Abstract
Brain-derived neurotrophic factor (BDNF) is a neurotrophin hypothesized to play an important role in mammalian sleep expression and regulation. In order to investigate the role of the truncated receptor for BDNF, TrkB.T1, in mammalian sleep, we examined sleep architecture and sleep regulation in adult mice constitutively lacking this receptor. We find that TrkB.T1 knockout mice have increased REM sleep time, reduced REM sleep latency, and reduced sleep continuity. These results demonstrate a novel role for the TrkB.T1 receptor in sleep expression and provide new insights into the relationship between BDNF, psychiatric illness, and sleep.
Keywords: TrkB, TrkB.T1, brain-derived neurotrophic factor, neurotrophins, REM sleep
neurotrophins are neuropeptides that promote brain cell survival and synaptic plasticity (44). They have also been implicated in mammalian sleep (1, 12, 31, 33, 37, 43). Among the neurotrophins, substantial evidence supports a role for brain-derived neurotrophic factor (BDNF). BDNF can influence sleep by affecting sleep expression or sleep homeostasis, a regulatory process that controls the accumulation and discharge of sleep drive (2). For example, sleep deprivation (SD) increases the expression of BDNF mRNA in the rodent brain (6, 21, 37, 51). Intracerebroventricular injection of BDNF leads to increases in non-rapid eye movement (NREM) sleep in rats and increases both NREM and rapid eye movement (REM) sleep in rabbits (31). The effects of BDNF on indices of sleep need [e.g., NREM slow-wave activity (SWA) (2, 17)] are less clear. BDNF reduces NREM SWA when administered intracerebroventricularly (31) but triggers a localized increase in SWA when injected intracortically (12). Moreover, in humans a single nucleotide polymorphism that reduces BDNF release is associated with less stage 4 NREM sleep and lower NREM SWA (1).
The precise signaling pathways governing the effects of BDNF on sleep are unknown. BDNF exerts its effects through two predominant isoforms of the TrkB receptor (13, 24). One isoform contains a tyrosine kinase (TrkB.FL) and a second, truncated isoform lacks the kinase domain (TrkB.T1). TrkB.FL is predominantly expressed in neurons, whereas TrkB.T1 shows substantially higher expression in glia (13). This differential expression may be important for the effects of TrkB.T1. When expressed in neurons, TrkB.T1 is thought to decrease TrkB.FL function (10, 11). However, in astrocytes TrkB.T1 triggers intracellular signaling cascades [e.g., via Rho guanosine triphosphatases (GTPases) and inositol triphosphate-mediated calcium release (39, 46)], which may alter astrocyte function and morphology (8, 38). Astrocytes, in turn, play a role in sleep homeostasis and mediate cognitive impairments incurred by sleep loss (22). Both TrkB.FL and TrkB.T1 receptors are expressed in brain regions important for mammalian sleep regulation (50, 56), but their relative contribution to sleep is poorly understood.
To explore the role of the TrkB.T1 receptor in sleep, we examined sleep expression and homeostasis in mice constitutively lacking the TrkB.T1 receptor. TrkB.T1-null mice exhibit heightened anxiety and structural changes in the amygdala (5), yet sleep has not been previously examined in these mice. We find that TrkB.T1 knockout mice have normal NREM sleep time and NREM sleep homeostasis, but show multiple sleep alterations reported in mood disorders. These include increased REM sleep time, reduced REM sleep latency, and sleep fragmentation (i.e., shorter bouts of total sleep and wake), similar to the reduced sleep continuity reported in major depressive disorders (40). These results support a novel role of the TrkB.T1 in mammalian sleep and suggest that this BDNF receptor may provide a link between sleep abnormalities and psychiatric illness.
MATERIALS AND METHODS
Animals and surgery.
Transgenic C57/BL6 mice lacking the TrkB.T1 receptor (n = 10) and their wild-type siblings (n = 9) were obtained from Dr. Susan Dorsey (University of Maryland, Baltimore, MD). The generation of these mice has been previously described (10). Mice were housed in a satellite facility under standard environmental conditions (temperature: 22 ± 2°C, humidity: 30 ± 10%), maintained on a 12:12-h light-dark cycle with lights on at 0730 [zeitgeber time (ZT) 0], and provided food and water ad libitum. Male mice 5–7 mo in age underwent surgical implantation for polysomnographic sleep recordings. Briefly, animals were administered antibiotics and buprenorphine for pain management and then were anesthetized with isoflurane. Four stainless-steel wire EEG electrodes were implanted epidurally (A/P: +1.5 mm and −3.0 mm, M/L: ±1.7 mm, from bregma) and two EMG electrodes were inserted into the nuchal muscle, as previously described (14, 52). Animals were returned to their home cages, administered additional antibiotics and analgesics, and allowed to recover for 5 days prior to acclimation. All experiments were performed with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Office of Laboratory Animal Welfare Policy.
Polysomnographic recordings.
Each animal was singly housed under a strict 12:12-h light-dark cycle, connected to a lightweight, flexible cable and acclimated to recording conditions for 6 days. EEG and EMG signals were amplified and filtered (lowpass cutoffs at 100 Hz, highpass cutoffs at 0.3 and 10 Hz, respectively) on a model 15LT system (Astro-Med, West Warwick, RI), sampled at 256 Hz using Contec A/D cards and recorded with Vital Recorder (Kissei Comtec America, Irvine, CA).
We investigated sleep during baseline conditions and again in response to SD in order to determine the homeostatic response to sleep loss. Following 24 h of baseline recording, mice were sleep-deprived (SD) for 6 h beginning at lights on using gentle handling. Procedures used to keep the mice awake consisted of light brushing, tapping on cages, and the introduction of novel objects (17). We analyzed the 18-h recovery period immediately following SD for changes in sleep architecture and EEG activity.
Vigilance state scoring and analysis.
