Abstract
ANG II is thought to increase the susceptibility to hypertension-induced renal disease (HIRD) via blood pressure (BP)-dependent and BP-independent pathways; however, the quantitative relationships between BP and HIRD have not been examined in ANG II-infused hypertensive rats. We compared the relationship between radiotelemetrically measured BP and HIRD in Sprague-Dawley rats (Harlan) chronically administered ANG II (300–500 ng·kg−1·min−1, n = 19) for 4 wk versus another commonly employed pharmacological model of hypertension induced by the chronic administration of Nω-nitro-l-arginine methyl ester (l-NAME, 50 mg·kg−1·min−1, n = 23). Despite the significantly higher average systolic BP associated with ANG II (191.1 ± 3.2 mmHg) versus l-NAME (179.9 ± 2.5 mmHg) administration, the level of HIRD was very modest in the ANG II versus l-NAME model as evidenced by significantly less glomerular injury (6.6 ± 1.3% vs. 11.3 ± 1.5%, respectively), tubulointerstitial injury (0.3 ± 0.1 vs. 0.7 ± 0.1 injury score, respectively), proteinuria (66.3 ± 10.0 vs. 117.5 ± 10.1 mg/day, respectively), and serum creatinine levels (0.5 ± 0.04 vs. 0.9 ± 0.07 mg/dl, respectively). Given that HIRD severity is expected to be a function of renal microvascular BP transmission, BP-renal blood flow (RBF) relationships were examined in additional conscious rats administered ANG II (n = 7) or l-NAME (n = 8). Greater renal vasoconstriction was observed during ANG II versus l-NAME administration (41% vs. 23% decrease in RBF from baseline). Moreover, administration of ANG II, but not l-NAME, led to a unique BP-RBF pattern in which the most substantial decreases in RBF were observed during spontaneous increases in BP. We conclude that the hemodynamic effects of ANG II may mediate the strikingly low susceptibility to HIRD in the ANG II-infused model of hypertension in rats.
Keywords: blood pressure, renal blood flow, radiotelemetry, Nω-nitro-l-arginine methyl ester
angiotensin ii (ANG II) is thought to play a major role in the development and progression of chronic kidney disease (CKD), and its blockade is recommended as a primary strategy for reducing CKD progression (31, 40, 66). It has been postulated that in addition to causing direct hypertensive (barotrauma-mediated) injury, ANG II also activates other blood pressure (BP)-independent tissue injury pathways such as inflammation, oxidative stress, aberrant O2 utilization, hypoxia, and profibrotic signaling (11, 17, 31, 32, 42, 47, 59, 63). Thus, it is widely believed that susceptibility to renal injury is increased in ANG II excess states, and the ANG II infusion model has been extensively used to investigate such mechanisms. However, while the chronic administration of ANG II has been repeatedly shown to produce sustained hypertension and renal injury in rodents (29, 30, 36, 41, 46, 48, 52, 54, 57, 61, 62, 65), such studies have not directly addressed the issue of susceptibility to renal injury, per se, in ANG II-infused animals. Any increase in the physical pressure (i.e., BP) within the intrarenal vasculature, if of sufficient magnitude and regardless of cause, is expected to result in barotrauma and vascular injury (4, 15, 20, 33, 35). Therefore, the susceptibility to hypertensive-induced renal damage (HIRD) in disease states or models can only be directly assessed by an examination of the BP threshold for renal injury and the slope of the relationship between renal injury and BP (i.e., increase in renal injury/mmHg increase in BP) (5, 10). Such quantitative relationships between directly measured BP and HIRD have still not been defined in ANG II-infused models.
Given the plethora of postulated mechanisms of ANG II-mediated renal injury, the degree of renal injury observed in previous studies (29, 30, 36, 41, 46, 48, 52, 54, 57, 61, 62, 65) appears to be surprisingly modest for reasons that remain unclear. Accordingly, the goal of the present study was to define the quantitative relationships between BP and HIRD in ANG II-infused rats and compare it with another very frequently used model of HIRD (2, 3, 22, 56, 58, 70), the Nω-nitro-l-arginine methyl ester (l-NAME)-induced nitric oxide synthesis inhibition model, for which such relationships have been recently defined (2, 3, 22, 56, 58, 70). Given the importance of hemodynamic factors in determining quantitative BP transmission to the renal microvasculature, we also investigated the effects of ANG II versus l-NAME on BP-renal blood flow (RBF) relationships in conscious rats.
METHODS
Animals.
All experiments were performed on male Sprague-Dawley rats (Harlan) weighing 250–350 g and fed standard 1% NaCl Purina chow and provided water ad libitum. All animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Hines Veterans Affairs Institutional Animal Care and Use Committee.
Surgical procedures and experimental design.
To investigate the quantitative relationships between BP and HIRD, rats (n = 42) were anesthetized with isoflurane, and a BP radiotransmitter (model TA11PA-C40, Data Sciences, St. Paul, MN) was inserted, via the femoral artery, into the abdominal aorta below the level of the renal arteries for the continuous assessment of BP (sampled for 10 s every 10 min for 24 h/day). After rats had recovered from surgery, baseline BP (average of days 5–7 postsurgery), proteinuria (24-h urine collection, sulfosalicylic acid), and serum creatinine levels (colorimetric QuantiCrhom Creatinine Assay Kit, BioAssay Systems, Hayward, CA) were assessed. One group of rats (n = 23) was then continuously administered l-NAME via drinking water (500 mg/l) for 4 wk. Water intake in male Sprague-Dawley rats from Harlan is ∼100 ml·kg−1·day−1 in our animal facility (22); therefore, the dose of l-NAME in the present study was ∼50 mg·kg−1·day−1. Another group of rats (n = 19) was anesthetized with isoflurane, and an osmotic minipump (2ML4, Alzet) was implanted subcutaneously between the scapulae for the chronic administration of either 300 ng·kg−1·day−1 (n = 8) or 500 ng·kg−1·day−1 (n = 11) ANG II. These doses of ANG II and l-NAME were used because they elicit relatively similar increases in BP, as determined from preliminary studies. As there were no significant differences in the BP response or the extent of HIRD between rats administered 300 or 500 ng·kg−1·day−1 ANG II, these data were combined into a single group. A 24-h urine collection was conducted at 2 and 4 wk of hypertension for the determination of proteinuria. At 4 wk, a blood sample was obtained for a final serum creatinine measurement, and kidneys were perfused fixed with paraformaldehyde-lysine-phosphate for the histological assessment of renal injury.
