Abstract
Successive transfer in synthetic medium of spores and mycelial fragments from original toxin-producing cultures of Helminthosporium sacchari results in attenuated cultures which do not produce the host-specific toxin helminthosporoside. When attenuated cultures are grown on material obtained from the water wash of sugarcane leaves susceptible to this fungus, the production of heminthosporoside resumes. Compounds that activate toxin production in the fungus are present on the leaf surface and presumably arise via plant metabolism. One activator was identified as a novel free amine, serinol (2-amino-1,3-propanediol). It activates toxin production in attenuated cultures at 1 μM. Several experiments described in this report argue against the selection theory for the attenuation of cultures. The biological significance and some possible mechanisms for the activation of toxin biosynthesis are discussed.
Keywords: helminthosporoside, attenuation, plant disease resistance
Full text
PDF

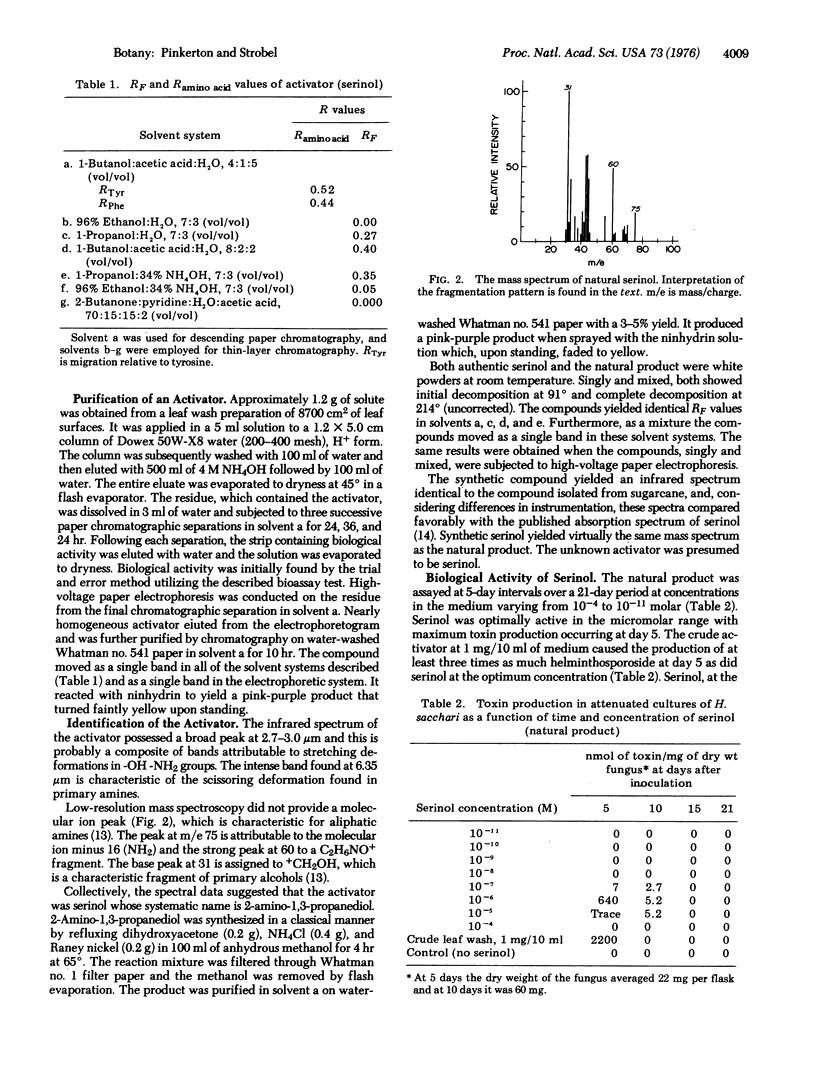


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Filner P. Semi-conservative replication of DNA in a higher plant cell. Exp Cell Res. 1965 Aug;39(1):33–39. doi: 10.1016/0014-4827(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Karr A. L., Karr D. B., Strobel G. A. Isolation and Partial Characterization of Four Host-specific Toxins of Helminthosporium maydis (Race T). Plant Physiol. 1974 Feb;53(2):250–257. doi: 10.1104/pp.53.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSI F., ZATTI M., GREENBAUM A. L. Evidence for the existence of the hexose monophosphate pathway for glucose metabolism in the normal and denervated skeletal muscle of rats. Biochem J. 1963 Apr;87:43–48. doi: 10.1042/bj0870043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqueullah M., McGrath R., Vining L. C. Biosynthesis of chloramphenicol. II. p-Aminophenylalanine as a precursor of the p-nitrophenylserinol moiety. Can J Biochem. 1967 Dec;45(12):1881–1889. doi: 10.1139/o67-221. [DOI] [PubMed] [Google Scholar]
- Smith T. A. The occurrence, metabolism and functions of amines in plants. Biol Rev Camb Philos Soc. 1971 May;46(2):201–241. doi: 10.1111/j.1469-185x.1971.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Steiner G. W., Strobel G. A. Helminthosporoside, a host-specific toxin from Helminthosporium sacchari. J Biol Chem. 1971 Jul 10;246(13):4350–4357. [PubMed] [Google Scholar]
- Strobel G. A. Biochemical basis of the resistance of sugarcane to eyespot disease. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1693–1696. doi: 10.1073/pnas.70.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]