We scored EEG and EMG recordings using SleepSign (Kissei Comtec) in 4-s epochs as wake, NREM sleep, or REM sleep according to previously reported methods (22). We first investigated the mean EEG power spectra of each state to ensure that no differences between knockout and wild-type mice affected the proper scoring of states. We performed a fast Fourier transform on EEG epochs convolved with a Hanning window to determine the signal power in frequencies from 0.5 to 20 Hz. The power spectra for each vigilance state was expressed as a percentage of the total power across all frequencies and states during the baseline recording (14). Epochs containing artifacts were excluded from spectral analysis. NREM SWA (0.5–4.0 Hz) was averaged in 2-h bins for baseline and recovery periods. SWA for individual animals was expressed as a percentage of values in the final 4 h of the baseline light phase, as described previously (16).
We next investigated sleep and wake architecture. Vigilance state time was expressed as a percentage of total recording time. “Total sleep” was calculated as combined NREM and REM sleep time. We calculated vigilance state bout frequency and duration according to previously published methods (52). Bouts of wake and NREM sleep were identified as consecutive epochs longer than 30 s in duration, while REM sleep bouts were identified as consecutive epochs greater than 20 s. Total sleep bouts were identified as 30 s or more of either NREM or REM sleep. A bout was considered terminated when it was interrupted by 30 s of any other state (e.g., the presence of 30 s of wakefulness in an ongoing NREM or total sleep bout) (52). We calculated these measures over 24 h, as well as separately for the light phase and dark phase. REM sleep latency was also calculated as previously described (52). Briefly, REM sleep latency was defined as the duration of NREM sleep preceding a bout of REM sleep, interrupted by periods of wakefulness shorter than 30 s. Initial inspection of the bout duration and REM latency data indicated that these data were not normally distributed. Therefore, we analyzed these data with empirical cumulative distribution functions using the ecdf function of the MatLab Statistics Toolbox (MathWorks, Natick, MA). All bouts for each vigilance state [in the 24-h baseline or 18-h recovery period after total sleep deprivation] were pooled across animals for each genotype.
NREM sleep homeostasis.
We calculated several metrics to identify potential differences in sleep homeostasis between genotypes. NREM SWA following SD was averaged in 2-h bins and normalized to the last 4 h of the baseline period (16). We then determined changes in total, NREM, and REM sleep time for the entire recording period and in 2-h bins (17). Compensatory changes in bout duration were also examined in a comparable manner for the entire 18-h recovery period (19).
REM sleep homeostasis.
In order to investigate REM sleep homeostasis directly, a subset of mice (n = 4 each genotype) underwent 6 h of selective REM sleep deprivation (RSD) 2 mo after the total sleep deprivation experiment. RSD began 2 h into the light phase when REM sleep pressure is highest (26). Vigilance state was monitored visually in real time using EEG and EMG, and mice were gently awakened upon entering REM sleep (26). The 4 h immediately following RSD were analyzed for compensatory changes in REM sleep time.
Immunofluorescence.
We collected brain tissue from the mice used in sleep experiments to examine regional TrkB.T1 receptor expression. Mice were anesthetized with isoflurane and euthanized with pentobarbital sodium (200 mg/kg) and then perfused transcardially with 20 ml of ice-cold PBS followed by 20 ml of ice-cold 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA). Brains were removed, postfixed overnight in 4% PFA in PBS at 4°C, washed and cryoprotected in 30% sucrose in PBS at 4°C. Tissue was frozen, cut into 30-μm coronal sections with a cryostat (Leica Microsystems, Buffalo Grove, IL), and processed as free-floating sections for immunofluorescence. Slices were washed with 0.25% Triton X-100 (Roche Diagnostics, Indianapolis, IN) in PBS-Tween (PBS-T), blocked with 5% normal donkey serum (Sigma-Aldrich, St. Louis, MO), and incubated with primary antibodies in PBS-T with 5% serum at 4°C overnight. We used a rabbit polyclonal antibody to TrkB.T1 (Santa Cruz Biotechnology, Dallas, TX; 1:50) and a goat polyclonal antibody to choline acetyltransferase (Millipore, Billerica, MA; 1:250) as primary antibodies. Slices were incubated with donkey Alexa fluorophore-conjugated secondary antibodies (Life Technologies, Grand Island, NY; 1:250) and coverslipped with Vectashield mounting media (Vector Laboratories, Burlingame, CA). Images were acquired sequentially with an SP5 laser-scanning confocal microscope (Leica Microsystems) using identical settings for images from both genotypes.
Statistical analyses.
We compared measures from wild-type and TrkB.T1 knockout mice with two-factor, repeated-measures ANOVAs with factors “genotype” and “time” when more than two comparisons were made. To evaluate the effects of selective REM sleep deprivation, we used repeated-measures ANOVAs with factors “recording day” and “time” within each genotype. We used the Holm-Sidak post hoc test when significant main effects or interactions were found using ANOVA. Wilcoxon-Mann-Whitney tests were used for single comparisons between genotypes since data were frequently nonparametric. We used Kolmogorov-Smirnov (K-S) tests to compare the distributions of bout lengths between genotypes. Statistics were calculated using SigmaPlot 11.0 (Systat Software, San Jose, CA) and the MatLab Statistics Toolbox (MatLab version R2013a; MathWorks).
RESULTS
Baseline spectral analysis, vigilance state time and sleep architecture.
