ANOVA (for treatment and shear) was completed. Differences between ENaC Po for flox control and CDNOS1KO with and without ET-1 were determined with a two-factor ANOVA. For the endothelin receptor binding assays, Bmax and Kd were calculated by nonlinear regression, assuming one binding site the least square fitting method (Prism, v6.0e, La Jolla, CA). As previously described (19), to calculate the expression of ETA and ETB, the Bmax for ET-3 binding represents ETB receptor binding and the difference between the Bmax for the ET-1 binding and ET-3 binding represents ETA receptor binding. The ET-1 excretion was compared with unpaired t-test. The criterion for significance was P ≤ 0.05.
RESULTS
Experimental design.
Figure 1 outlines the hypothetical scheme guiding this study and shows the points of intervention to test our hypothesis.
Regulation of shear stress-mediated increases in NO.
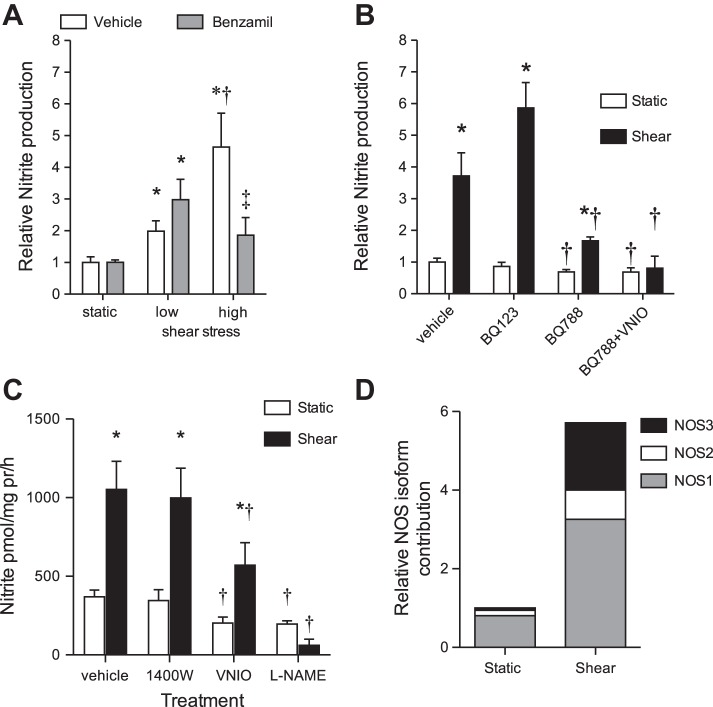
mIMCD-3 cells express ENaC (8). To determine whether ENaC is part of the cascade leading to the shear stress-mediated increase in NO, we inhibited ENaC with 1 μM amiloride or 0.2 μM benzamil under low (3 dyn/cm2) and high (30 dyn/cm2) shear stress. Inhibition of ENaC with benzamil prevented the 30 dyn/cm2 (high shear) shear-mediated increase in nitrite production (n = 4–8, P = 0.025) without any significant effect on nitrite production under static conditions (Fig. 2A). Likewise, inhibition of ENaC with amiloride also prevented the high shear-mediated increases in nitrite (static = 1.0 ± 0.4 pmol·mg pr−1·h−1, 30 dyn/cm2 = 1.19 ± 0.2 pmol·mg pr−1·h−1, P > 0.05). We further tested whether ENaC inhibition prevented a lower shear stress-mediated NO. Cells subjected to 3 dyn/cm2 for 1 h produced twice as much nitrite as static cells (Fig. 2A); however, inhibition of ENaC with benzamil did not prevent the 3-dyn/cm2-mediated increase in nitrite production (Fig. 2A, n = 8, P > 0.05). Given this finding, the remainder of our experiments was conducted using a high shear of 30 dyn/cm2 for 1 h.
Fig. 2.
