Abstract
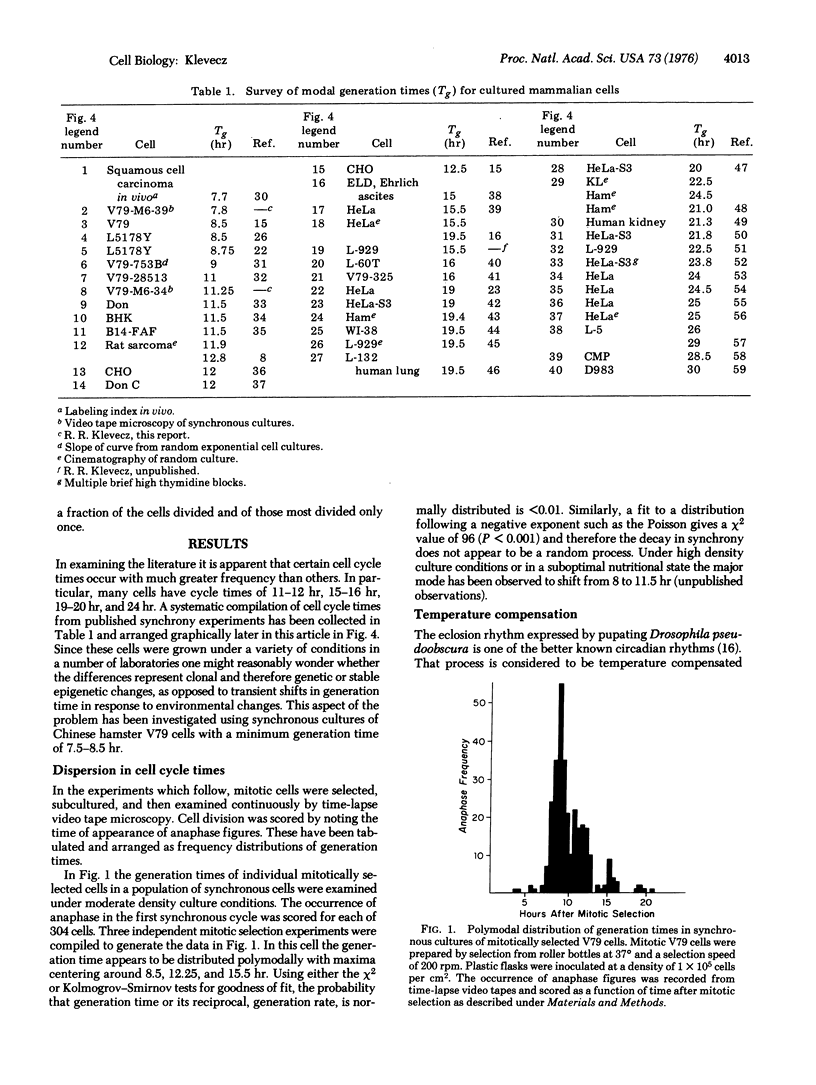
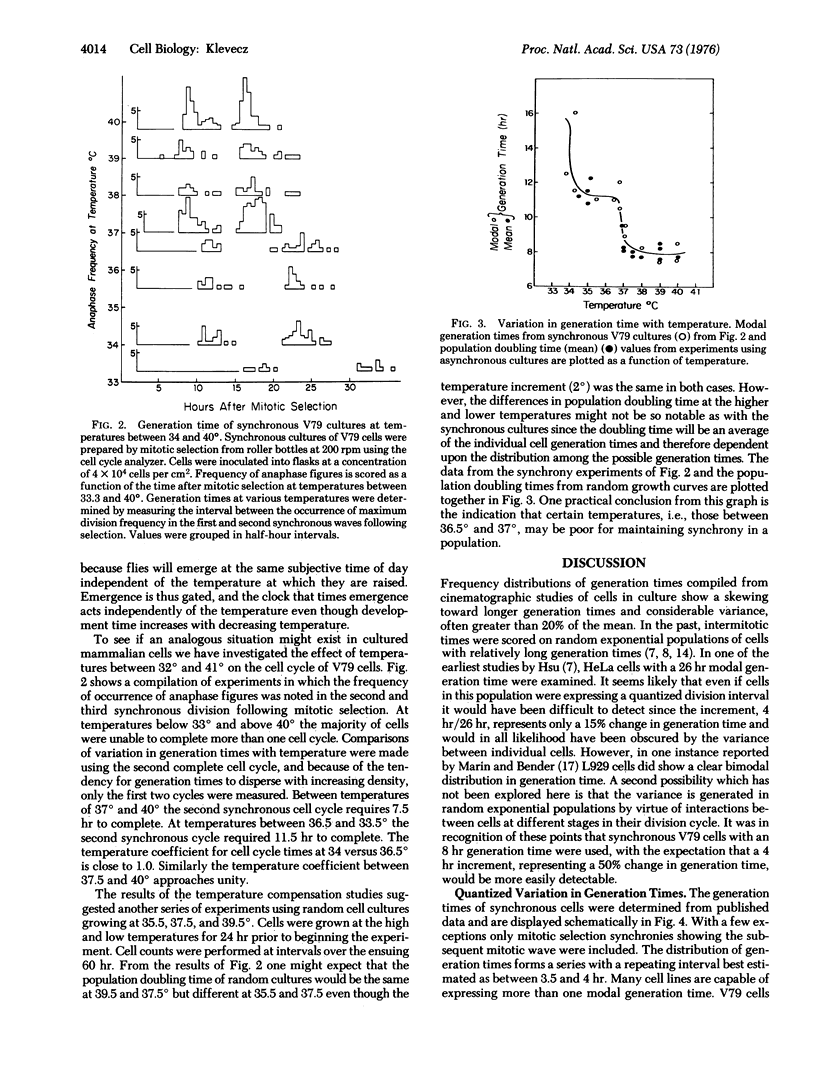
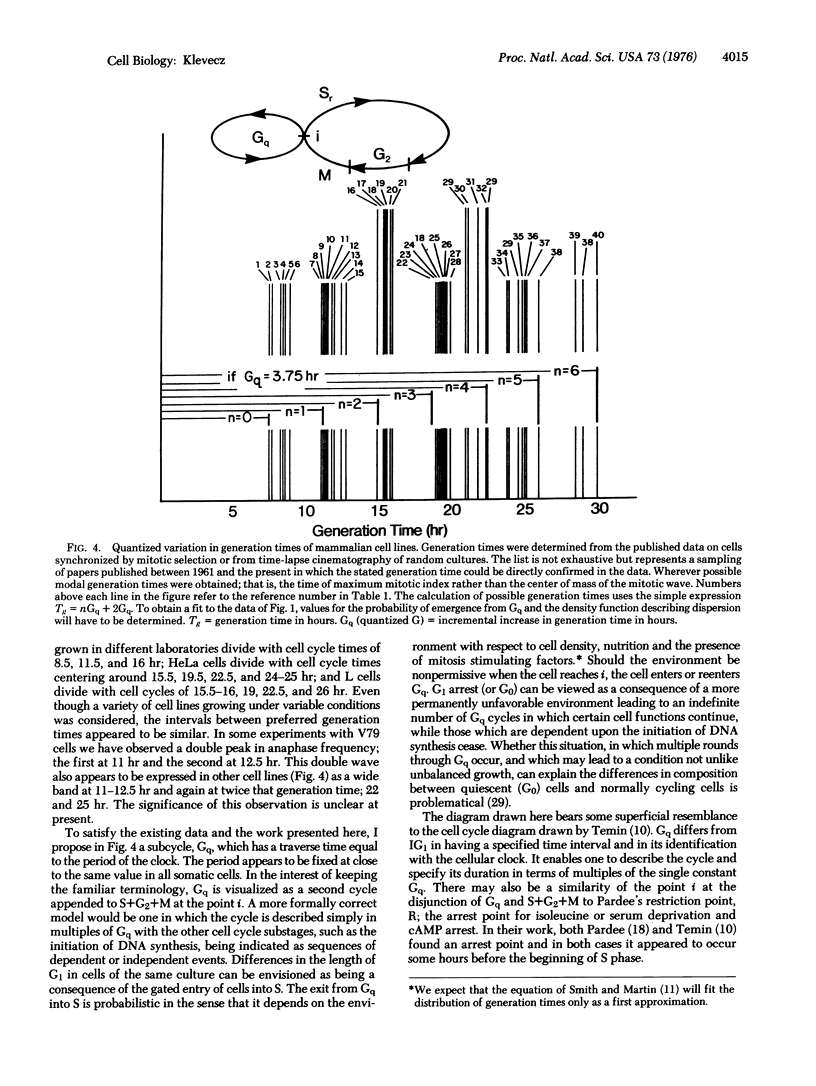
The distribution of possible generation times in mammalian cells does not appear to be continous within the limits of range for each cell type; rather, generation time is quantized in multiples of 3-4 hr. Synchronous cultures of Chinese hamster V79 cells were prepared using manual and automated methods to select and stage mitotic cells. Using synchronous cultures and time-lapse video tape microscopy, it was possible to show that generation times within a population of mitotically selected cells normally disperse in a quantized fashion, with intervals of 3-4 hr occurring between bursts in division. In addition, at temperatures above 37 degrees, V79 cells have a 7.5-8.5 hr modal cell cycle, while at temperatures from 36.5 degrees to 33.5 degrees the modal cell cycle is 11-12 hr long. A survey of the synchrony literature reveals that the tendency to preferred generation times holds between cell lines. The distribution of modal generation times from a variety of different cell types forms a series with a similar interval but with a greater range of values than that observed here for V79 cells. To satisfy the published data and the work presented here, I propose a subcycle, Gq, which has a traverse time equal to the period of the clock. The period appears to be fixed at close to the same value in all mammalian somatic cells. The timekeeping mechanism appears to be temperature compensated, since the time required to traverse Gq is constant at temperatures between 34 degrees and 39 degrees. It is suggested that cell cycle time increases at lower temperatures, lower serum concentration, and high cell densitite because the number of rounds of traverse through Gq increases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenlicht L. H., Baserga R. Changes in the G0 state of WI-38 fibroblasts at different times after confluence. Exp Cell Res. 1974 Dec;89(2):255–262. doi: 10.1016/0014-4827(74)90789-7. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Bernacki R. J. Glycosidase activity. Glycosidase acitivity in L5178Y mouse leukemic cells and the activity of acid phosphatase, beta-galactosidase and beta-N-acetylgalactosaminidase and beta-N-acetylglucosaminidase in a synchronous L5178Y cell population. Exp Cell Res. 1970 Aug;61(2):379–386. doi: 10.1016/0014-4827(70)90461-1. [DOI] [PubMed] [Google Scholar]