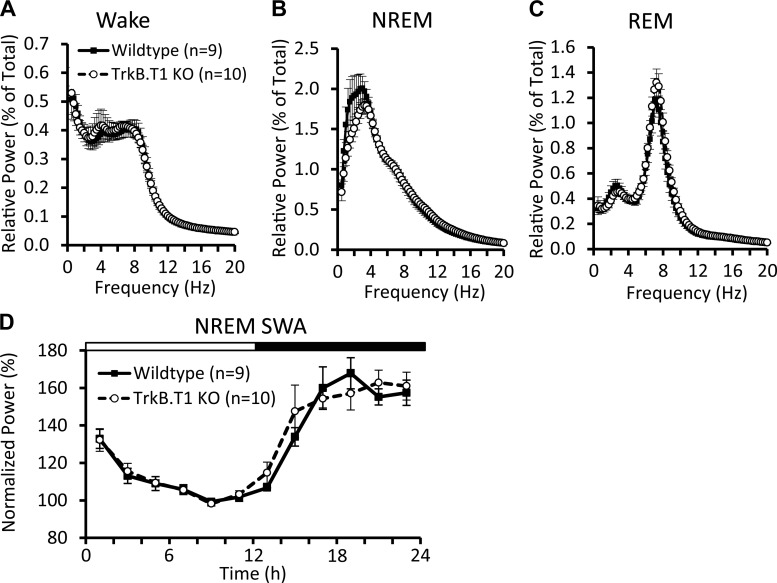
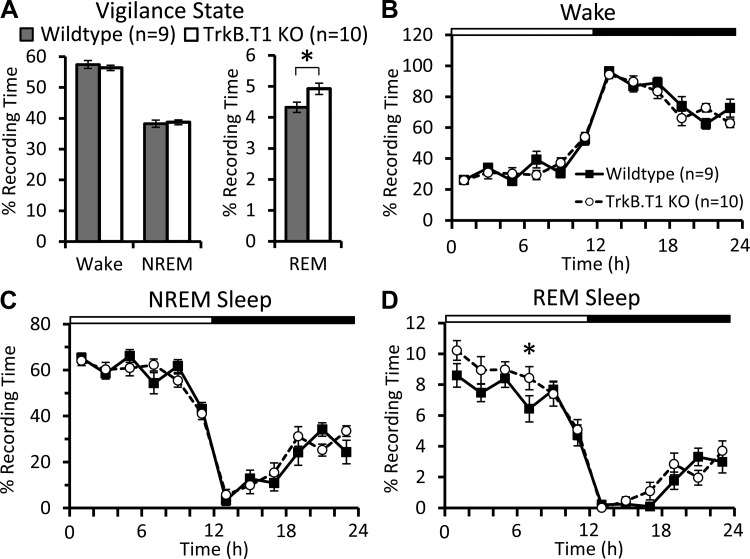
Spectral analysis of sleep and wake EEG revealed no significant differences between the knockout and wild-type mice (Fig. 1, A–C), nor any significant differences in the variation of NREM SWA across the 24-h day (Fig. 1D). There were also no differences in Wake or NREM sleep time as a percentage of total recording time (Fig. 2, A–C). However, we did find that knockout mice spent significantly more time in REM sleep (Fig. 2A). This difference in REM sleep was most apparent in the light phase (Table 1; Fig. 2D).
Fig. 1.
EEG power spectra and non-rapid eye movement (NREM) sleep slow-wave activity (SWA) during the baseline recording. Normalized EEG power during wake (A), NREM (B), and rapid eye movement (REM; C) sleep, expressed as a percentage of total power across all states. TrkB.T1 knockout and wild-type mice have comparable EEG spectra in all three states. D: NREM SWA across the baseline period, normalized to the NREM SWA in the final 4 h of the light phase. TrkB.T1 knockout mice show similar variation in SWA across the light-dark cycle. Values are expressed as means ± SE.
Fig. 2.
Vigilance state time during the baseline period. A: time spent in wake, NREM, and REM sleep during the 24-h baseline period. TrkB.T1 knockout mice spend significantly more time in REM sleep compared with wild-type siblings (Mann-Whitney U-test, *P < 0.05). Wake (B), NREM (C) and REM (D) sleep time across the baseline period, analyzed in 2-h bins. D: two-way repeated-measures ANOVA with factors “genotype” and “time” revealed that knockout mice spend significantly more time in REM than wild-type mice [Fgenotype(1,17) = 5.867; P = 0.027]. White and black bars at top represent the light-dark cycle. Values are expressed as means ± SE.
Table 1.
Vigilance state time and bout duration in TrkB.T1 knockout and wild-type mice
| Time (% Recording) |
Bout Duration (Minutes) |
Bouts (Count) |
||||
|---|---|---|---|---|---|---|
| L | D | L | D | L | D | |
| Baseline | ||||||
| Total Sleep | ||||||
| WT | 65.4 ± 1.1 | 19.7 ± 1.9 | 19.2 ± 2.7 | 12.7 ± 2.3 | 28.6 ± 3.9 | 13.8 ± 2.3 |
| KO | 65.5 ± 1.7 | 21.8 ± 1.3 | 17.0 ± 2.5 | 9.0 ± 1.0 | 34.5 ± 6.0 | 20.3 ± 3.7 |
| Wake | ||||||
| WT | 34.6 ± 1.1 | 80.3 ± 1.9 | 7.7 ± 1.0 | 51.2 ± 17.1 | 30.8 ± 4.0 | 16.2 ± 2.7 |
| KO | 34.5 ± 1.7 | 78.2 ± 1.3 | 6.5 ± 0.7 | 30.4 ± 4.4 | 37.6 ± 5.9 | 22.1 ± 3.7 |
| NREM | ||||||
| WT | 58.2 ± 1.0 | 18.3 ± 1.8 | 7.5 ± 0.4 | 7.8 ± 0.6 | 56.6 ± 2.7 | 17.9 ± 2.2 |