ENaC and the ETB receptor are necessary for shear stress-mediated nitrite production. Shear stress (low = 3 dyn/cm2 or high = 30 dyn/cm2) or static conditions (0 dyn/cm2) applied to mouse inner medullary CD segment 3 cells (mIMCD-3) for 1 h. A: pretreatment (30 min) of cells with the ENaC inhibitor benzamil (0.2 μM) blunted the shear stress-mediated increase in mIMCD-3 nitrite under high shear stress (n = 4–8, P = 0.025). Benzamil did not prevent the shear stress-mediated increase in nitrite under low shear stress, and there was no significant effect under static conditions (n = 4–8, P > 0.5). *Represents a significant increase compared with the corresponding static condition. †Represents a significant difference from the corresponding low shear stress group. ‡Represents significant difference between vehicle and benzamil groups with high shear. B: pretreatment for 30 min with the ETB antagonist BQ788 (1 μM), but not the ETA antagonist BQ123 (1 μM), reduced nitrite production in static conditions and blunted the high shear stress increase in nitrite production. ETB receptor blockade and inhibition of nitric oxide synthase (NOS)1 with 1 μM VNIO abolished the shear stress-mediated increase in nitrite (n = 5, *P < 0.05 compared with vehicle static, †P < 0.05 compared with corresponding vehicle). C: NOS inhibitors were given for 30 min before sample collection. Inhibition of NOS2 by 100 nM 1400W had no significant effect on static or high shear stress-mediated nitrite production; however, NOS1 inhibition by 1 μM VNIO significantly blunted static and shear stress-mediated NO production. Inhibition of total NOS by 1 mM l-NAME significantly reduced nitrite production in static and high shear conditions (n = 5–11, P < 0.05). *Represents a significant increase compared with the corresponding static condition. †Represents a significant difference from the corresponding vehicle group. D: % contribution of NOS1, NOS2, and NOS3 on nitrite production under static and shear stress conditions.
Under static and shear stress conditions, mIMCD-3 nitrite production can be partially inhibited by acute ETB receptor blockade with BQ788, but not with acute ETA receptor blockade (BQ123) (Fig. 2B). BQ788 reduced nitrite production in static cells by 30% and shear stress cells by 55%. BQ123 tended to increase shear stress-mediated nitrite production compared with vehicle (n = 6, P = 0.078). Combined BQ788 and VNIO treatment reduced nitrite production in static cells by 32% and abolished the shear stress-induced nitrite production (Fig. 2B).
Under static conditions, 1400W (a selective NOS2 inhibitor) did not significantly affect nitrite production; however, VNIO (a selective NOS1 inhibitor) and l-NAME (inhibitor of all NOS isoforms) significantly reduced nitrite production (n = 5–11, P < 0.05; Fig. 2C). Similar results were found under shear conditions, with no significant effect of 1400W (P > 0.05), but a significant reduction in nitrite in the VNIO and l-NAME cells (n = 5–10, P < 0.05; Fig. 2C). From these data, we calculated that in static conditions ∼80% of the nitrite is derived from NOS1 (Fig. 2D). While under shear conditions, there is a 360% increase in NOS1-derived NO, and a 171% increase in NOS3-derived NO (Fig. 2D).
ENaC activity in flox and CDNOS1KO mice.
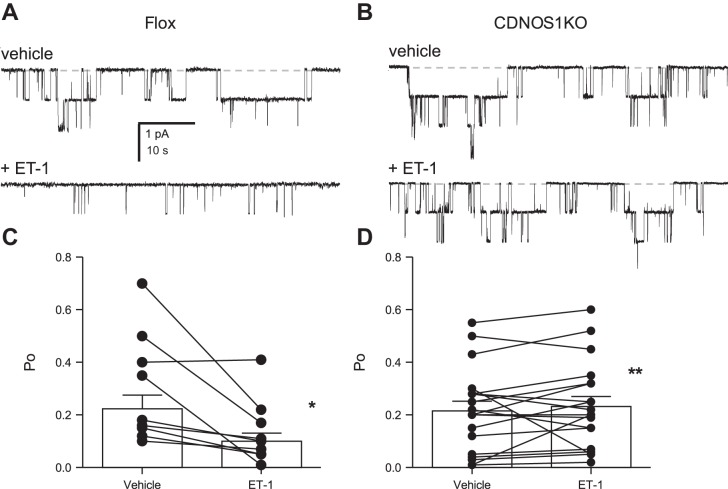
We determined whether principal cell NOS1 was necessary for the ET-1-dependent inhibition of ENaC activity by using patch-clamp electrophysiology. Table 1 reports the resting activity of ENaC in principal cells in isolated, split-open CCD isolated from flox control and CDNOS1KO mice maintained with a normal-sodium diet. Before acute 20 nM ET-1 treatment, ENaC activity was not different between genotypes, as there were equal open Po and number of channels (n) in the patches. Representative current traces of ENaC in flox control and CDNOS1KO mice before and after acute treatment with 20 nM ET-1 are shown in Fig. 3, A-B. Summarized results of the effects of ET-1 on the Po of ENaC in principal cells from control and CDNOS1KO mice are shown in Fig. 3, C–D. Consistent with our previous findings (5), ET-1 significantly decreases the Po of ENaC in principal cells from flox control mice. In contrast, ET-1 is without effect on the Po of ENaC in CDNOS1KO mice (Fig. 3, C–D). Moreover, the activity of ENaC in principal cells from CDNOS1KO mice in the presence of ET-1 is significantly greater than that in control mice treated with ET-1. These results demonstrate that normal expression and function of NOS1 in principal cells are required for ET-1 inhibition of ENaC.
