Abstract
Background
Poor self-rated health (SRH) and elevated inflammation and morbidity and mortality are robustly associated in middle- and older-aged adults. Less is known about SRH-elevated inflammation associations during young adulthood and whether these linkages differ by sex.
Methods
Data came from the National Longitudinal Study of Adolescent Health. At Wave IV, young adults aged 24–34 reported their SRH, acute and chronic illnesses, and sociodemographic and psychological characteristics relevant to health. Trained fieldworkers assessed medication use, BMI, waist circumference, and also collected bloodspots from which high-sensitivity CRP (hs-CRP) was assayed. The sample size for the present analyses was N=13,236.
Results
Descriptive and bivariate analyses revealed a graded association between SRH and hs-CRP: Lower ratings of SRH were associated with a higher proportion of participants with hs-CRP > 3 mg/L and higher mean levels of hs-CRP. Associations between SRH and hs-CRP remained significant when acute and chronic illnesses, medication use, and health behaviors were taken into account. When BMI was taken into account, the association between SRH and hs-CRP association fully attenuated in females; a small, but significant association between SRH and hs-CRP remained in males.
Conclusion
Poor SRH and elevated hs-CRP are associated in young adults, adjusting for other health status measures, medication use, and health behavior. In males, SRH provided information about elevated hs-CRP that was independent of BMI. In females, BMI may be a better surrogate indicator of global health and pro-inflammatory influences compared to SRH.
Keywords: self-rated health, C-reactive protein, inflammation, sex differences, young adulthood, cardiovascular risk, Add Health
Self-rated health (SRH) measures a person’s subjective, global perception of their health, and is often assessed using a single item with a four- or five-point scale (e.g., Jylhä et al., 2006). Despite criticisms of single-item assessments, SRH is a notably robust predictor of future morbidity and mortality in both community-dwelling, generally healthy and also patient samples of adults, even with extensive controls for objective indicators of health status (e.g., Benjamins et al., 2004; Benyamini and Idler, 1999; DeSalvo et al., 2006; Idler and Benyamini, 1997). Several reasons may account for these robust predictions (for recent reviews, see Benyamini, 2011; Jylhä, 2009). First, SRH ratings elicit inclusive “summary ratings” of health, which likely capture diagnosed diseases, severity and comorbidity of conditions, but also self-perceived but not-yet-diagnosable bodily sensations related to disease processes. Second, SRH ratings are dynamic, capturing chronic, but also recent or intermittent health problems that may otherwise be missed during one-time “objective” assessments. Finally, SRH ratings reflect and also influence behavioral (e.g., physical activity) and emotional (e.g., depression) factors and also a person’s perceived resources (e.g., social support) that may predict later health (Benyamini, 2011; Jylhä, 2009).
Recent research from mostly middle and older adulthood has highlighted pro-inflammatory cytokines and their products, including the acute phase reactant C-reactive protein as key biological intermediaries in the link between SRH and morbidity and mortality (e.g., Christian et al., 2011; Cohen et al., 1997; Janszky et al., 2005; Jylhä et al., 2006; Lekander et al., 2004; Tanno et al., 2012; Unden et al., 2007). For example, in a study of generally healthy subjects in middle and older adulthood, those with the highest levels of Interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP) also reported the poorest SRH (Christian et al., 2011). This association remained significant after accounting for health status (e.g., self-reported diseases), health behaviors (e.g., smoking, physical activity and other substance use), socioeconomic factors indicative of disease risk (e.g., race, marital status), and psychological characteristics that could predispose one to both, poor ratings of health and later disease (e.g. depression, neuroticism). Inflammation induces subjective feelings of sickness (Dantzer and Kelley, 2007), and it is also a marker of disease risk (Ridker, 2007; Shah et al., 2009)—thus, it is a highly plausible biological mechanism in the SRH-morbidity/mortality link (e.g., Christian et al., 2011). Indeed, both, elevated inflammation—including hs-CRP—and also less than “excellent” or “very good” SRH are linked with morbidity and mortality from diabetes, heart disease, stroke, and cancer (Benjamins et al., 2004; Benyamini and Idler, 1999; Erlinger et al., 2004; Jylhä, 2009; Pearson et al., 2003; Pradhan et al., 2002; Yeh and Willerson, 2003).