| KO | 57.3 ± 1.6 | 20.1 ± 1.3 | 7.1 ± 0.5 | 6.4 ± 0.5 | 60.6 ± 4.4 | 24.2 ± 3.1 |
| REM | ||||||
| WT | 7.2 ± 0.3* | 1.4 ± 0.2 | 1.4 ± 0.1 | 1.5 ± 0.1 | 37.2 ± 1.8 | 7.1 ± 0.8 |
| KO | 8.2 ± 0.3* | 1.7 ± 0.2 | 1.5 ± 0.0 | 1.5 ± 0.1 | 38.8 ± 1.7 | 7.7 ± 0.8 |
| Recovery | ||||||
| Total Sleep | ||||||
| WT | 70.4 ± 1.0 | 29.1 ± 2.2 | 24.0 ± 2.6 | 10.9 ± 0.8 | 11.3 ± 1.2 | 19.6 ± 1.8 |
| KO | 72.5 ± 2.1 | 33.3 ± 3.1 | 21.2 ± 3.0 | 11.4 ± 1.1 | 14.1 ± 1.8 | 23.4 ± 3.8 |
| Wake | ||||||
| WT | 29.6 ± 1.0 | 70.9 ± 2.2 | 8.0 ± 0.7 | 20.3 ± 1.7 | 12.1 ± 1.2 | 21.4 ± 1.7 |
| KO | 27.5 ± 2.1 | 66.7 ± 3.1 | 6.4 ± 0.8 | 20.7 ± 4.0 | 15.0 ± 2.0 | 25.9 ± 3.7 |
| NREM | ||||||
| WT | 61.7 ± 1.1 | 26.3 ± 2.0 | 7.8 ± 0.3 | 6.3 ± 0.2 | 28.2 ± 0.7 | 29.8 ± 2.1 |
| KO | 62.8 ± 1.9 | 29.5 ± 2.8 | 7.3 ± 0.3 | 6.4 ± 0.2 | 30.9 ± 1.6 | 33.5 ± 3.7 |
| REM | ||||||
| WT | 8.8 ± 0.2 | 2.8 ± 0.2* | 1.5 ± 0.1 | 1.3 ± 0.1* | 21.4 ± 0.7 | 15.4 ± 1.2 |
| KO | 9.7 ± 0.4 | 3.8 ± 0.3* | 1.5 ± 0.1 | 1.5 ± 0.1* | 22.8 ± 1.3 | 17.9 ± 1.5 |
Values are expressed as means ± SE. Sleep parameters were determined by polysomnography during a baseline recording and during recovery from sleep deprivation.
L, light cycle; D, dark cycle; WT, wild-type (n = 9); KO, TrkB.T1 knockout (n = 10).
P < 0.05 between WT and KO values, Mann-Whitney U-test.
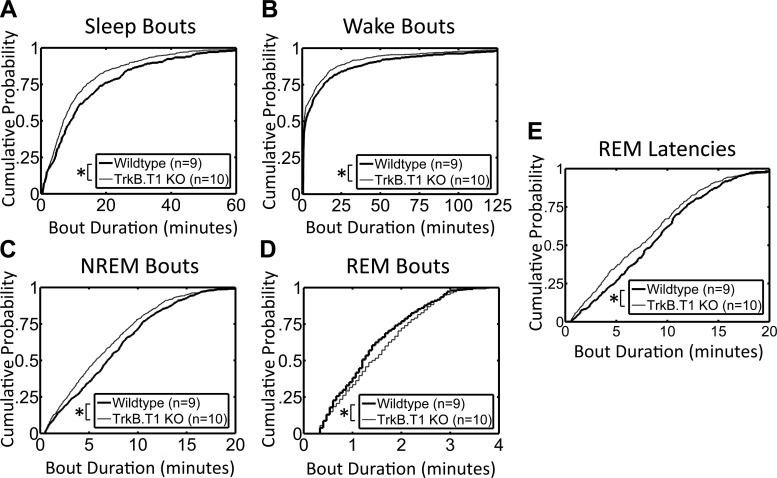
Vigilance state bout durations and REM latencies were also different between the two groups of mice. Mean values for the vigilance states were similar between the two groups of mice (Table 1), but cumulative histogram analyses revealed that total sleep, Wake, and NREM sleep bouts were significantly skewed toward shorter duration in the knockout mice (Fig. 3, A–C). In contrast, the distribution of REM bouts was skewed toward longer duration (Fig. 3D). REM sleep latencies were also significantly skewed to shorter duration in the TrkB.T1 knockout mice (Fig. 3E).
Fig. 3.
Vigilance state bout durations during the baseline period. Total sleep (A), wake (B), and NREM sleep (C) bouts are significantly different in TrkB.T1 knockout mice compared with wild-type (*P < 0.05, Kolmogorov-Smirnov tests). Bouts during all three states are skewed toward shorter duration in knockout mice. D: REM sleep bouts in knockout mice are skewed toward longer duration (*P < 0.05, Kolmogorov-Smirnov test). E: distribution of REM sleep latencies in knockout mice is significantly skewed to shorter latencies (*P < 0.05, Kolmogorov-Smirnov test).
Sleep homeostasis in the TrkB.T1 knockout mice.