Table. 1.
ENaC activity in flox control vs. CDNOS1KO mice
| Genotype | NPo | N | Po | f2 |
|---|---|---|---|---|
| Flox | 0.72 ± 0.18 | 2.52 ± 0.32 | 0.23 ± 0.03 | 0.50 (25/50) |
| CDNOS1KO | 0.74 ± 0.15 | 2.47 ± 0.27 | 0.24 ± 0.04 | 0.48 (47/91) |
Data are means ± SE. All groups maintained with regular chow containing 0.32% [Na+]. 2f = frequency (patches with at least one active channel/total number of viable seals for that condition).
Fig. 3.
NOS1 is necessary for ET-1-mediated inhibition of ENaC. Representative current traces from cell-attached patches made on the apical membrane of principal cells in split-open murine collecting ducts from flox control (A; n = 6 mice, n = 10 patches) and CDNOS1KO (B) mice (n = 6 mice, n = 16 patches) before (top) and after (bottom) treatment with 20 nM ET-1. The closed state is denoted with a dashed line. Inward current is downward. The holding potential for these patches was −Vp = −60 mV. Summary graphs of ENaC Po in control (C) and CDNOS1KO (D) mice before (vehicle) and after ET-1. Data are from experiments identical to that in A–B. *Significantly decreased compared with before ET-1. **Significantly greater than flox control under identical conditions.
Endothelin excretion and EDN1, EDNR mRNA expression in the kidney.
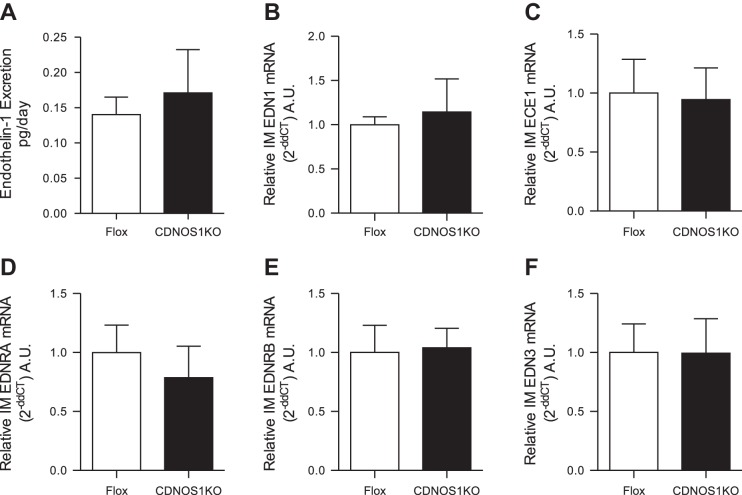
Urinary ET-1 excretion is a measure of renal ET-1 production (1, 30). To determine whether CD NOS1 is integral in renal ET-1 production, urinary ET-1 excretion from CDNOS1KO and flox mice was determined. On a standard chow diet with normal sodium, flox and CDNOS1KO mice excrete similar amounts of ET-1 (n = 10, P = 0.65; Fig. 4A).
Fig. 4.