Self-Rated Health in Young Adult Males and Females
Less is known about SRH as an indicator of disease risk and its biological manifestations in the earlier life course. Young adults are generally viewed as healthy. Nevertheless, SRH declines between adolescence and young adulthood (Bauldry et al., 2012), and in recent reports, rates of hypertension in US young adults were unexpectedly high (Nguyen et al., 2011). Indeed, many young adults in the community rate their health as less than “very good”—perhaps because they are experiencing subjective feelings of sickness induced by physiological changes during the early, “pre-diagnostic” stages of chronic disease (Konsman et al., 2002) or because they have received information that they are at high risk for chronic disease. It is during these early stages—before clinical endpoints are reached—when interventions to halt or reverse disease progression could be highly beneficial and cost-effective. Indeed, although elevated inflammatory proteins in the early life course are associated with early vascular changes involved in atherosclerosis, these changes may still be reversible (e.g., Kapiotis et al., 2006; Reinehr et al., 2006; Wunsch et al., 2006).
When testing SRH-inflammatory marker associations in young adults, potential sex differences must be considered. Levels of hs-CRP are almost twice as high in young adult females compared to young adult males (Ford et al., 2003; Shanahan et al., 2013), with 26% of females as compared to 10% of males meeting the disease-risk cut-off of CRP > 3 mg/L (Shanahan et al., 2013). Furthermore, correlates of hs-CRP during young adulthood are partially sex-differentiated. Female young adults face a multitude of potentially pro-inflammatory influences, ranging from actual chronic disease processes to use of oral contraceptives and pregnancy-related hormonal/metabolic shifts (Shanahan et al., 2013). In turn, male young adults face fewer pro-inflammatory factors, and BMI is less strongly associated with hs-CRP in this group.
Here we use a US nationally-representative sample of females and males to test whether SRH and levels of systemic inflammation—measured via hs-CRP in this study—are associated during young adulthood. Our analyses control for potential confounders of this association (reviewed, for example, in Christian et al., 2011; Jylhä, 2009; O'Connor et al., 2009), including health conditions and medication use, health behaviors, BMI, and also sociodemographic and psychological characteristics (e.g., low education, depressive symptoms, neuroticism).
Methods and Materials
Participants and Procedures
Data came from Waves I and IV of the National Longitudinal Study of Adolescent Health (Add Health, see Harris et al., 2009). Wave I of Add Health is a nationally representative sample of adolescents enrolled in middle school or high school in the US in 1994. The National Quality Education Database, which lists all US high schools, provided the sampling frame. Eighty high schools were randomly selected out of all high schools with an 11th grade and at least 30 students enrolled. These 80 high schools were paired with middle schools that fed into their student body. Together, 145 schools hosted an in-school survey, yielding 90,118 student respondents in grades 7–12 in 1994.
Approximately 200 students from each school were randomly selected for in-depth in-home interviews, resulting in N=20,745 (Wave I). The only variables from Wave I used by the present analyses are the race/ethnicity variables. Wave IV was collected when respondents were 24–34 years old (14 years after Wave I). Of the eligible respondents from Wave I, 93% were relocated, 80% were re-interviewed; resulting in 15,701 in-home interviews. Wave IV blood samples were obtained at the end of each interview, as described in the Add Health documentation of biomarker collection procedures (Harris and Udry, 2013). Dried blood spots were mailed to and assayed at the University of Washington Medical Center Immunology Lab. Written consent was obtained from young adults at Wave IV.