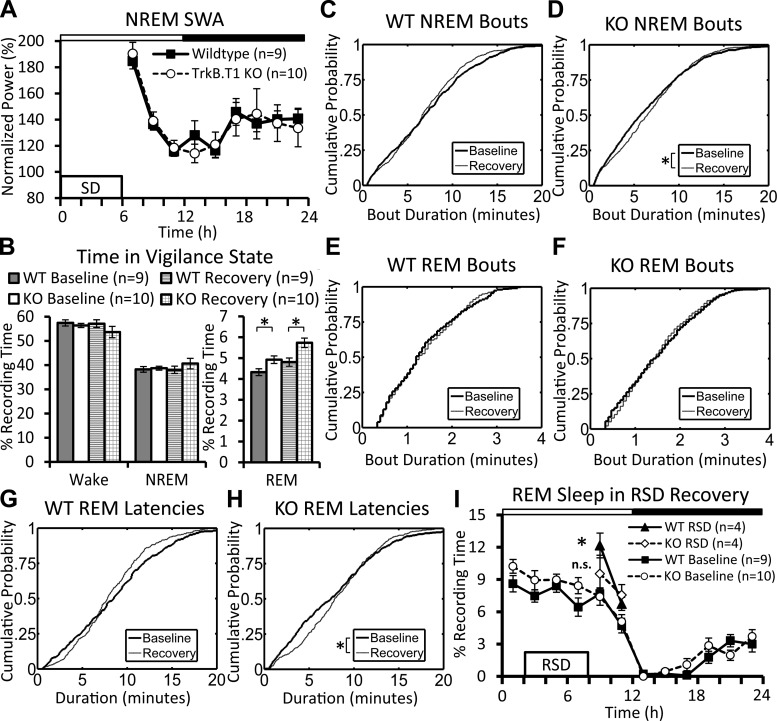
We examined several indices of sleep homeostasis to determine whether sleep regulation was altered in TrkB.T1 knockout mice. There was no difference in the effectiveness of SD between knockout and wild-type mice (wake time during SD: 98.0 ± 0.6% and 99.0 ± 0.4%, respectively; means ± SE). As shown in Fig. 4A, wild-type and knockout mice responded with a similar increase in SWA during the recovery period. We also examined compensatory increases in sleep time following SD. When hours ZT 6 to ZT 24 were compared, the two groups of mice also showed similar compensatory increases in total sleep time between the baseline and recovery periods (knockout: 34.5 ± 1.0% and 46.3 ± 2.3%; wild-type mice: 32.9 ± 1.4% and 42.9 ± 1.6%; P < 0.001, Mann-Whitney U-tests). There were also no significant differences in compensatory changes in wake or NREM sleep time between the groups of mice (Fig. 4B). REM sleep remained elevated in knockout mice during the recovery period compared with wild-type (Fig. 4B). This was mainly restricted to the latter half of the dark phase (Table 1). A cumulative histogram analyses of bout durations showed that NREM sleep bouts in knockout (but not wild-type) mice were significantly skewed to longer durations during the recovery period (relative to baseline, Fig. 4D). In addition, average REM sleep bout durations were significantly higher during the dark period in knockout mice relative to wild-type values (Table 1). REM sleep latencies were also skewed toward longer latencies in the knockout mice relative to baseline (Fig. 4H), but this did not occur in wild-type mice (Fig. 4G).
Fig. 4.
Homeostatic response to sleep deprivation (SD) and selective REM sleep deprivation (RSD). A: NREM SWA during the 18-h recovery from 6 h of total SD. TrkB.T1 knockout and wild-type mice show a comparable homeostatic increase in SWA following SD. SWA is normalized to the final 4 h of the baseline light phase; values are expressed as means ± SE. B: time spent in wake, NREM, and REM sleep during the baseline and 18-h recovery periods. Values from the recovery period are plotted along with 24-h baseline values from Fig. 2A. Knockout mice spend significantly more time in REM sleep than wild-type siblings in baseline and recovery (Mann-Whitney U-tests, *P < 0.05). Values are expressed as means ± SE. C–F: cumulative distribution of NREM (C, D) and REM (E, F) sleep bouts during the baseline and recovery periods, plotted separately for wild-type (C, E) and knockout (D, F) mice. D: distribution of NREM sleep bout durations in the knockout mice is significantly skewed toward longer durations in the knockout mice (between baseline and recovery, *P < 0.05, Kolmogorov-Smirnov test). G and H: cumulative distribution of REM sleep latencies between baseline and recovery in wild-type (G) and knockout (H) mice. G: there was no difference between the distribution of REM sleep latencies in wild-type mice. H: distribution of REM sleep latencies is significantly skewed to longer duration during recovery in knockout mice (*P < 0.05, Kolmogorov-Smirnov test). I: REM sleep time following selective RSD. The 4-h recovery period is plotted along with baseline values from Fig. 2D. Comparisons using two-way repeated-measures ANOVAs with factors “recording day” and “time” revealed that wild-type mice display significantly more REM sleep during RSD recovery than baseline [Frecording day(1,3) = 76.88; P = 0.003], whereas the knockout mice displayed no such increase [Frecording day(1,3) = 4.127; P = 0.135]. Values are expressed as means ± SE.
We performed a supplementary selective RSD experiment to more directly probe REM sleep homeostasis in the TrkB.T1 knockout mice. There was no difference in the effectiveness of the 6-h RSD between knockout and wild-type mice (REM sleep time during RSD: 2.2 ± 0.4% and 2.7 ± 0.5%, respectively; mean ± SE). Wild-type mice showed a significant increase above baseline values in REM sleep during recovery from RSD, whereas the knockout mice displayed a blunted rebound (Fig. 4I).
TrkB.T1 immunolabeling.