ET-1 excretion, ET, and ET receptor mRNA is similar in flox and CDNOS1KO mice. A: urinary ET-1 excretion from flox control (n = 10) and CDNOS1KO mice (n = 10) on a normal-sodium diet was similar between the genotypes (P = 0.65). B–F: relative to flox mice, CDNOS1KO mice express similar levels of EDN1 (B), EDNRA (C), EDNRB (D), ECE1 (E), and EDN3 (F) in the inner medulla (n = 5–6).
EDN1 (prepro-EDN1), EDNRA, and ENDRB mRNA expression was determined in the renal cortex and inner medulla of flox and CDNOS1KO mice on a normal-salt diet. There were no statistically significant differences between flox and CDNOS1KO mice for cortical mRNA expression of EDN1 (flox = 1.0 ± 0.4 AU vs. CDNOS1KO = 1.0 ± 0.3 AU), EDNRA (flox = 1.0 ± 0.2 AU vs. CDNOS1KO = 1.0 ± 0.1 AU), or EDNRB (flox = 1.0 ± 0.4 AU vs. CDNOS1KO = 0.9 ± 0.3 AU). Likewise, EDN1, EDNRA, EDNRB, ECE1, or EDN3 mRNA expression were not significantly different in inner medullary tissue from flox and CDNOS1KO mice (Fig. 4, B–E).
Endothelin receptor binding analysis.
To determine whether deletion of CD NOS1 alters inner medullary membrane expression of ET receptors, radioligand binding assays were performed. Flox control mice and CDNOS1KO had similar specific binding of ET-1 curves (Fig. 5A). The Bmax and Kd were 590 ± 120 fmol/mg protein and 0.075 ± 0.05 nM, respectively, for the flox mice (n = 6). The CDNOS1KO mice had a Bmax of 546 ± 95 fmol/mg protein and a Kd of 0.11 ± 0.05 nM (n = 6; Fig. 5A). Flox and CDNOS1KO mice also had similar ET-3-specific binding curves (Fig. 5B). The flox control mice and CDNOS1KO mice had similar Bmax (453 ± 49 and 470 ± 52 fmol/mg protein, n = 6, P > 0.05) and Kd values (0.06 ± 0.03 and 0.08 ± 0.03 nM; Fig. 5B). These mice express ∼20% ETA receptors and ∼80% ETB receptors in the inner medulla, regardless of genotype (Fig. 5C).