An additional group of male Sprague-Dawley rats from Harlan (n = 15) was chronically instrumented with a BP radiotransmitter and a RBF transducer (model 1RB, Transonic Systems, Ithaca, NY), which was placed on the left renal artery and packed in Dacron mesh to ensure proper alignment of the transducer and vessel (9, 24). The transducer cable was secured to the back muscles, routed subcutaneously, exteriorized at the back of the neck, and connected to a flowmeter (model T106, Transonic Systems) during RBF recordings. Rats recovered for 1 wk after the implantation of the BP transmitter and RBF probe. BP and RBF were then obtained (200 Hz) for 3 h on one to three separate occasions at 24-h intervals in conscious rats. After these baseline BP-RBF measurements, one group of rats (n = 8) was anesthetized and implanted with osmotic minipumps to chronically deliver ANG II (500 ng·kg−1·day−1) for 2 wk, whereas another group (n = 7) was administered l-NAME via drinking water (50 mg·kg−1·day−1) for 2 wk. Three days after the induction of hypertension, BP and RBF recordings were again obtained at 200 Hz for a 3-h period and repeated every 2–3 days for 2 wk.
Assessment of renal injury.
Renal injury was assessed in a blinded fashion by one of the investigators (M. Picken) using 4-μm-thick sections stained with periodic acid-Schiff. As previously described (16, 23, 25, 27), glomerular injury was expressed as the total percentage of 100 glomeruli exhibiting lesions of either glomerulosclerosis/necrosis and/or ischemic glomerulosclerosis, as previously described (16). We have previously shown that assessment of the severity of glomerular injury using this method compared with a semiquantitative glomerular injury score based on the percentage of glomerular surface involved yields comparable results (26). Tubulointerstitial injury and fibrosis were assessed on a semiquantitative scale of 0–4. The vascular injury score was calculated as the number of interlobular or afferent arterioles exhibiting acute disruptive injury (lesions of necrosis, thrombosis, aneurysmal dilatation, and/or onion skinning) per 100 glomeruli.
Ambient renal hemodynamics.
As previously described (22, 55), mean arterial pressure (MAP), renal vascular resistance (RVR), and RBF were averaged for each rat over the two to three separate 3-h recordings at baseline and over the six to seven separate 3-h recordings during ANG II and l-NAME administration.
Time-varying BP-RBF relationships.
As previously described (55), each BP and RBF recording was downsampled (20 Hz) and divided into 10-s segments with 50% overlap between successive segments. Average BP and RBF were calculated for each 10-s segment over the entire 3-h recording. The resulting average RBF values for each 10-s segment were associated with a 10-mmHg range (bin) corresponding to the average segment MAP value. The RBF-BP bin data were averaged over the baseline and multiple recordings during ANG II or l-NAME administration for each rat. The RBF-BP bin data at baseline and during ANG II or l-NAME administration were averaged across rats. RBF values are expressed as a percentage of the respective RBF observed when BP was between 100 and 110 mmHg during baseline recordings.
Statistical analysis.
Results are means ± SE. Statistical comparisons between groups were performed using two-way repeated-measures ANOVA. A nonparametric Mann-Whitney test was used to evaluate differences in renal injury parameters between groups. Linear regression analysis was used to calculate the slope of the relationship between glomerular injury and BP for each group (increases in glomerular injury/mmHg increases in systolic BP). Analysis of covariance was used to compare the slopes and x-intercepts between groups. One-way repeated-measures ANOVA was used to evaluate time-varying BP-RBF relationships within baseline, ANG II, and l-NAME groups. If necessary, post hoc comparisons were made using a Student-Newman-Keuls test. P values of <0.05 were considered statistically significant.
RESULTS
Body weight, kidney weight, and serum creatinine levels.
Baseline body weight and serum creatinine levels were not significantly different between groups (Table 1). Rats administered ANG II gained a significantly greater amount of body weight compared with rats administered l-NAME over the 4-wk protocol. Absolute kidney weight was significantly less in rats administered l-NAME (1.3 ± 0.05 g) versus ANG II (1.6 ± 0.05 g) at the completion of the study; however, no differences were observed when kidney weight was normalized to body weight (Table 1). Final serum creatinine values were significantly greater in the l-NAME versus ANG II model at the completion of the study, suggesting a greater degree of renal impairment (Table 1).
Table 1.
Body weight, serum creatinine levels, and kidney weight in ANG II- and l-NAME-infused rats
| Body Weight, g |
Serum Creatinine, mg/dl |
||||
|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Right Kidney Weight/Body Weight, g/kg | |
| ANG II | 272 ± 5 | 332 ± 10* | 0.25 ± 0.03 | 0.54 ± 0.04* | 4.8 ± 0.1 |
| l-NAME | 265 ± 4 | 294 ± 12*† | 0.26 ± 0.03 | 0.92 ± 0.07*† | 4.6 ± 0.1 |
Values are expressed as means ± SE; n = 19 ANG II (300–500 ng·kg−1·min−1) hypertensive rats and 23 Nω-nitro-l-arginine methyl ester (l-NAME; 50 mg·kg−1·min−1) hypertensive rats.
P < 0.05, maximum vs. baseline;
P < 0.05, maximum vs. ANG II.
Hypertension and renal injury.