- Brent T. P., Butler J. A., Crathorn A. R. Effects of irradiation on synthesis of deoxyribonucleic acid and mitosis in synchronous cultures of HeLa cells. Nature. 1966 Apr 23;210(5034):393–395. doi: 10.1038/210393a0. [DOI] [PubMed] [Google Scholar]
- Brown J. M. The effect of acute x-irradiation on the cell proliferation kinetics of induced carcinomas and their normal counterpart. Radiat Res. 1970 Sep;43(3):627–653. [PubMed] [Google Scholar]
- Burns F. J., Tannock I. F. On the existence of a G 0 -phase in the cell cycle. Cell Tissue Kinet. 1970 Oct;3(4):321–334. doi: 10.1111/j.1365-2184.1970.tb00340.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., ESTABROOK R. W., GHOSH A. DAMPED SINUSOIDAL OSCILLATIONS OF CYTOPLASMIC REDUCED PYRIDINE NUCLEOTIDE IN YEAST CELLS. Proc Natl Acad Sci U S A. 1964 Jun;51:1244–1251. doi: 10.1073/pnas.51.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill J. R., Studzinski G. P. Thymidine as synchronizing agent. 3. Persistence of cell cycle patterns of phosphatase activities and elevation of nuclease activity during inhibition of DNA synthesis. J Cell Physiol. 1970 Jun;75(3):297–303. doi: 10.1002/jcp.1040750306. [DOI] [PubMed] [Google Scholar]
- DAWSON K. B., MADOC-JONES H., FIELD E. O. VARIATIONS IN THE GENERATION TIMES OF A STRAIN OF RAT SARCOMA CELLS IN CULTURE. Exp Cell Res. 1965 Apr;38:75–84. doi: 10.1016/0014-4827(65)90429-5. [DOI] [PubMed] [Google Scholar]
- Dewey W. C., Furman S. C., Miller H. H. Comparison of lethality and chromosomal damage induced by x-rays in synchronized Chinese hamster cells in vitro. Radiat Res. 1970 Sep;43(3):561–581. [PubMed] [Google Scholar]
- Djordjevic B., Djordjevic O. Chromosomal aberrations in synchronized mammalian cells treated with 5-bromo-deoxyuridine and irradiated by ultra-violet light. Nature. 1965 Jun 12;206(989):1165–1166. doi: 10.1038/2061165a0. [DOI] [PubMed] [Google Scholar]
- Elkind M. M., Kano E. Actinomycin D and radiation fractionation studies in asynchronous and synchronized Chinese hamster cells. Radiat Res. 1970 Nov;44(2):484–497. [PubMed] [Google Scholar]
- Engelberg J. On deterministic origins of mitotic variability. J Theor Biol. 1968 Aug;20(2):249–259. doi: 10.1016/0022-5193(68)90194-x. [DOI] [PubMed] [Google Scholar]
- Friedman S. J., Bellantone R. A., Canellakis E. S. Ornithine decarboxylase activity in synchronously growing Don C cells. Biochim Biophys Acta. 1972 Jan 28;261(1):188–193. doi: 10.1016/0304-4165(72)90329-7. [DOI] [PubMed] [Google Scholar]