Fig. 5.
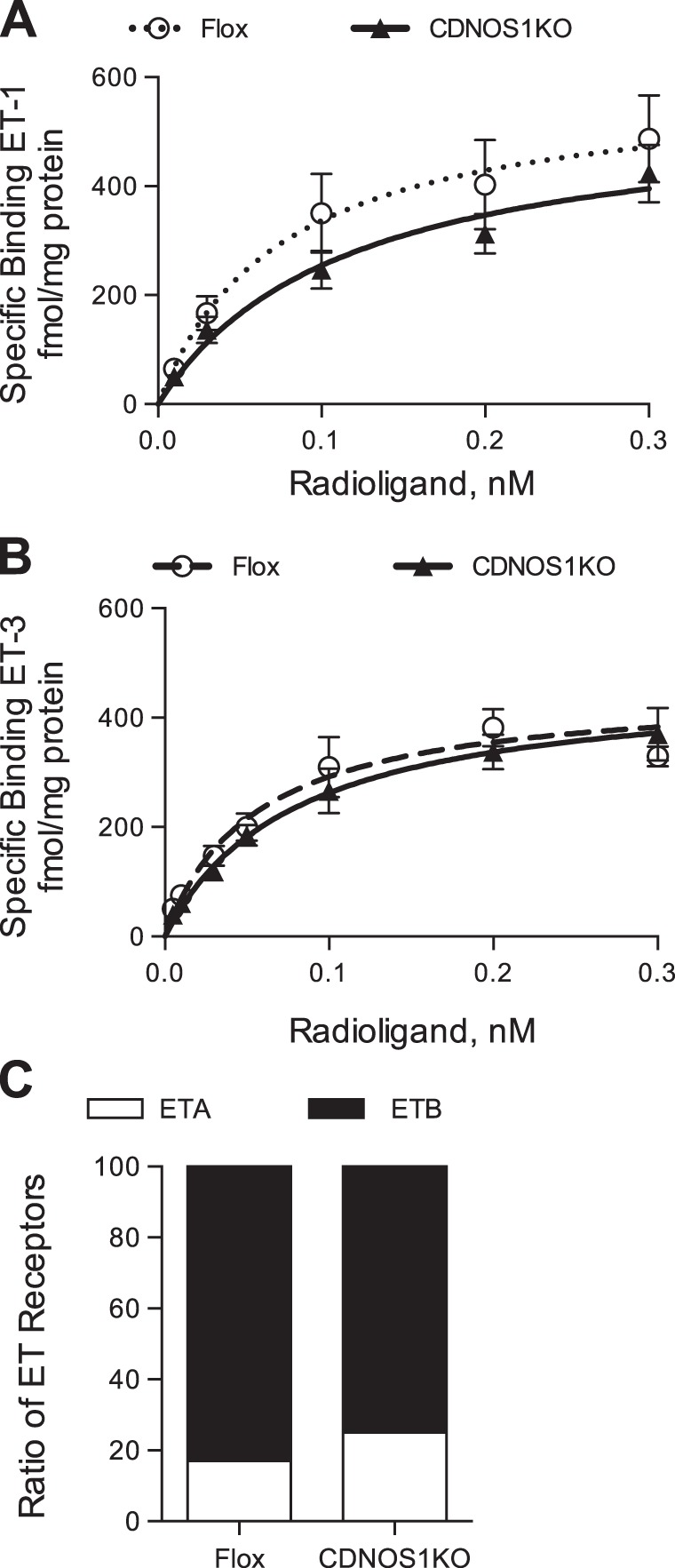
Endothelin receptor expression is similar in flox and CDNOS1KO mice. Inner medullary membrane homogenates were used in the radioligand binding assay. A: ET-1-specific binding curves for flox (○, dashed lines) and CDNOS1KO mice (▲, solid line). B: ET-3-specific binding curves for flox and CDNOS1KO mice. C: percent expression of ETA and ETB receptors in the inner medulla.
DISCUSSION
Three major findings were elucidated in this study. First, increased acute shear stress against the IMCD epithelium increases NOS1- and NOS3-derived NO production that is dependent on ENaC. Second, deletion of NOS1 in the CD results in an inappropriate maintenance of CCD ENaC activation after ET-1 stimulation. These data reveal that CD NOS1 is a critical component of the ET-1-mediated inhibition of ENaC Po. Third, deletion of CD NOS1 does not affect renal ET-1 production or ET receptor expression. We provide a hypothetical working scheme (Fig. 6) of these findings.
Fig. 6.
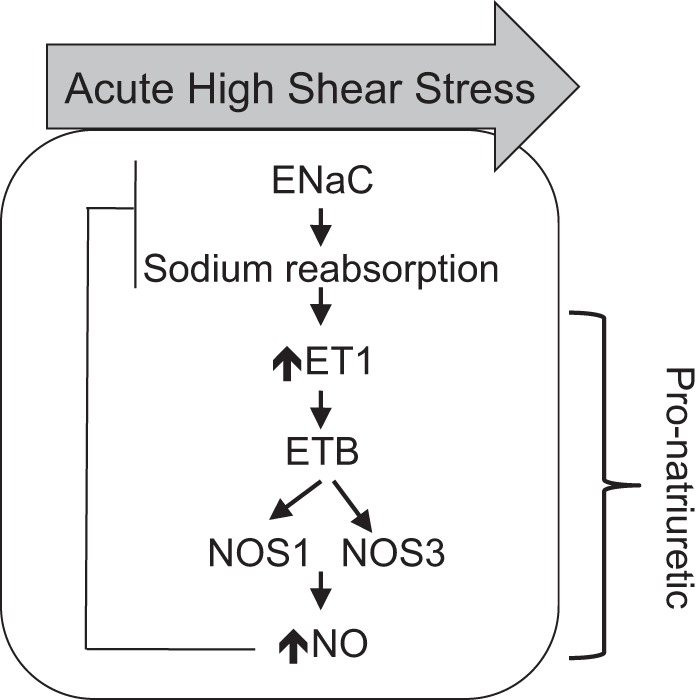
Hypothetical working model of ENaC negative feedback regulation. With an increase in acute high shear stress, ENaC in the apical membrane of the collecting duct initially leads to the stimulation of ET-1 and the ETB receptor resulting in an increase in NO via NOS1. There is also a small but significant increase in NOS3-derived NO; however, it is the NOS1-derived NO that acts in a negative feedback loop to decrease ENaC Po and promotes natriuresis. The downstream targets of NOS3-derived NO remain to be elucidated.