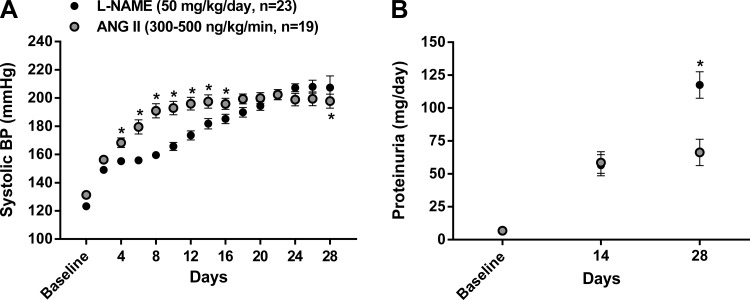
The average BP at baseline and during the 4-wk of ANG II (n = 19) and l-NAME (n = 23) administration (presented as 2-day averages) is shown in Fig. 1A. While both ANG II and l-NAME led to sustained increases in BP over the 4-wk protocol, BP was significantly greater between days 4 and 15 in ANG II-infused rats. However, BP levels plateaued in ANG II-infused rats after ∼2 wk but continued to rise in l-NAME-infused rats such that significantly higher values were achieved over the last 2 days compared with ANG II-infused rats. The average systolic BP over the entire 4-wk protocol was significantly (P < 0.05) higher in ANG II (191.1 ± 3.2 mmHg) versus l-NAME (179.9 ± 2.5 mmHg) hypertensive rats.
Fig. 1.
Blood pressure (BP) and proteinuria at baseline and during chronic administration of Nω-nitro-l-arginine methyl ester (l-NAME) or ANG II. A: radiotelemetrically measured systolic BP at baseline and averaged over 48-h intervals during the 4-wk administration of l-NAME or ANG II. *P < 0.05 vs. l-NAME at the respective time point. B: 24-h proteinuria at baseline and at the second and fourth wk of l-NAME or ANG II. Values are expressed as means ± SE. *P < 0.05 vs. ANG II at 4 wk.
As shown in Fig. 1B, ANG II and l-NAME led to similar increases in proteinuria by 2 wk; however, proteinuria reached significantly greater levels at 4 wk in rats administered l-NAME despite the higher 4-wk average systolic BP observed in ANG II-infused rats. Moreover, the similar level of proteinuria observed at 2 wk of hypertension coincided with a significantly lower average systolic BP in l-NAME- versus ANG II-infused rats (180 ± 3 vs. 197 ± 1 mmHg, respectively, averaged over days 12–16 of hypertension).
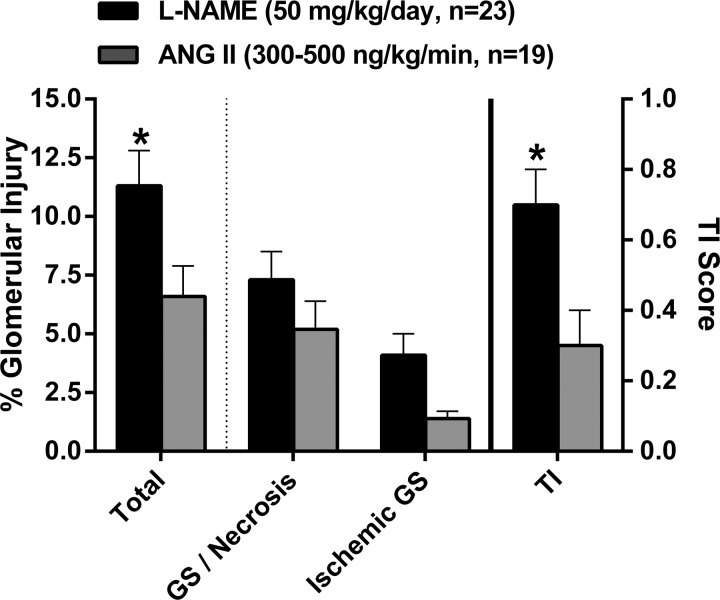
The pattern and magnitude of HIRD in ANG II and l-NAME hypertensive rats are shown in Fig. 2. In both models of hypertension, a pattern of malignant nephrosclerosis was observed with acute disruptive vascular injury, glomerular injury (∼75% glomerulosclerosis/necrosis and ∼25% ischemic glomerulosclerosis), and tubulointerstitial injury. Total glomerular injury (sum of glomerulosclerosis/necrosis and ischemic glomerulosclerosis) was significantly higher in l-NAME versus ANG II hypertensive rats. While both types of glomerular injury were higher in l-NAME-administered rats, the individual differences did not reach statistical significance. The magnitude of tubulointerstitial injury paralleled the level of glomerular injury in both models of hypertension and was 2.3-fold greater (P < 0.05) in rats administered l-NAME versus ANG II. In contrast, both the magnitude (average number of vessels exhibiting vascular injury per 100 glomeruli evaluated) and incidence of acute disruptive vascular injury were similar between l-NAME (2.1 ± 0.5, 12 of 23 rats) and ANG II (2.0 ± 0.7, 10 of 19 rats) hypertensive rats. In summary, these data demonstrate a very modest and significantly lower level of glomerular and tubulointerstitial injury in ANG II versus l-NAME hypertensive rats, despite a higher average systolic BP in the ANG II model.
Fig. 2.
Summary of semiquantitative analysis of renal injury in l-NAME and ANG II hypertensive rats. Total glomerular injury (in %), consisting of both glomerulosclerosis (GS)/necrosis and ischemic GS, and tubulointerstitial injury (TI) were greater in l-NAME versus ANG II hypertensive rats. Values are expressed as means ± SE. *P < 0.05 vs. ANG II.