- Galavazi G., Bootsma D. Synchronization of mammalian cells in vitro by inhibition of the DNA synthesis. II. Population dynamics. Exp Cell Res. 1966 Feb;41(2):438–451. doi: 10.1016/s0014-4827(66)80150-7. [DOI] [PubMed] [Google Scholar]
- Griffin M. J., Ber R. Cell cycle events in the hydrocortisone regulation of alkaline phosphatase in HeLa S3 cells. J Cell Biol. 1969 Feb;40(2):297–304. doi: 10.1083/jcb.40.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU T. C. Generation time of HeLa cells determined from cine records. Tex Rep Biol Med. 1960;18:31–33. [PubMed] [Google Scholar]
- Hand R. Regulation of DNA replication on subchromosomal units of mammalian cells. J Cell Biol. 1975 Jan;64(1):89–97. doi: 10.1083/jcb.64.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLANDER D., ZETTERBERG A. QUANTITATIVE CYTOCHEMICAL STUDIES ON INTERPHASE GROWTH. I. DETERMINATION OF DNA, RNA AND MASS CONTENT OF AGE DETERMINED MOUSE FIBROBLASTS IN VITRO AND OF INTERCELLULAR VARIATION IN GENERATION TIME. Exp Cell Res. 1965 May;38:272–284. doi: 10.1016/0014-4827(65)90403-9. [DOI] [PubMed] [Google Scholar]
- KUBITSCHEK H. E. Normal distribution of cell generation rate. Exp Cell Res. 1962 Mar;26:439–450. doi: 10.1016/0014-4827(62)90150-7. [DOI] [PubMed] [Google Scholar]
- Kapp L. N., Okada S. Factors affecting acid phosphatase activity in exponential and synchronized L5178Y mouse leukemia cells. Exp Cell Res. 1972 Jun;72(2):465–472. doi: 10.1016/0014-4827(72)90015-8. [DOI] [PubMed] [Google Scholar]
- Kasten F. H., Strasser F. F. Nucleic acid synthetic patterns in synchronized mammalian cells. Nature. 1966 Jul 9;211(5045):135–140. doi: 10.1038/211135a0. [DOI] [PubMed] [Google Scholar]
- Kelly F., Legator M. Effect of N-methyl-N'-nitro-N-nitrosoguanidine on the cell cycle and chromosomes of human embryonic lung cell. Mutat Res. 1970 Sep;10(3):237–246. doi: 10.1016/0027-5107(70)90120-x. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Stambuk B. K. Synchronization of HeLa cells by vinblastine sulfate. Exp Cell Res. 1966 Nov-Dec;44(2):631–634. doi: 10.1016/0014-4827(66)90470-8. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R., Kapp L. N. Intermittent DNA synthesis and periodic expression of enzyme activity in the cell cycle of WI-38. J Cell Biol. 1973 Sep;58(3):564–573. doi: 10.1083/jcb.58.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevecz R. R., Keniston B. A. The temporal structure of S phase. Cell. 1975 Jun;5(2):195–203. doi: 10.1016/0092-8674(75)90027-6. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R. Temporal coordination of DNA replication with enzyme synthesis in diploid and heteroploid cells. Science. 1969 Dec 19;166(3912):1536–1538. doi: 10.1126/science.166.3912.1536. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R. Temporal order in mammalian cells. I. The periodic synthesis of lactate dehydrogenase in the cell cycle. J Cell Biol. 1969 Nov;43(2):207–219. doi: 10.1083/jcb.43.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuv J., Sinclair W. K. X-ray sensitivity of synchronized Chinese hamster cells irradiated during hypoxia. Radiat Res. 1968 Oct;36(1):45–54. [PubMed] [Google Scholar]
- LaBella F. S., Sanwal M. Isolation of nerve endings from the posterior pituitary gland. Electron microscopy of fractions obtained by centrifugation. J Cell Biol. 1965 Jun;25(3 Suppl):179–193. doi: 10.1083/jcb.25.3.179. [DOI] [PubMed] [Google Scholar]
- Lange C. S. On the relative importance of repair and progressin in Elkind recovery as measured in synchronous HeLa cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(1):61–79. doi: 10.1080/09553007014550071. [DOI] [PubMed] [Google Scholar]
- Lesser B., Brent T. P. Cold storage as a method for accumulating mitotic HeLa cells without impairing subsequent synchronous growth. Exp Cell Res. 1970 Oct;62(2):470–473. doi: 10.1016/0014-4827(70)90580-x. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B. Radiosensitivity of Ehrlich ascites tumor cells. I. Variation in x-ray sensitivity during the cell-cycle. Int J Radiat Biol Relat Stud Phys Chem Med. 1968;14(2):133–148. doi: 10.1080/09553006814550941. [DOI] [PubMed] [Google Scholar]
- Marin G., Bender M. A. Radiation-induced mammalian cell death: lapse-time cinemicrographic observations. Exp Cell Res. 1966 Sep;43(2):413–423. doi: 10.1016/0014-4827(66)90068-1. [DOI] [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Noland B. J., Walters R. A., Tobey R. A., Hardin J. M., Shepherd G. R. Effects of ionizing radiation upon intracellular levels of soluble microtubule protein in cultured mammalian cells. Exp Cell Res. 1974 Apr;85(2):234–238. doi: 10.1016/0014-4827(74)90122-0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis T. Populations of interacting oscillators and circadian rhythms. J Theor Biol. 1969 Mar;22(3):418–436. doi: 10.1016/0022-5193(69)90014-9. [DOI] [PubMed] [Google Scholar]