These data provide evidence for a novel negative feedback loop to promote natriuresis during acute high shear conditions that are associated with increased tubular flow. This increase in fluid flow along the renal epithelium results in a 10-fold increase in shear stress-mediated NO production yet the mechanism of this increase in NO production was undetermined (6). We present here that in static conditions, the majority (∼80%) of the NO is derived from NOS1. However, during an increase in shear stress, NOS1 as well as NOS3 significantly contribute to the NO production. We found that isolated IMCD from CDNOS1KO mice produce ∼70% less NO than the IMCD from flox control mice under basal conditions (10). Furthermore, urinary NOx excretion (the metabolites of NO) was ∼60% lower in the CDNOS1KO mouse on both normal- and high-salt diets (10). Taken together with our current findings, these data suggest that NOS1 is the predominant contributor to NO production in the CD and a small but significant portion is also derived from NOS3. Yet, although NOS3 is active, it apparently does not compensate for the loss of NOS1 in the CD in the CDNOS1KO mouse.
Under static conditions, ET-1 stimulates NO production in the IMCD (11, 34). Lyon-Roberts et al. (22) recently reported that shear stress increases ET-1 in the CCD and here we present that inhibition of the ETB receptor significantly attenuates the shear stress-induced NO production in the IMCD. These data indicate that the ET-1/ETB/NO signaling cascade would promote natriuresis and diuresis in the CD under high shear conditions. In addition, shear stress was determined to increase ENaC Po and sodium reabsorption in perfused CCDs (24) and if sodium transport is not inhibited, 80–96% of the delivered sodium may be reabsorbed (7). The sodium reabsorption via ENaC during increased shear stress is an important, and likely initial step in the cascade leading to natriuresis. Shear stress-induced increases in ET-1 in the CCD were determined to be dependent on ENaC and on the mitochondrial sodium-calcium exchanger (27), suggesting that increases in intracellular sodium are integral in stimulating ET-1 production. Here, we present that in the IMCD, shear stress-induced NO production is also mediated via ENaC, but only under high shear stress. When cells were subjected to lower shear stress (3 dyn/cm2), there was a significant increase in ENaC-independent NO production. Thus, we propose that during high shear stress states, ENaC activation leads to stimulation of the ETB/NOS pathways. The stimulated ETB/NOS pathway leads to an increase in NO and closes the feedback loop to inhibit ENaC Po promoting sodium excretion (Fig. 6).
Previous studies determined that exogenous NO inhibits sodium (33) and chloride (28) flux in the CCD via an amiloride- or benzamil-sensitive channel. These data are consistent with the hypothesis that NO inhibits ENaC-dependent sodium reabsorption. Moreover, the NOS isoform putatively involved in the inhibition of ENaC has not been identified. Our CDNOS1KO mouse is a novel model that presents with reduced urinary NOx excretion on normal-sodium diets and similar blood pressure to flox control mice (14). To determine whether CD NOS1 regulates ENaC, we determined resting ENaC activity and the response of ENaC to acute ET-1 treatment in CDNOS1KO and flox control mice using patch-clamp electrophysiology. ET-1 inhibits the Po of ENaC in wild-type mice (5) and this is mediated via the ETB receptor (4). Basal activity of ENaC was not different between flox control and CDNOS1KO mice, suggesting that there is little effect of NOS1 on resting ENaC activity. In response to acute ET-1, flox control mice significantly reduced ENaC Po as expected; however, CDNOS1KO mice were unresponsive to ET-1 and ENaC activity was maintained. Taken together, these data suggest that in states of elevated ET-1 production and/or ETB receptor stimulation ENaC Po is reduced via a NOS1-dependent mechanism.