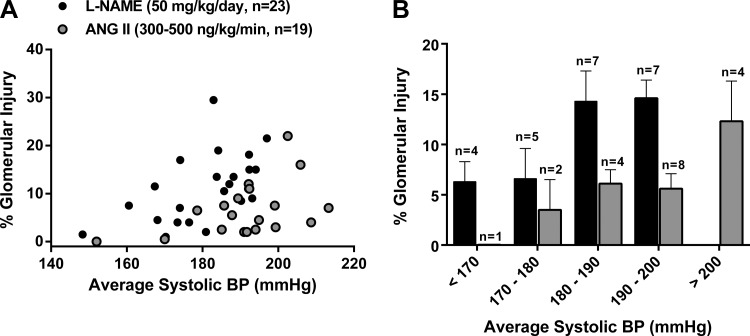
The quantitative relationships between BP and glomerular injury are shown in Fig. 3A. Significant correlations between BP and renal injury were observed in l-NAME (r2 = 0.29, P < 0.01) and ANG II (r2 = 0.21, P < 0.05) hypertensive rats. The slope of the relationship between BP and renal injury, while higher in rats administered l-NAME (y = 0.31x − 45.21), was not statistically different to that observed in rats administered ANG II (y = 0.19x − 28.88). However, the x-intercept of the relationship between BP and renal injury was significantly higher in ANG II versus l-NAME hypertensive rats, indicating an increased BP threshold for the development of renal injury. The increased BP threshold for hypertensive glomerular injury in ANG II hypertensive rats is also shown in Fig. 3B, which shows the average percent glomerular injury plotted within 10-mmHg systolic BP bins corresponding to the average systolic BP achieved during the duration of hypertension. Similar differences in the threshold for vascular injury were also noted. For example, the average systolic BP of the rats in which vascular injury was observed was 198.3 ± 2.9 mmHg in the ANG II model (10 of 19 rats) and 183.1 ± 3.8 in the l-NAME model (12 of 23 rats). The average systolic BP in rats in which no evidence of vascular injury was found was 183.4 ± 2.6 mmHg in ANG II and 178.1 ± 2.9 mmHg in l-NAME hypertensive rats. Collectively, these data clearly demonstrate a very modest level of HIRD and the higher BP threshold required for the development of such injury in the ANG II versus l-NAME model of hypertension in rats.
Fig. 3.
A: relationship between systolic BP (averaged over 4-wk hypertension) and total glomerular injury (in %) in rats administered l-NAME versus ANG II. Whereas no significant differences were observed with respect to the slope of this relationship (l-NAME: 0.31 ± 0.11 vs. ANG II: 0.19 ± 0.09), significant (P < 0.001) differences were seen with respect to the x-intercept when y = 0 (l-NAME: 145 mmHg vs. ANG II: 156 mmHg). B: relationship between total glomerular injury (in %) and the corresponding 4-wk average systolic BP plotted within 10-mmHg systolic BP bins (n = number of rats whose BP fell within the corresponding 10-mmHg BP range). Values are expressed as means ± SE. *P < 0.05 vs. ANG II.
Renal hemodynamics in conscious rats administered ANG II and l-NAME.
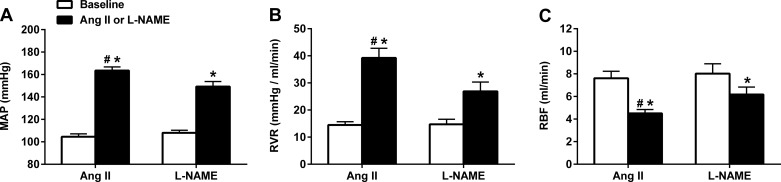
The average MAP, RBF, and calculated RVR obtained from BP-RBF recordings in conscious rats before and during either ANG II (n = 7) or l-NAME (n = 8) administration are shown in Fig. 4. The average BP increase over the 2-wk protocol was significantly greater in rats administered ANG II versus l-NAME (56% vs. 38% increase from baseline, respectively; Fig. 4A), similar to the different BP responses observed during the first 2 wk of ANG II and l-NAME administration in the rats shown in Fig. 1A. However, despite the higher BP, ANG II led to a greater reduction in RBF compared with l-NAME (−41% vs. −23% reduction from baseline, respectively; Fig. 4C). Clearly, this reduction in RBF was due to greater increases in RVR in ANG II versus l-NAME hypertension (171% vs. 82% increase from baseline, respectively; Fig. 4B).
Fig. 4.
Hemodynamic effects of l-NAME (50 mg·kg−1·day−1, n = 8) and ANG II (500 ng·kg−1·day−1, n = 7) in conscious chronically instrumented rats. ANG II led to a significant ∼1.5-fold greater increase in mean arterial pressure (MAP; A) and ∼2-fold greater increase in renal vascular resistance (RVR; B), which resulted in an ∼1.5-fold greater decrease in renal blood flow (RBF; C). Values are expressed as means ± SE. *P < 0.05 vs. the respective baseline; #P < 0.05 vs. l-NAME.
Time-varying BP-RBF relationships in conscious rats administered ANG II and l-NAME.
