To the Editor:
Reduced health-related quality of life (HRQoL), including impaired cognitive, social, physical, behavioural and emotional functioning, is common in children treated for medulloblastoma [7,2,1] and has been associated with clinical factors such as surgical resection and treatment modality [2,6,5]. Recent biological advances have allowed the distinction of medulloblastomas into four consensus molecular subgroups – WNT, SHH, Group 3 and Group 4 – which display distinct molecular, clinical, and pathological disease characteristics [3,9,4,8]. Together, these obervations raise the hypothesis that HRQoL in medulloblastoma survivors may be related to their underlying tumour biology.
We have previously reported the extensive characterisation of clinical outcomes [10], HRQoL [2], and biomarker-driven prognostication schemes [4] for children with medulloblastoma treated on the SIOP-UKCCSG PNET3 clinical trial. Moreover, we have recently described, in this journal, the robust assignment of molecular subgroup status in these patients using immunohistochemical [3,4] and DNA methylation profiling [8] methods. A combined analysis of these datasets thus provides a first opportunity to explore relationships between HRQoL and tumour biology in a trials setting.
We identified 39 SIOP-UKCCSG PNET3 survivors for whom clinical, HRQoL, and molecular subgroup data had been assessed (Supplementary Table 1). Tumours were categorised into the SHH, WNT and non-SHH/non-WNT subgroups (comprising Group 3 and Group 4 tumours) as previously described [3,4,8]. We combined Groups 3 and 4 together as only a subset of those classified as non-SHH/WNT [3] were sub-classified as group 3 or 4[8] leaving small numbers with similar HRQoL scores in the two groups. Age at diagnosis, age at HRQoL [2] assessment, time from diagnosis to HRQoL [2] assessment, gender, pre-operative neurology, post-operative complications, extent of resection, pathological subtype, tumour metastatic stage, MYC and MYCN amplification status, and treatment received were documented and assessed within each molecular subgroup (Supplementary Table 1).
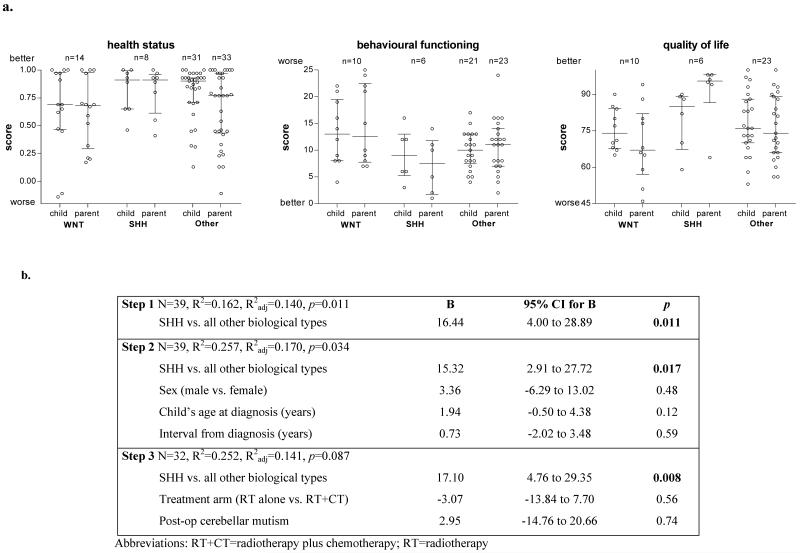
The distribution of scores for quality of survival (at a mean interval of seven years from diagnosis) using the Health Utilities Index total utility score (HUI3; a measure of health status), the Strengths and Difficulties Questionnaire total difficulties score (SDQ; a measure of emotional and behavioural difficulties), and the Pediatric Quality of Life Inventory total score (PedsQL; a measure of HRQoL) [2], showed differences in patterns between tumour molecular subgroups. The SHH subgroup showed a trend to better functioning across all indices tested (Figure 1, Supplementary Table 2), and univariate analyses (Kruskal-Wallis tests) revealed the overall inter-group difference for the parent-reported PedsQL total score for HRQoL was statistically significant (Supplementary Table 2). Subsequent comparisons of these HRQoL scores between each of three molecular subgroups (Mann-Whitney U tests) showed significant differences between SHH and WNT, and between SHH and non-SHH/non-WNT (Supplementary Table 2).