Assessment
C-reactive Protein
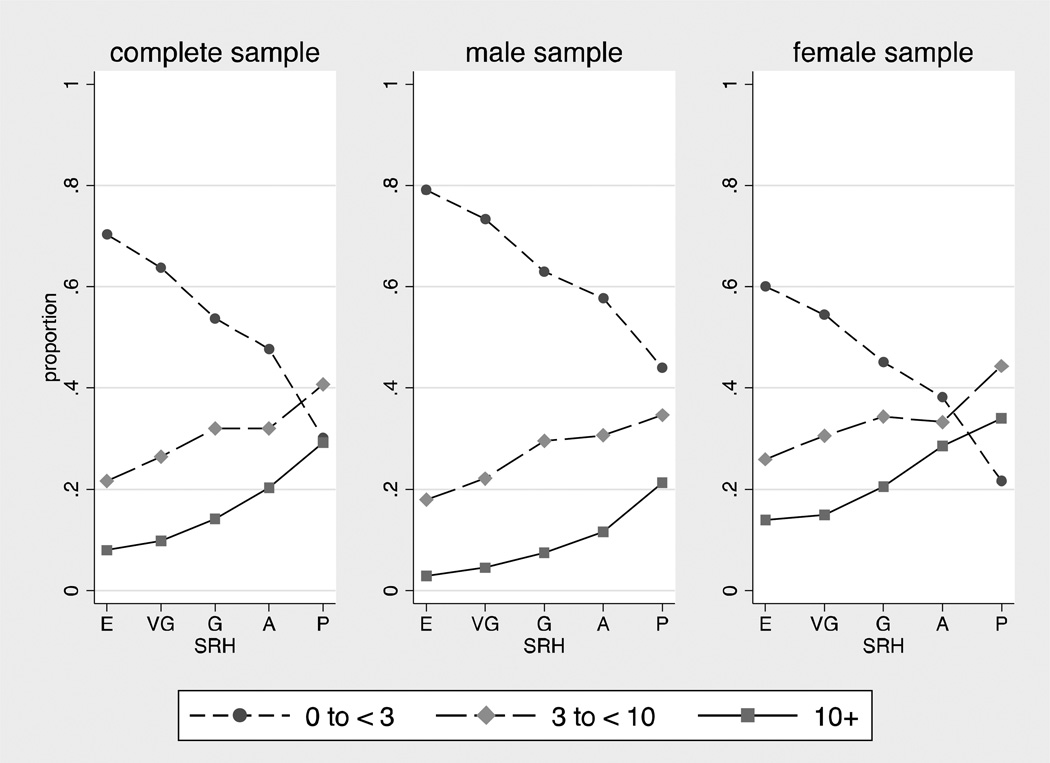
An in-depth documentation of the Add Health hs-CRP assay and quality control are available online (Whitsel et al., 2013). Briefly, a sandwich ELISA method was adapted from a previously published method (McDade et al., 2004). Values from dried blood spots and paired plasma samples were highly correlated (r=.98) in a cross-validation study. Intra-assay variation was 8.1% and inter-assay variation was 11%. In one first distinct set of descriptive analyses that established bivariate SRH-CRP associations, we divided the CRP continuum into three distinctive groups: low hs-CRP (≤ 3 mg/L; N=7,899, 60%), elevated hs-CRP (3 to < 10 mg/L; N=3,650, 28%), and very high hs-CRP (≥ 10 mg/L; N=1,687, 12% results shown in Figure 1). In all other analyses, including all of the weighted logistic regression analyses, we used a continuous, log-transformed variable of hs-CRP that included the full range of values. In follow-up analyses, we tested all models using a log-transformed continuous hs-CRP < 10 mg/L as the outcome variable (results available in Supplement 4).
Figure 1.
Weighted proportion of cases in low (< 3 mg/L), elevated (3 to < 10 mg/L), and very high (≥ 10mg/L) hs-CRP categories across levels of self-rated health.
E = Excellent, VG = Very Good, G = Good, A = Average, P = Poor
Self-rated health was assessed by asking “In general, how is your health.” Possible responses were 5=excellent, 4=very good, 3=good, 2=fair, and 1=poor.
Health conditions were assessed with checklists of recent and chronic health conditions, as is common in this type of field-based, epidemiological research. For self-reported diagnoses, participants were asked whether a doctor, nurse or other health care provider had ever told them that they suffered from a given condition. The subclinical symptoms scale counted whether participants reported having had a fever, night sweats, nausea or vomiting or diarrhea, blood in stool or in urine, frequent urination, and skin rash or abscess in the past two weeks. The infectious/inflammatory diseases scale counted lifetime diagnosis of asthma or chronic bronchitis or emphysema, lifetime diagnosis of hepatitis C, and also gum disease, active infection, injury, acute illness, surgery, and active seasonal allergies in the past four weeks. Other illnesses counted self-reported diagnoses of cancer or lymphoma or leukemia, high blood cholesterol or triglycerides or lipids, high blood pressure or hypertension, high blood sugar or diabetes, heart disease, migraine headaches, epilepsy or another seizure disorder, and HIV/AIDS. Counts > 3 for the three health conditions variables were collapsed to a value of “3.” The first two measures were constructed in accordance with the Add Health documentation (Whitsel et al., 2013). The other illnesses variable captures the remaining health conditions that were assessed.
Medication use was primarily recorded by interviewers from medications/containers provided by participants. A minority of participants (22%) recalled their medication use. The medication use variable indicated whether respondents were taking (1) NSAID/Salicylate medication in the past 24 hours, or (2) NSAID/Salicylate medications, (3) Cox-2 Inhibitor medications, (4) Inhaled Corticosteroid medications, (5) Corticotropin/Glucocorticoid medications, (6) Antirheumatic/Antipsoriatic medications, and (7) Immunosuppressive medications in the past four weeks. This indicator for medication use was constructed in accordance with the Add Health documentation (Whitsel et al., 2013).