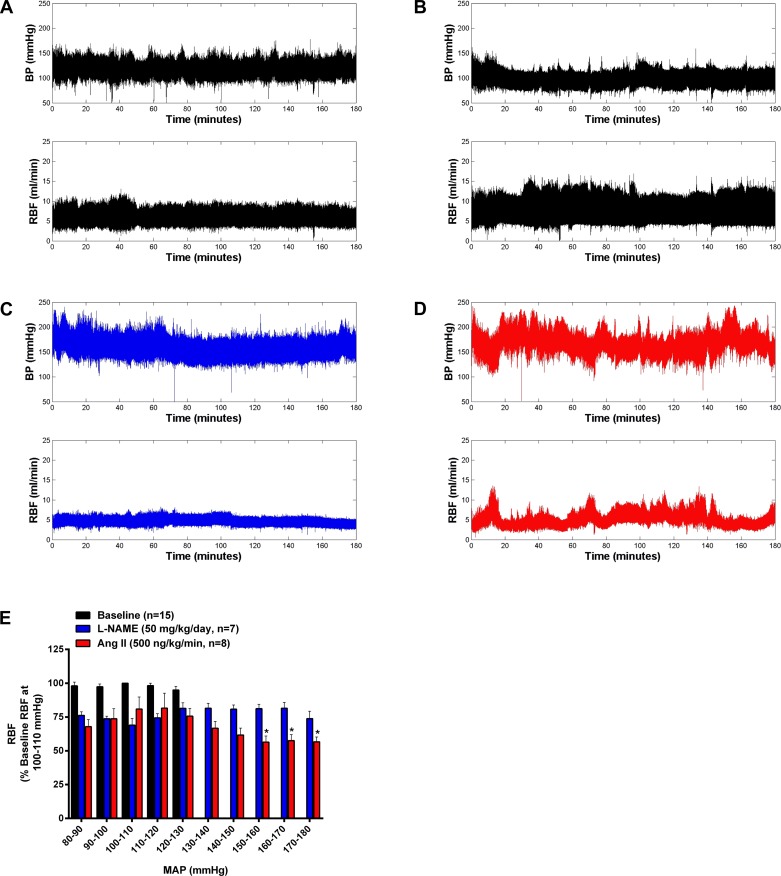
The analysis of BP-RBF relationships in conscious rats before and during ANG II or l-NAME administration is shown in Fig. 5. No significant differences in baseline BP-RBF relationships were observed in the two groups of rats before the administration of ANG II and l-NAME; therefore, these baseline data were combined for presentation clarity (Fig. 5E). RBF was well preserved over the entire BP range during baseline measurements, as shown in Fig. 5E, with individual representative BP-RBF recordings before l-NAME or ANG II administration shown in Fig. 5, A and B. In addition to the significant reductions in average RBF observed during l-NAME and ANG II infusion (Fig. 4C), such significant reductions in RBF are also evident in the BP-RBF bin analysis (Fig. 5E) as well as in the representative BP-RBF tracings shown in Fig. 5, C and D, respectively. However, differences in the BP-RBF patterns across the range of spontaneous BP fluctuations were apparent. As shown in Fig. 5E, l-NAME led to a similar ∼25% reduction in RBF versus pre-L-NAME values across the entire range of BP fluctuations. Conversely, while ANG II led to a similar ∼25% reduction in RBF versus pre-ANG II values when BP was between 80 and 130 mmHg, further reductions in RBF were observed during spontaneously fluctuations in which BP exceeded 130 mmHg. Indeed, when BP was between 150 and 180 mmHg during ANG II infusion, RBF was significantly lower than values observed when BP was between 100 and 120 mmHg. In summary, marked differences in BP-RBF patterns were observed in two commonly used models of hypertension associated with renal vasoconstriction. Similar to a recent report from our laboratory (55), these data demonstrate an enhanced renal vasoconstriction response during episodes of spontaneous increases in BP in conscious ANG II-induced hypertensive rats.
Fig. 5.
Effects of l-NAME and ANG II on BP-RBF relationships in conscious chronically instrumented rats. A–D: representative BP-RBF recordings at baseline (A and B) and during l-NAME (C) or ANG II (D). No differences were seen in BP-RBF relationships during baseline recordings in both groups, so these data were averaged. Both l-NAME and ANG II led to significant reductions in RBF (expressed as percent changes from RBF when MAP was within 100–100 mmHg) compared with baseline values (E). However, the pattern of changes in RBF during spontaneous fluctuations in BP was markedly different during ANG II and l-NAME infusion. Whereas l-NAME led to a similar ∼25% reduction in RBF across the entire range of BP fluctuations, RBF further decreased during episodes of BP elevations in rats administered ANG II. Values are expressed as means ± SE. *P < 0.05 vs. RBF values when BP was within 100–110 and 110–120 mmHg during ANG II administration.
DISCUSSION
A surprisingly modest degree of renal injury was seen in ANG II-infused rats despite the severe hypertension and presumable activation of the plethora of BP-dependent and BP-independent deleterious mechanisms that have been postulated in ANG II-mediated renal damage (11, 17, 31, 32, 42, 47, 59, 63). Although somewhat counterintuitive, these results are nevertheless consistent with several previous studies that have reported similar modest levels of renal injury in ANG II-infused rodents even with higher levels of NaCl intake than used in the present study (29, 30, 36, 41, 46, 48, 52, 54, 57, 61, 62, 65). Indeed, a previous study (54) that compared HIRD in ANG II- versus norepinephrine-infused rats noted that the susceptibility to BP-induced glomerular injury was significantly less in the ANG II model despite the comparable hypertension. However, the apparent discordance between the abundance of postulated mechanisms for ANG II-mediated injury and the relative paucity of observed histological injury has not been directly addressed and the underlying mechanisms have not been investigated.
The significantly higher BP threshold for HIRD and relatively flat slope of the relationship between BP and HIRD in ANG II-infused rats indicate a reduced susceptibility to HIRD compared with rats that receive l-NAME. The primary determinants of such susceptibility to HIRD are expected to be 1) the severity of hypertension, 2) the degree to which the systemic hypertension is transmitted to the renal microvasculature, and 3) the local tissue injury response to a given degree of renal microvasculature BP exposure (5). In this regard, the severity of hypertension was, if anything, greater in the ANG II group (Fig. 1), and there is little evidence to suggest that loss of nitric oxide has more deleterious effects on the local tissue injury response to a given level of BP compared with elevated ANG II levels. Therefore, the reduced susceptibility to HIRD in the ANG II versus l-NAME model is most likely to be due to differences in renal hemodynamic factors that govern the degree of renal microvasculature BP transmission. Consistent with such interpretations, the overall greater constriction observed in ANG II-infused rats would be expected to reduce BP transmission and thus barotrauma-mediated injury compared with rats administered l-NAME (5, 8–10, 51). Moreover, in contrast to the relatively stable vasoconstriction observed in rats administered l-NAME, ANG II infusion was associated with a greater variability in BP-RBF relationships with the more severe vasoconstriction occurring during spontaneous BP elevations (21, 45, 53, 55, 64). Such hemodynamic patterns, which we have also recently described with more modest doses of ANG II (125 ng·kg−1·min−1) (55), are expected to provide more effective protection against renal microvasculature transmission of hypertensive episodes and barotrauma-mediated glomerular injury (5, 10). There is also evidence that ANG II may affect more upstream vascular segments (interlobar, arcuate, and interlobular vessels), as demonstrated by the vascular casting study performed by Wilson and Heptinstall (67), which would result in a greater dissipation of the severity of hypertension along the length of the renal vasculature and microvasculature (14, 39, 67). Indeed, as early as 1964, one of the pioneering investigators of hypertensive organ damage, Byrom (13), suggested that the extreme vasoconstriction in upstream renal vascular segments associated with ANG II infusion may protect the more distal vessels by reducing renal BP transmission. The lack of similar renal vasoconstriction observed in conscious rats administered sympathomimetic agents (43, 55) may account for the greater degree of glomerular injury in norepinephrine- versus ANG II-infused rats previously noted (54). It should also be emphasized that a modest, but nevertheless significant, increase in outer medullary tubulointerstitial injury was observed in ANG II- versus norepinephrine-infused rats. While the mechanisms remain to be elucidated, one possibility is that the extensive renal vasoconstriction observed during ANG II infusion may have led to greater levels of ischemia-induced tubulointerstitial injury compared with norepinephrine. Indeed, we have previously suggested that reduced barotrauma-mediated but increased ischemia-mediated renal injury may be a feature of hypertensive models associated with significant renal vasoconstriction (10, 22). Nevertheless, the overall protective importance of renal vasoconstriction is dramatically illustrated by the severe consequences of ANG II infusion in Notch3-deficient mice, which have impaired vasoconstrictor responses (12).