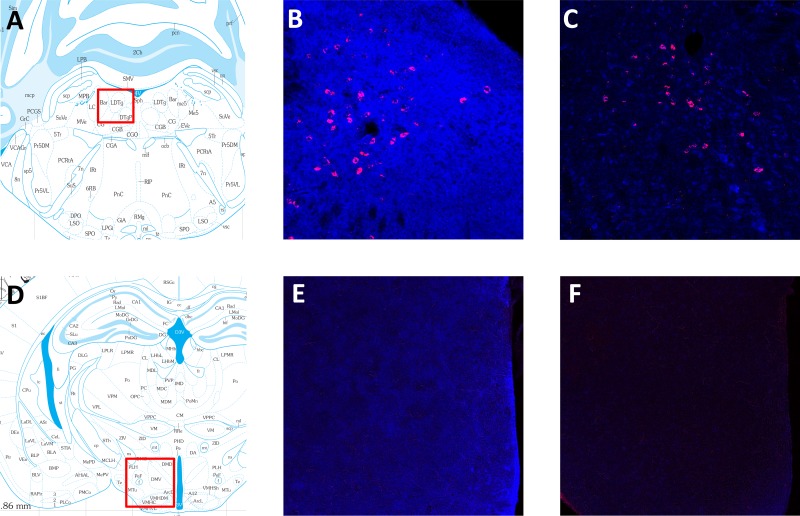
We examined brain slices from wild-type and knockout mice labeled for TrkB.T1 to determine regions where the loss of TrkB.T1 may affect REM sleep. Widespread TrkB.T1 labeling was observed in wild-type mice, and selected brain areas known to play a role in REM sleep regulation were examined further (36, 49). In wild-type mice, TrkB.T1 labeling was observed in several brain stem nuclei, including the cholinergic neurons of the laterodorsal tegmental area (LDT; Fig. 5B) and pedunculotegmental nucleus (data not shown). Additionally, the caudal pontine and oral pontine reticular nuclei, subcoeruleus nucleus and ventrolateral periaqueductal gray all displayed substantial TrkB.T1 labeling (data not shown). In the forebrain, wild-type mice also displayed TrkB.T1 expression in the lateral hypothalamus (Fig. 5E), preoptic hypothalamus, and amygdala (data not shown), although at lower intensity than in the brain stem. As expected, TrkB.T1 labeling in the brain stem and forebrain of knockout mice was at background levels (Fig. 5, C and F).
Fig. 5.
TrkB.T1 immunofluorescence in wild-type and TrkB.T1 knockout mice. A–C: TrkB.T1 (blue) and choline acetyltransferase (ChAT; red) immunofluorescence labeling in the caudal laterodorsal tegmental nucleus (LDT). A: approximate location (red box) where images of the LDT were taken. B: Wild-type mice show intense TrkB.T1 labeling around the LDT, indicated by ChAT neurons. C: labeling is at background levels in TrkB.T1 knockout mice. D–F: TrkB.T1 labeling in the lateral hypothalamus (LH). D: approximate location (red box) of images of the LH. E: wild-type mice show moderate TrkB.T1 labeling. F: labeling is at background levels in TrkB.T1 knockout mice. Atlas images in A and D were adapted from Ref. 41, and definition of additional brain regions can be found there.
DISCUSSION
In this study, we determined whether the neurotrophin receptor TrkB.T1 plays a role in sleep. We found that TrkB.T1 deletion increases REM sleep time, REM bout duration, and sleep fragmentation, and decreases REM sleep latency. Deletion of TrkB.T1 also resulted in subtle abnormalities in sleep homeostasis. NREM SWA (a classic index of sleep homeostasis) was similar in wild-type and knockout mice in the baseline period and after sleep deprivation. However, knockout mice showed changes in NREM bout duration and REM sleep latencies during recovery that were not observed in wild-type mice. Selective REM sleep deprivation also produced an attenuated “REM rebound” in knockout mice. While we cannot exclude the possibility that embryonic deletion of the TrkB.T1 receptor influenced the adult phenotype, this seems unlikely for the following reasons. Overt developmental abnormalities are not observed in TrkB.T1 knockout mice (5), and indirect compensatory effects are limited by the late postnatal onset of TrkB.T1 expression (50). We discuss the implications of our main findings below.
These results provide new insights into the role of BDNF in sleep expression and regulation. Whole brain and cortical BDNF protein and mRNA increase with wakefulness (6, 21, 51). BDNF increases NREM sleep time when administered intracerebroventricularly (31) and may influence NREM SWA (1, 12, 25, 31). The fact that TrkB.T1 deletion had no impact on NREM sleep time or NREM SWA indicates that TrkB.T1 does not play a major role in these aspects of sleep. On the other hand, NREM sleep bouts after SD were skewed toward longer duration in knockout mice (but not wild-type), suggesting that other differences in compensation may exist. An additional, unusual finding is the fragmentation of sleep combined with increased REM sleep time, longer REM bout duration, and decreased REM sleep latency in the TrkB.T1 knockout mice. Other mutant mice exhibit altered REM sleep time or bout duration (3, 9, 27, 28), and sleep fragmentation itself is a relatively common finding among studies of knockout mice (reviewed in Ref. 29). Consequently, it is possible that the changes in REM sleep and sleep fragmentation are unrelated, or mediated, by multiple effects of a constitutive knockout. To our knowledge, however, this entire suite of changes has not been previously found following deletion of a single membrane-bound receptor.
Our results provide new clues about the relationship between sleep, BDNF and psychiatric illness. Manipulating the expression or activity of TrkB isoforms has been used to explore mouse models of major depressive disorder (MDD) (reviewed in Ref. 33), but the relationship between these models and sleep has not been explored. We demonstrate that TrkB.T1 knockout mice display increased REM sleep and REM bout duration, decreased latency to REM sleep, and abnormal REM sleep regulation. These sleep abnormalities are reported in depressed patients and animal models of depression (42, 53), suggesting that reduced signaling through TrkB.T1 may mediate some changes in sleep architecture associated with psychiatric illness. Moreover, the 15–20% increase in REM sleep above wild-type values that TrkB.T1 knockout mice exhibit is similar to the increase observed in rodent models of MDD (53) and human studies of MDD (18, 45). It is unknown whether this amount of REM sleep directly influences MDD or is merely a risk factor for its progression. However, it is interesting to note that the majority of antidepressant drugs suppress REM sleep, and normalization of REM sleep parameters is a strong correlate of remission (40). Our results were obtained in male mice, and it will be important to examine sleep in female TrkB.T1 knockout mice in the future given the higher prevalence of MDD in women.
Our findings also provide insights into the relationship between stress and changes in REM sleep. Stress-mediated activation of the amygdala is known to influence REM sleep, as mice exposed to a controllable stressor exhibit increased REM sleep time (48). This increase is thought to result from heightened activity in the central nucleus of the amygdala and its projections to brain stem nuclei important for sleep (47). Interestingly, TrkB.T1 knockout mice spend less time in the open arm of the elevated plus maze (5), an indication of increased anxiety, and are known to have altered neuronal morphology in the basolateral amygdala (5). Thus, our results link BDNF receptor-dependent changes in anxiety to sleep abnormalities seen in mood disorders.