In the endothelium, NO appears to inhibit ET-1 production (2, 16, 26, 29). We reasoned then that genetic deletion of NOS1 from the CD may result in an altered renal ET-1 system. Although CDNOS1KO mice have reduced urinary NOx and CD NO production compared with flox control mice (10), the CDNOS1KO mice excrete similar levels of urinary ET-1 and have similar medullary ET receptor profiles (both mRNA and protein levels). Thus, we conclude that CD NOS1 most likely does not regulate ET-1 production or ET receptor expression. Additionally, these data indicate that the unresponsiveness to acute ET-1 treatment on ENaC Po in the CDNOS1KO mouse was not because of diminished ETB receptor expression.
One potential paradox to our study is the fact that ENaC is highly expressed in the CCD while ET-1 and NO are highly produced by the IMCD. ET-1 (22, 27) and NOS1 are expressed in the CCD (10, 15). In the IMCD, ENaC subunits are expressed (9) and amiloride inhibits sodium reabsorption in the IMCD as determined by in vivo microcatheterization of the IMCD (32, 36). Thus, there is overlap among the paracrine and ENaC systems along the length of the CD. Finally, NO has been shown to inhibit amiloride-sensitive sodium flux (presumably ENaC dependent) in the CCD (33).
In conclusion, we propose the following model of ENaC negative feedback regulation based on data from the published literature and the data from this study (Fig. 6). With an increase in acute, high shear stress across the CD epithelium, there is an increase in sodium delivery (7) and sodium reabsorption via ENaC (24). If ENaC remains open, then 80–96% of the sodium delivered will be reabsorbed (7). In times where excess sodium must be excreted to maintain balance, it is critical that ENaC Po be decreased. This can be accomplished by increases in ET-1 (27) and activation of the ETB receptor, which stimulates NOS1-mediated NO, and in turn decreases ENaC Po promoting natriuresis. Thus, NOS1 is critical for fluid-electrolyte balance through direct actions on CD ion transport/channel activity.
GRANTS
This study was supported by American Heart Association Postdoctoral Fellowship to K. A. Hyndman and National Institutes of Health Program Project Grant on Endothelin Control of Renal Excretory and Hemodynamic Function (P01 HL95499) to J. S. Pollock and J. D. Stockand.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.A.H., J.D.S., and J.S.P. conception and design of research; K.A.H., V.B., and E.M. performed experiments; K.A.H., V.B., E.M., and J.D.S. analyzed data; K.A.H., V.B., E.M., J.D.S., and J.S.P. interpreted results of experiments; K.A.H., V.B., and E.M. prepared figures; K.A.H. and J.S.P. drafted manuscript; K.A.H., J.D.S., and J.S.P. edited and revised manuscript; K.A.H., V.B., E.M., J.D.S., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the technical support of Melissa Durley-Giles, Amy Dukes, and Dr. Wararat Kittikulsuth.
REFERENCES
- 1.Benigni A, Perico N, Gaspari F, Zoja C, Bellizzi L, Gabanelli M, Remuzzi G. Increased renal endothelin production in rats with reduced renal mass. Am J Physiol Renal Fluid Electrolyte Physiol 260: F331–F339, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg FC, Wolf K, Sandner P, Lorenz C, Riegger GA, Pfeifer M. The NO donor molsidomine reduces endothelin-1 gene expression in chronic hypoxic rat lungs. Am J Physiol Lung Cell Mol Physiol 280: L258–L263, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Brenman JE, Xia H, Chao DS, Black SM, Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci 19: 224–231, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol 302: C188–C194, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279: F270–F274, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Cupples WA, Sonnenberg H. Load dependency of sodium chloride reabsorption by medullary collecting duct in rat. Am J Physiol Renal Fluid Electrolyte Physiol 253: F642–F648, 1987. [DOI] [PubMed] [Google Scholar]