In addition to the ambient tone of the preglomerular vasculature, its ability to appropriately and proportionately respond to BP changes (i.e., RBF autoregulation) is expected to be the other major hemodynamic determinant of BP transmission (5). Recent studies showing that ANG II impairs RBF autoregulation have been interpreted as providing a mechanism for enhancing HIRD in ANG II excess states (29, 30, 36, 38, 50, 61, 65). However, such interpretations are difficult to reconcile with the paucity of renal damage seen in ANG II-infused rats in both the present study and previous studies. An explanation for this apparent discrepancy may relate to the nature of renal autoregulatory impairment that has been observed in the vasoconstricted vasculature of anesthetized ANG II-infused rats and in ex vivo kidney preparations of ANG II-infused rats (29, 30, 36, 38, 50, 52, 65). While these studies indeed demonstrated an impairment of renal autoregulatory function (i.e., a failure of the renal vasculature to respond to changes in BP) after 1 or 2 wk of ANG II infusion in rats, the autoregulatory impairment was manifest as a reduced ability to vasodilate as renal perfusion pressure was lowered. The implications of impaired autoregulation of this nature are completely opposite to impaired autoregulation in a vasodilated renal vasculature that fails to appropriately vasoconstrict when renal perfusion pressure increases (e.g., the remnant kidney). Whereas the latter impairment increases the susceptibility to barotrauma-mediated injury, the former impairment would be expected to reduce microvascular BP transmission and possibly increase the susceptibility to ischemia-mediated injury (5, 10, 51, 55).
Ambient efferent arteriolar resistance is an additional hemodynamic factor that may impact the severity of glomerular hypertension and injury in hypertensive models. While both ANG II and l-NAME (3, 18, 49) are known to cause efferent vasoconstriction, ANG II is thought to be a more selective and potent efferent vasoconstrictor and would have been expected to cause greater glomerular hypertension and injury. The rather modest levels of glomerular injury actually observed in ANG II-infused rats is accordingly more consistent with a greater vascocontrictive effect on the preglomerular vasculature, at least in the exogenously infused ANG II model, as has indeed been shown by a large number of laboratories (18, 34, 37, 44, 50). The significantly lower level of proteinuria, a robust marker of glomerular capillary pressure (69), observed in ANG II versus l-NAME hypertensive rats at 4 wk is also consistent with a greater preglomerular vascocontrictor effect.
The dominant role played by preglomerular tone and autoregulatory ability in determining the pattern and severity of HIRD is also evident when the ANG II model is compared with the 5/6 renal ablation model. The preglomerular vasodilation and impaired autoregulatory ability characteristic of this model result in a greatly reduced BP threshold for HIRD (∼125 mmHg) and a much steeper slope of the relationship between BP and HIRD (6, 7, 25–27). However, perhaps a more relevant comparison is with another model of HIRD with intact renal mass, the salt-supplemented stroke-prone spontaneously hypertensive rat. More modest levels of renal injury as well as a smaller slopes of the relationship between BP and HIRD were observed in ANG II, as well as l-NAME, hypertensive rats (slope: 0.19 ± .09 and 0.31 ± .11 change in renal injury/change in mmHg, respectively) compared with that recently reported in salt-supplemented stroke-prone spontaneously hypertensive rats with a similar duration and magnitude of hypertension (slope: 1.2 ± 0.2 change in renal injury/change in mmHg) (28). This differential susceptibility to HIRD may also be explained by differences in ambient renal vascular tone (10, 45) given that renal vasoconstriction, similar to that observed in rats administered ANG II and l-NAME, is not observed in salt-supplemented stroke-prone spontaneously hypertensive rats (1).
The morphological pattern of HIRD observed in the present study provides additional insights regarding the pathogenesis of such injury (5, 28). We have suggested that a malignant nephrosclerosis pattern of HIRD, as seen in the present study, supports a barotrauma-mediated pathogenesis and develops when the magnitude of hypertension exceeds both the vascular threshold for acute disruptive injury and autoregulatory capacity to protect glomerular capillaries (5, 8, 23, 28). The evidence that the most severe cases of HIRD were observed primarily in those rats whose average 4-wk systolic BP exceeded the approximate levels of 180 and 200 mmHg in the l-NAME and ANG II model, respectively, supports this concept. Such a pattern of BP and renal injury strongly infers that renal autoregulatory mechanisms, in addition to the ambient tone of the renal microvasculature, largely prevented the transmission of systemic BP to the renal microvasculature as long as BP remained within the autoregulatory range and below the threshold for acute disruptive vascular injury in both models (5, 8, 10, 45).
Finally, these data strongly suggest that the extensive use of chronic ANG II infusion as a model to investigate the pathogenesis of progressive renal damage may not be merited due to the dominant and protective preglomerular vasoconstriction observed in such models. Yet, it is of note that ANG II infusion is the most commonly used model of hypertension in research grants sponsored by the Vascular Biology and Hypertension Branch within the National Heart, Lung, and Blood Institute of the National Institutes of Health (19). In this context, it may also be important to note that the phenotype of renal injury observed after chronic infusion of pharmacological doses of ANG II is different from that seen in most states of CKD progression in humans, which are characterized by progressive glomerulosclerosis and normal, suppressed, or only mildly elevated renin levels. Accordingly, the BP-independent deleterious effects of ANG II in CKD states have been attributed to local renin-angiotensin system activation in renal tissues (31, 42, 50, 59, 60, 68). Regardless of the merits of the claims of BP independence in such studies (5, 6, 10, 23, 26), the ANG II infusion model does not seem to be representative of CKD states. However, it does provide a demonstration of the predominant quantitative importance of physical BP transmission in the pathogenesis of HIRD.