Figure 1.
a. Quality of survival outcomes seven years after diagnosis by molecular subgroup in children with medulloblastoma treated on the PNET3 trial. Bars medians and interquartile ranges, circles individual data points, scores total scores for each measure. b. Regression model for prediction of parent-reported health-related quality of life (HRQoL) scores seven years after treatment in the PNET3 study.
We next examined this inter-group difference in parent-reported HRQoL using multivariate analyses, that included factors previously associated with worse HRQoL outcomes in childhood brain tumours [1], to determine whether the impact of the SHH subgroup remained. Predictors were placed in the model in a hierarchical forward step-wise fashion and were retained if p<0.1, beginning with SHH vs. all others at step one, followed by gender, age at diagnosis, and interval from diagnosis at step two and, lastly at step 3, by treatment given (either craniospinal irradiation alone or craniospinal irradiation plus chemotherapy) and the presence (or not) of cerebellar mutism (Figure 1). At each step, SHH remained the only significant predictor of parent-report HRQoL.
In spite of WNT patients displaying a better prognosis [4,8], our analysis indicates patients with SHH subgroup tumours are associated with better parent-report HRQoL, even after taking into consideration other possible HRQoL predictors. The larger effect on parent-than self-report of HRQoL is consistent with the larger effects reported by parents in all 108 patients that participated in the PNET3 QoS study [2]. Further studies to expand and validate these observations, and to determine any clinico-biological correlates and mechanisms, are now paramount. Such studies should also determine whether tumour location in the cerebellum, not available to us for the sample described here, and recently reported to vary with tumour subtype [11], may be on the pathway from biological tumour subtype to HRQoL. Although the modest cohort size precluded more detailed analysis, our initial investigations suggest that combined biological and quality of survival investigations have the potential to inform our understanding of HRQoL outcomes, and could impact subgroup-directed disease management strategies in the future.
Supplementary Material
Acknowledgements
This work was conducted on behalf of the UK Children’s Cancer and Leukemia Group (CCLG), and was supported by grants from The Brain Tumour Charity and Cancer Research UK. Medulloblastomas investigated in this study were provided as part of CCLG approved biological study BS-2007-04. This study was conducted with ethics committee approval from Newcastle/North Tyneside and Trent RECs (study reference numbers 07/Q0905/71; MREC/02/4/019). The task of identifying associations between biological subtype and quality of survival was made possible by funding from the European Union’s Seventh Framework Programme (FP7/2007-13) under the project ENCCA, grant agreement HEALTH-F2-2011-261474.
References
- 1.Bhat SR, Goodwin TL, Burwinkle TM, et al. Profile of daily life in children with brain tumors: An assessment of health-related quality of life. J Clin Oncol. 2005;23:5493–500. doi: 10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]
- 2.Bull KS, Spoudeas HA, Yadegarfar G, et al. Reduction of health status 7 years after addition of chemotherapy to craniospinal irradiation for medulloblastoma: A follow-up study in PNET 3 trial survivors - On behalf of the CCLG (formerly UKCCSG) J Clin Oncol. 2007;25:4239–45. doi: 10.1200/JCO.2006.08.7684. [DOI] [PubMed] [Google Scholar]
- 3.Ellison DW, Dalton J, Kocak M, et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–96. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–07. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy C, Bull K, Chevignard M, et al. Quality of survival and growth in children and young adults in the PNET4 European controlled trial of hyperfractionated versus conventional radiation therapy for standard-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2014;88:292–300. doi: 10.1016/j.ijrobp.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Konczak J, Schoch B, Dimitrova A, et al. Functional recovery of children and adolescents after cerebellar tumour resection. Brain. 2005;128:1428–41. doi: 10.1093/brain/awh385. [DOI] [PubMed] [Google Scholar]
- 7.Palmer SL, Armstrong C, Onar-Thomas A, et al. Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol. 2013;31:3494–500. doi: 10.1200/JCO.2012.47.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwalbe EC, Williamson D, Lindsey JC, et al. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol. 2013;125:359–71. doi: 10.1007/s00401-012-1077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor RE, Bailey CC, Robinson K, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology United Kingdom Children’s Cancer Study Group PNET-3 study. J Clin Oncol. 2003;21:1581–91. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 11.Wefers AK1, Warmuth-Metz M, Pöschl J, et al. Subgroup-specific localization of human medulloblastoma based on pre-operative MRI. Acta Neuropathol. 2014 Apr 4; doi: 10.1007/s00401-014-1271-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.