- 8.Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–F673, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension 62: 91–98, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyndman KA, MacDonell AH, Pollock JS. Extracellular signal-regulated kinases 1/2 signaling pathways are not involved in endothelin regulation of mouse inner medullary collecting duct nitric oxide production. Life Sci 91: 578–582, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyndman KA, Musall JB, Xue J, Pollock JS. Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1. Am J Physiol Renal Physiol 301: F118–F124, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyndman KA, Xue J, MacDonell A, Speed JS, Jin C, Pollock JS. Distinct regulation of inner medullary collecting duct nitric oxide production from mice and rats. Clin Exp Pharmacol Physiol 40: 233–239, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarry A, Renaudin K, Denis MG, Robard M, Buffin-Meyer B, Karam G, Buzelin F, Paris H, Laboisse CL, Vallette G. Expression of NOS1 and soluble guanylyl cyclase by human kidney epithelial cells: morphological evidence for an autocrine/paracrine action of nitric oxide. Kidney Int 64: 170–180, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol 286: L984–L991, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura K, Tanaka T, Kato J, Eto T, Tanaka K. Regional distribution of immunoreactive endothelin in porcine tissue: abundance in inner medulla of kidney. Biochem Biophys Res Commun 161: 348–352, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Kittikulsuth W, Pollock JS, Pollock DM. Loss of renal medullary endothelin B receptor function during salt deprivation is regulated by angiotensin II. Am J Physiol Renal Physiol 303: F659–F666, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kittikulsuth W, Pollock JS, Pollock DM. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension 58: 212–218, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohan DE, Hughes AK, Perkins SL. Characterization of endothelin receptors in the inner medullary collecting duct of the rat. J Biol Chem 267: 12336–12340, 1992. [PubMed] [Google Scholar]
- 22.Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension 27: 688–692, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Oberbaumer I, Moser D, Bachmann S. Nitric oxide synthase 1 mRNA: tissue-specific variants from rat with alternative first exons. Biol Chem 379: 913–919, 1998. [PubMed] [Google Scholar]
- 26.Ohkita M, Takaoka M, Sugii M, Shiota Y, Nojiri R, Matsumura Y. The role of nuclear factor-kappa B in the regulation of endothelin-1 production by nitric oxide. Eur J Pharmacol 472: 159–164, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Pandit MM, Strait KA, Matsuda T, Kohan DE. Na delivery and ENaC mediate flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 302: F1325–F1330, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pech V, Thumova M, Kim YH, Agazatian D, Hummler E, Rossier BC, Weinstein AM, Nanami M, Wall SM. ENaC inhibition stimulates Cl− secretion in the mouse cortical collecting duct through an NKCC1-dependent mechanism. Am J Physiol Renal Physiol 303: F45–F55, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raoch V, Rodriguez-Pascual F, Lopez-Martinez V, Medrano-Andres D, Rodriguez-Puyol M, Lamas S, Rodriguez-Puyol D, Lopez-Ongil S. Nitric oxide decreases the expression of endothelin-converting enzyme-1 through mRNA destabilization. Arterioscler Thromb Vasc Biol 31: 2577–2585, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Serneri GG, Modesti PA, Cecioni I, Biagini D, Migliorini A, Costoli A, Colella A, Naldoni A, Paoletti P. Plasma endothelin and renal endothelin are two distinct systems involved in volume homeostasis. Am J Physiol Heart Circ Physiol 268: H1829–H1837, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Sonnenberg H. Mechanisms of release and renal tubular action of atrial natriuretic factor. Fed Proc 45: 2106–2110, 1986. [PubMed] [Google Scholar]
- 32.Sonnenberg H, Honrath U, Wilson DR. In vivo microperfusion of inner medullary collecting duct in rats: effect of amiloride and ANF. Am J Physiol Renal Fluid Electrolyte Physiol 259: F222–F226, 1990. [DOI] [PubMed] [Google Scholar]
- 33.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol Renal Physiol 290: F1315–F1319, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Unique endothelin receptor binding in kidneys of ETB receptor deficient rats. Am J Physiol Regul Integr Comp Physiol 284: R674–R681, 2003. [DOI] [PubMed] [Google Scholar]
- 36.West ML, Sonnenberg H, Veress A, Halperin ML. The relationship between the plasma potassium concentration and renal potassium excretion in the adrenalectomized rat. Clin Sci (Lond) 72: 577–583, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Wu F, Park F, Cowley AW Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol Renal Physiol 276: F874–F881, 1999. [DOI] [PubMed] [Google Scholar]