GRANTS
This work was supported by a Career Development Award 1IK2BX001285 (to A. J. Polichnowski) and a Merit Review Award (to K. A. Griffin) from the Office of Research and Development of the Department of Veterans Affairs and by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-40426 (to A. K. Bidani) and DK-61653 (to K. A. Griffin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J.P., K.A.G., and A.K.B. conception and design of research; A.J.P. and H.L.-V. performed experiments; A.J.P., K.A.G., M.M.P., J.L., G.A.W., and A.K.B. analyzed data; A.J.P., K.A.G., M.M.P., J.L., G.A.W., and A.K.B. interpreted results of experiments; A.J.P., J.L., and G.A.W. prepared figures; A.J.P., K.A.G., and A.K.B. drafted manuscript; A.J.P., K.A.G., and A.K.B. edited and revised manuscript; A.J.P., K.A.G., M.M.P., J.L., G.A.W., and A.K.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Theresa Herbst for technical support and Martha Prado for secretarial support.
REFERENCES
- 1.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol Renal Physiol 289: F305–F313, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol 294: F1–F9, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 90: 278–281, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beilin LJ, Goldby FS, Mohring J. High arterial pressure versus humoral factors in the pathogenesis of the vascular lesions of malignant hypertension. Clin Sci Mol Med 52: 111–117, 1977. [DOI] [PubMed] [Google Scholar]
- 5.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bidani AK, Griffin KA, Bakris G, Picken MM. Lack of evidence of blood pressure-independent protection by renin-angiotensin system blockade after renal ablation. Kidney Int 57: 1651–1661, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol Renal Fluid Electrolyte Physiol 265: F391–F398, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. “Step” vs. “dynamic” autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol 285: F113–F120, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 22: 1–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Boulos N, Helle F, Dussaule JC, Placier S, Milliez P, Djudjaj S, Guerrot D, Joutel A, Ronco P, Boffa JJ, Chatziantoniou C. Notch3 is essential for regulation of the renal vascular tone. Hypertension 57: 1176–1182, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Byrom FB. Angiotensin and renal vascular damage. Br J Exp Pathol 45: 7–12, 1964. [PMC free article] [PubMed] [Google Scholar]
- 14.Byrom FB. The evolution of acute hypertensive arterial disease. Prog Cardiovasc Dis 17: 31–37, 1974. [DOI] [PubMed] [Google Scholar]
- 15.Byrom FB. The Hypertensive Vascular Crisis: an Experimental Study. Heinemann Monograph London: Pitman, 1969. [Google Scholar]
- 16.Churchill PC, Churchill MC, Bidani AK, Griffin KA, Picken M, Pravenec M, Kren V, St Lezin E, Wang JM, Wang N, Kurtz TW. Genetic susceptibility to hypertension-induced renal damage in the rat. Evidence based on kidney-specific genome transfer. J Clin Invest 100: 1373–1382, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng A, Arndt MA, Satriano J, Singh P, Rieg T, Thomson S, Tang T, Blantz RC. Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade. Am J Physiol Renal Physiol 299: F1365–F1373, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denton KM, Anderson WP, Sinniah R. Effects of angiotensin II on regional afferent and efferent arteriole dimensions and the glomerular pole. Am J Physiol Regul Integr Comp Physiol 279: R629–R638, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Galis ZS, Thrasher T, Reid DM, Stanley DV, Oh YS. Investing in high blood pressure research: a national institutes of health perspective. Hypertension 61: 757–761, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giese J. Acute hypertensive vascular disease. 2. Studies on vascular reaction patterns and permeability changes by means of vital microscopy and colloidal tracer technique. Acta Pathol Microbiol Scand 62: 497–515, 1964. [DOI] [PubMed] [Google Scholar]
- 21.Grady HC, Bullivant EM. Renal blood flow varies during normal activity in conscious unrestrained rats. Am J Physiol Regul Integr Comp Physiol 262: R926–R932, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Griffin K, Polichnowski A, Licea-Vargas H, Picken M, Long J, Williamson G, Bidani A. Large BP-dependent and -independent differences in susceptibility to nephropathy after nitric oxide inhibition in Sprague-Dawley rats from two major suppliers. Am J Physiol Renal Physiol 302: F173–F182, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure-dependent. Hypertension 41: 201–206, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK. Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol Renal Physiol 286: F1136–F1143, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol 4: 2023–2031, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Griffin KA, Picken M, Bidani AK. Radiotelemetric BP monitoring, antihypertensives and glomeruloprotection in remnant kidney model. Kidney Int 46: 1010–1018, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest 96: 793–800, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin KA, Polichnowski A, Litbarg N, Picken M, Venkatachalam MA, Bidani AK. Critical blood pressure threshold dependence of hypertensive injury and repair in a malignant nephrosclerosis model. Hypertension 64: 801–807, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in ANG II-infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Renal Physiol 298: F1276–F1284, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan Z, Giddens MI, Osmond DA, Cook AK, Hobbs JL, Zhang S, Yamamoto T, Pollock JS, Pollock DM, Inscho EW. Immunosuppression preserves renal autoregulatory function and microvascular P2X1 receptor reactivity in ANG II-hypertensive rats. Am J Physiol Renal Physiol 304: F801–F807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris RC, Neilson EG. Toward a unified theory of renal progression. Annu Rev Med 57: 365–380, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Haugen EN, Croatt AJ, Nath KA. Angiotensin II induces renal oxidant stress in vivo and heme oxygenase-1 in vivo and in vitro. Kidney Int 58: 144–152, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Heptinstall RH, Hill GS. Steroid-induced hypertension in the rat. A study of the effects of renal artery constriction on hypertension caused by deoxycorticosterone. Lab Invest 16: 751–767, 1967. [PubMed] [Google Scholar]