- Pittendrigh C. S. Circadian systems. I. The driving oscillation and its assay in Drosophila pseudoobscura. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1762–1767. doi: 10.1073/pnas.58.4.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C. S. ON TEMPERATURE INDEPENDENCE IN THE CLOCK SYSTEM CONTROLLING EMERGENCE TIME IN DROSOPHILA. Proc Natl Acad Sci U S A. 1954 Oct;40(10):1018–1029. doi: 10.1073/pnas.40.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B. The effects of low concentrations of actinomycin D on the progress of cells through the cell cycle. Cell Tissue Kinet. 1970 Oct;3(4):335–347. doi: 10.1111/j.1365-2184.1970.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Scharff M. D., Robbins E. Synthesis of ribosomal RNA in synchronized HeLa cells. Nature. 1965 Oct 30;208(5009):464–466. doi: 10.1038/208464a0. [DOI] [PubMed] [Google Scholar]
- Sinclair W. K. Protection by cysteamine against lethal x-ray damage during the cell cycle of Chinese hamster cells. Radiat Res. 1969 Jul;39(1):135–154. [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward D. L., Humphrey R. M. Induction of thymine dimers in synchronized populations of Chinese hamster cells. Nature. 1966 Oct 15;212(5059):298–300. doi: 10.1038/212298b0. [DOI] [PubMed] [Google Scholar]
- Steward D. L., Shaeffer J. R., Humphrey R. M. Breakdown and assembly of polyribosomes in synchronized Chinese hamster cells. Science. 1968 Aug 23;161(3843):791–793. doi: 10.1126/science.161.3843.791. [DOI] [PubMed] [Google Scholar]
- TILL J. E., WHITMORE G. F., GULYAS S. Deoxyribonucleic acid synthesis in individual L-strain mouse cells. II. Effects of thymidine starvation. Biochim Biophys Acta. 1963 Jun 25;72:277–289. [PubMed] [Google Scholar]
- Temin H. M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971 Oct;78(2):161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- Walters R. A., Tobey R. A. Radiosensitivity of mammalian cells. IV. Effects of x-irradiation on the DNA synthetic period in synchronized cells. Biophys J. 1970 Jun;10(6):556–562. doi: 10.1016/S0006-3495(70)86319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmsley A. M., Phillips B., Pasternak C. A. The use of zonal centrifugation to study membrane formation during the life cycle of mammalian cells. Synthesis of 'marker' enzymes and other components of cellular organelles. Biochem J. 1970 Dec;120(4):683–688. doi: 10.1042/bj1200683. [DOI] [PMC free article] [PubMed] [Google Scholar]