Perspectives and Significance
Our results raise several interesting questions. First, where in the brain do TrkB.T1 receptors exert their effects on sleep? Brain stem nuclei are central to REM sleep generation and regulation (reviewed in Refs. 36 and 49). In the brain stem, the neurotrophins NGF and NT-3 are known to elicit REM sleep when injected into the pontine brain stem of the cat (54, 55). It is unknown whether BDNF also induces REM sleep when injected in a similar fashion, but extensive labeling for TrkB was reported in the same region as receptors for NGF and NT-3 (56). Other brain regions that influence REM sleep in rodents include the lateral hypothalamus (7), extended ventrolateral preoptic nucleus (35), median preoptic nucleus (20), and central nucleus of the amygdala (48). We find that the TrkB.T1 receptor is highly expressed in comparable brain stem and forebrain regions in mice (Fig. 3), which is suggestive of brain regions where the loss of TrkB.T1 may influence REM sleep.
A second question is what are the cellular events triggered by TrkB.T1 that alter sleep? The deletion of TrkB.T1 likely has two effects. The first is a loss of dominant-negative inhibition of the TrkB.FL receptor in neurons, the second is the absence of TrkB.T1-mediated signaling cascades in astrocytes. Regarding the first possibility, there is evidence that the effects of BDNF on sleep depend on tyrosine kinase activation. Specifically, the increase in NREM SWA after cortical BDNF injection is prevented by coadministration of the tyrosine kinase inhibitor K252a (12). The second possibility is that disruption of TrkB.T1 signaling in astrocytes contributes to our results. TrkB.T1 signaling mechanisms in astrocytes include the activation of Rho GTPases (39), inositol triphosphate-mediated intracellular calcium release (46), and the production of nitric oxide (8). The intracellular release of calcium from astrocytes is thought to trigger the release of glial signaling molecules (23), including, possibly, several cytokines and growth factors known to influence sleep (15, 30, 32). This suggests that the deletion of TrkB.T1 may reduce the release of glial signaling molecules, which has been implicated in MDD (4).
GRANTS
This work was supported by the National Institutes of Health Grant MH-099544 to M. G. Frank.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.J.W., S.G.D., and M.G.F. conception and design of research; A.J.W. and K.H. performed experiments; A.J.W. analyzed data; A.J.W. and M.G.F. interpreted results of experiments; A.J.W. prepared figures; A.J.W. and M.G.F. drafted manuscript; A.J.W., S.G.D., and M.G.F. edited and revised manuscript; A.J.W., S.G.D., and M.G.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Tammi Coleman for her technical expertise and Jason Gerstner for helpful comments.
REFERENCES
- 1.Bachmann V, Klein C, Bodenmann S, Schäfer N, Berger W, Brugger P, Landolt HP. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep 35: 335–344, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borbély AA. A two process model of sleep regulation. Hum Neurobiol 1: 195–204, 1982. [PubMed] [Google Scholar]
- 3.Boutrel B, Monaca C, Hen R, Hamon M, Adrien J. Involvement of 5-HT1A receptors in homeostatic and stress-induced adaptive regulations of paradoxical sleep: studies in 5-HT1A knock-out mice. J Neurosci 22: 4686–4692, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, Yan HC, Gao YB, Liu JH, Li XW, Sun LR, Zeng YN, Zhu XH, Gao TM. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med 19: 773–777, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Carim-Todd L, Bath KG, Fulgenzi G, Yanpallewar S, Jing D, Barrick a C, Becker J, Buckley H, Dorsey SG, Lee FS, Tessarollo L. Endogenous truncated TrkB. T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J Neurosci 29: 678–685, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res 885: 303–321, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Clément O, Sapin E, Libourel PA, Arthaud S, Brischoux F, Fort P, Luppi PH. The lateral hypothalamic area controls paradoxical (REM) sleep by means of descending projections to brainstem GABAergic neurons. J Neurosci 32: 16,763–16,774, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo E, Cordiglieri C, Melli G, Newcombe J, Krumbholz M, Parada LF, Medico E, Hohlfeld R, Meinl E, Farina C. Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. J Exp Med 209: 521–535, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deboer T, Fontana A, Tobler I. Tumor necrosis factor (TNF) ligand and TNF receptor deficiency affects sleep and the sleep EEG. J Neurophysiol 88: 839–846, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dorsey SG, Renn CL, Carim-Todd L, Barrick CA, Bambrick L, Krueger BK, Ward CW, Tessarollo L. In vivo restoration of physiological levels of truncated TrkB. T1 receptor rescues neuronal cell death in a trisomic mouse model. Neuron 51: 21–28, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci 16: 3123–3129, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci 28: 4088–4095, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenner BM. Truncated TrkB: beyond a dominant negative receptor. Cytokine Growth Factor Rev 23: 15–24, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Frank MG, Stryker MP, Tecott LH. Sleep and sleep homeostasis in mice lacking the 5-HT2c receptor. Neuropsychopharmacology 27: 869–873, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank MG. Astroglial regulation of sleep homeostasis. Curr Opin Neurobiol 23: 812–818, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci 21: 2610–2621, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franken P, Dijk DJ, Tobler I, Borbély AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol Regul Integr Comp Physiol 261: R198–R208, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Gann H, Riemann D, Hohagen F, Dressing H, Müller WE, Berger M. The sleep structure of patients with anxiety disorders compared with that of healthy controls and depressive patients under baseline conditions and after cholinergic stimulation. J Affect Disord 26: 179–189, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Gvilia I, Suntsova N, Angara B, McGinty D, Szymusiak R. Maturation of sleep homeostasis in developing rats: a role for preoptic area neurons. Am J Physiol Regul Integr Comp Physiol 300: R885–R894, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci 26: 3037–3044, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hairston IS, Peyron C, Denning DP, Ruby NF, Flores J, Sapolsky RM, Heller HC, O'Hara BF. Sleep deprivation effects on growth factor expression in neonatal rats: a potential role for BDNF in the mediation of delta power. J Neurophysiol 91: 1586–1595, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Halassa MM, Florian C, Fellin T, Munoz JR, Abel T, Haydon PG, Frank MG, Lee SY. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61: 213–219, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11: 227–238, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72: 609–642, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep 30: 129–139, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Jego S, Salvert D, Renouard L, Mori M, Goutagny R, Luppi PH, Fort P. Tuberal hypothalamic neurons secreting the satiety molecule Nesfatin-1 are critically involved in paradoxical (REM) sleep homeostasis. PLos One 7: e52525, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jhaveri KA, Ramkumar V, Trammell RA, Toth LA. Spontaneous, homeostatic, and inflammation-induced sleep in NF-κB p50 knockout mice. Am J Physiol Regul Integr Comp Physiol 291: R1516–R1526, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kapás L, Bohnet SG, Traynor TR, Majde a J, Szentirmai E, Magrath P, Taishi P, Krueger JM. Spontaneous and influenza virus-induced sleep are altered in TNF-alpha double-receptor-deficient mice. J Appl Physiol 105: 1187–1198, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly JM, Bianchi MT. Mammalian sleep genetics. Neurogenetics 13: 287–326, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des 14: 3408–3416, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kushikata T, Fang J, Krueger JM. Brain-derived neurotrophic factor enhances spontaneous sleep in rats and rabbits. Am J Physiol Regul Integr Comp Physiol 276: R1334–R1338, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kushikata T, Kubota T, Fang J, Krueger JM. Glial cell line-derived neurotrophic factor promotes sleep in rats and rabbits. Am J Physiol Regul Integr Comp Physiol 280: R1001–R1006, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Kushikata T, Kubota T, Fang J, Krueger JM. Neurotrophins 3 and 4 enhance non-rapid eye movement sleep in rabbits. Neurosci Lett 346: 161–164, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Lindholm JSO, Castrén E. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci 8: 143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu J, Greco a M, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci 20: 3830–3842, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luppi PH, Clement O, Sapin E, Peyron C, Gervasoni D, Léger L, Fort P. Brainstem mechanisms of paradoxical (REM) sleep generation. Pflügers Arch 463: 43–52, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Martinowich K, Schloesser RJ, Jimenez DV, Weinberger DR, Lu B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol Brain 4: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohira K, Funatsu N, Homma KJ, Sahara Y, Hayashi M, Kaneko T, Nakamura S. Truncated TrkB-T1 regulates the morphology of neocortical layer I astrocytes in adult rat brain slices. Eur J Neurosci 25: 406–416, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Ohira K, Kumanogoh H, Sahara Y, Homma KJ, Hirai H, Nakamura S, Hayashi M. A truncated tropomyosin-related kinase B receptor, T1, regulates glial cell morphology via Rho GDP dissociation inhibitor 1. J Neurosci 25: 1343–1353, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev 17: 377–390, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Compact 3rd ed.London: Elsevier Academic, 2008. [Google Scholar]
- 42.Popa D, El Yacoubi M, Vaugeois JM, Hamon M, Adrien J. Homeostatic regulation of sleep in a genetic model of depression in the mouse: effects of muscarinic and 5-HT1A receptor activation. Neuropsychopharmacology 31: 1637–1646, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Ramos OV, Torterolo P, Lim V, Chase MH, Sampogna S, Yamuy J. The role of mesopontine NGF in sleep and wakefulness. Brain Res 1413: 9–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361: 1545–1564, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riemann D, Hohagen F, Bahro M, Berger M. Sleep in depression: the influence of age, gender and diagnostic subtype on baseline sleep and the cholinergic REM induction test with RS 86. Eur Arch Psychiatry Clin Neurosci 243: 279–290, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A. Truncated TrkB-T1 mediates calcium signalling in glia cells. Nature 426: 74–78, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Sanford LD, Parris B, Tang X. GABAergic regulation of the central nucleus of the amygdala: implications for sleep control. Brain Res 956: 276–284, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep 33: 621–630, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron 68: 1023–1042, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience 132: 613–624, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Taishi P, Sanchez C, Wang Y, Fang J, Harding JW, Krueger JM. Conditions that affect sleep alter the expression of molecules associated with synaptic plasticity. Am J Physiol Regul Integr Comp Physiol 281: R839–R845, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Veasey SC, Valladares O, Fenik P, Kapfhamer D, Sanford L, Benington J, Bucan M. An automated system for recording and analysis of sleep in mice. Sleep 23: 1025–1040, 2000. [PubMed] [Google Scholar]
- 53.Vogel G, Neill D, Kors D, Hagler M. REM sleep abnormalities in a new animal model of endogenous depression. Neurosci Biobehav Rev 14: 77–83, 1990. [DOI] [PubMed] [Google Scholar]
- 54.Yamuy J, Morales F, Chase M. Induction of rapid eye movement sleep by the microinjection of nerve growth factor into the pontine reticular formation of the cat. Neuroscience 66: 9–13, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Yamuy J, Rojas MJ, Torterolo P, Sampogna S, Chase MH. Induction of rapid eye movement sleep by neurotrophin-3 and its co-localization with choline acetyltransferase in mesopontine neurons. Neuroscience 115: 85–95, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Yamuy J, Sampogna S, Chase MH. Neurotrophin-receptor immunoreactive neurons in mesopontine regions involved in the control of behavioral states. Brain Res 866: 1–14, 2000. [DOI] [PubMed] [Google Scholar]