- 34.Heyeraas KJ, Aukland K. Interlobular arterial resistance: influence of renal arterial pressure and angiotensin II. Kidney Int 31: 1291–1298, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Hill GS. Studies on the pathogenesis of hypertensive vascular disease. Effect of high-pressure intra-arterial injections in rats. Circ Res 27: 657–668, 1970. [DOI] [PubMed] [Google Scholar]
- 36.Inscho EW, Cook AK, Clarke A, Zhang S, Guan Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in angiotensin II-infused hypertensive rats fed a high-salt diet. Hypertension 57: 780–787, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inscho EW, Imig JD, Cook AK. Afferent and efferent arteriolar vasoconstriction to angiotensin II and norepinephrine involves release of Ca2+ from intracellular stores. Hypertension 29: 222–227, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol 10, Suppl 11: S178–S183, 1999. [PubMed] [Google Scholar]
- 39.Jacobsen JC, Beierholm U, Mikkelsen R, Gustafsson F, Alstrom P, Holstein-Rathlou NH. “Sausage-string” appearance of arteries and arterioles can be caused by an instability of the blood vessel wall. Am J Physiol Regul Integr Comp Physiol 283: R1118–R1130, 2002. [DOI] [PubMed] [Google Scholar]
- 40.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311: 507–520, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension 19: 464–474, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Kagami S. Involvement of glomerular renin-angiotensin system (RAS) activation in the development and progression of glomerular injury. Clin Exp Nephrol 16: 214–220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinjans JC, Smits JF, Kasbergen CM, van Essen H, Struyker-Boudier HA. Evaluation of renal function during intrarenal norepinephrine infusion in conscious rats. Renal Physiol 7: 243–250, 1984. [DOI] [PubMed] [Google Scholar]
- 44.Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: actions of angiotensin II. Am J Physiol Renal Physiol 273: F307–F314, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller PL, Rennke HG, Meyer TW. Glomerular hypertrophy accelerates hypertensive glomerular injury in rats. Am J Physiol Renal Fluid Electrolyte Physiol 261: F459–F465, 1991. [DOI] [PubMed] [Google Scholar]
- 47.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol 6: 667–678, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Mori T, Cowley AW Jr.. Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Myers BD, Deen WM, Brenner BM. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res 37: 101–110, 1975. [DOI] [PubMed] [Google Scholar]
- 50.Navar LG. Intrarenal renin-angiotensin system in regulation of glomerular function. Curr Opin Nephrol Hypertens 23: 38–45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olson JL, Wilson SK, Heptinstall RH. Relation of glomerular injury to preglomerular resistance in experimental hypertension. Kidney Int 29: 849–857, 1986. [DOI] [PubMed] [Google Scholar]
- 52.Osmond DA, Zhang S, Pollock JS, Yamamoto T, De Miguel C, Inscho EW. Clopidogrel preserves whole kidney autoregulatory behavior in ANG II-induced hypertension. Am J Physiol Renal Physiol 306: F619–F628, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pires SL, Barres C, Sassard J, Julien C. Renal blood flow dynamics and arterial pressure lability in the conscious rat. Hypertension 38: 147–152, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Polichnowski AJ, Cowley AW Jr.. Pressure-induced renal injury in angiotensin II versus norepinephrine-induced hypertensive rats. Hypertension 54: 1269–1277, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polichnowski AJ, Griffin KA, Long J, Williamson GA, Bidani AK. Blood pressure-renal blood flow relationships in conscious angiotensin II- and phenylephrine-infused rats. Am J Physiol Renal Physiol 305: F1074–F1084, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raij L. Nitric oxide, salt sensitivity, and cardiorenal injury in hypertension. Semin Nephrol 19: 296–303, 1999. [PubMed] [Google Scholar]
- 57.Rajapakse NW, De Miguel C, Das S, Mattson DL. Exogenous l-arginine ameliorates angiotensin II-induced hypertension and renal damage in rats. Hypertension 52: 1084–1090, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 20: 298–303, 1992. [DOI] [PubMed] [Google Scholar]
- 59.Ruster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 22: 1189–1199, 2011. [DOI] [PubMed] [Google Scholar]
- 60.Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol 17: 2985–2991, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Saeed A, Dibona GF, Marcussen N, Guron G. High-NaCl intake impairs dynamic autoregulation of renal blood flow in ANG II-infused rats. Am J Physiol Regul Integr Comp Physiol 299: R1142–R1149, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Sasser JM, Moningka NC, Cunningham MW Jr, Croker B, Baylis C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Regul Integr Comp Physiol 298: R740–R746, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh P, Deng A, Blantz RC, Thomson SC. Unexpected effect of angiotensin AT1 receptor blockade on tubuloglomerular feedback in early subtotal nephrectomy. Am J Physiol Renal Physiol 296: F1158–F1165, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skarlatos S, Metting PJ, Britton SL. Spontaneous pressure-flow patterns in the kidney of conscious rats. Am J Physiol Heart Circ Physiol 265: H2151–H2159, 1993. [DOI] [PubMed] [Google Scholar]
- 65.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol 279: F319–F325, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Wheeler DC, Becker GJ. Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int 83: 377–383, 2013. [DOI] [PubMed] [Google Scholar]
- 67.Wilson SK, Heptinstall RH. Effects of acute, angiotensin-induced hypertension on intrarenal arteries in the rat. Kidney Int 25: 492–501, 1984. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558–1565, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Yoshioka T, Rennke HG, Salant DJ, Deen WM, Ichikawa I. Role of abnormally high transmural pressure in the permselectivity defect of glomerular capillary wall: a study in early passive Heymann nephritis. Circ Res 61: 531–538, 1987. [DOI] [PubMed] [Google Scholar]
- 70.Zatz R, Baylis C. Chronic nitric oxide inhibition model six years on. Hypertension 32: 958–964, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]