Health behaviors
Physical activity was calculated as the sum of two items: “In the past seven days, how many times did you participate in individual sports such as running, wrestling, swimming, cross-country skiing, cycle racing, or martial arts?” and “In the past seven days, how many times did you participate in gymnastics, weight lifting, or strength training?” Responses for the individual items were 0=not at all, 1=1 time, 2=2 times, 3=3 times, 4=4 times, 5=5 times, 6=6 times, and 7=7 or more times. The sum of the two items could range from 0 to 14. Drinking behavior was assessed as the extent of drinking in the past year. Possible responses included 0= never, 1=1 or 2 days in the past 12 months, 2=once a month or less, 3=2 or 3 days a month, 4=1 or 2 days a week, 5=3 to 5 days a week, and 6=almost every day. The dichotomous current smoking variable indicated whether the subject had smoked at least one cigarette/day for the past 30 days. The dichotomous past smoking variable indicated whether the subject had ever smoked. The dichotomous insomnia variable indicated whether the subject reported difficulties either falling asleep or staying asleep on a daily or almost-every-day basis.
Adiposity-related variables were assessed by trained field interviewers. BMI was calculated as weight (kg)/height (m2). A squared BMI term was created in order to allow for effects of extreme obesity (Ishii et al., 2012). Waist circumference—an additional, and perhaps better indicator of health-related adiposity—was measured in centimeters.
Demographic variables
Dummy variables coded different racial/ethnic groups assessed at Wave 1 of the study: White, Black, Asian, American Indian, Hispanic, and other. Participants who indicated being Hispanic were coded into this category regardless of whether they indicated any other racial category. Young adults reported their marital status. Educational attainment was assessed on an 11-point scale (1=less than 8th grade to 11=doctoral degree).
Psychological characteristics
Depressive symptoms were measured with four indicators from the Center for Epidemiologic Studies Depression Scale (CES-D) items with adequade Cronbach’s as described by Peirreira (2005). Items included “could not shake off the blues,” “felt depressed,” “felt happy,” and “felt sad” in the past week. Neuroticism was assessed using Mini- International Personality Item Pool (IPIP) items, and consisted of the simple sum with appropriate reverse coding, with Cronbach’s α=.62 in the Add Health study (Baldasaro et al., 2013; Donnellan et al., 2006).
Finally, females reported whether they were currently pregnant or taking oral contraceptives. Table 1 shows the means and standard deviations or N and weighted % for all study variables. Supplement 1 also shows the prevalence of each level of each illness variable.
Table 1.
Descriptive statistics* for hs-CRP, self-rated health, additional measures of health and health behaviors, and demographic and psychological characteristics.
| Overall N=13,236 |
Male N=6,055 |
Female N=7,181 |
||||
|---|---|---|---|---|---|---|
| Continuous Variables | ||||||
| Mean | SD | Mean | SD | Mean | SD | |
| Variables Used in Main Analyses | ||||||
| hs-CRP (range: 0–205) | 4.73 | 8.61 | 3.27 | 6.74 | 6.16 | 9.73 |
| Self-Rated Health | 2.36 | 0.92 | 2.33 | 0.91 | 2.40 | 0.92 |
| Subclinical symptoms | 0.46 | 0.73 | 0.39 | 0.68 | 0.53 | 0.77 |
| Infect./Inflamm. Illness | 0.46 | 0.67 | 0.42 | 0.63 | 0.50 | 0.70 |
| Other illness | 0.40 | 0.67 | 0.36 | 0.64 | 0.45 | 0.70 |
| Physical activity** | 1.58 | 2.61 | 2.04 | 2.98 | 1.13 | 2.16 |
| Alcohol use*** | 2.32 | 1.80 | 2.67 | 1.87 | 1.97 | 1.67 |
| BMI | 29.22 | 7.62 | 29.10 | 6.77 | 29.33 | 8.26 |
| BMI2 | 911.54 | 532.51 | 894.06 | 467.72 | 928.80 | 581.07 |
| Waist circumference | 98.71 | 17.60 | 99.92 | 16.24 | 97.50 | 18.60 |
| Variables Used in Sensitivity Analyses | ||||||
| hs-CRP (range: 0–9.99) | 2.52 | 2.41 | 2.19 | 2.16 | 2.90 | 2.58 |
| Age | 28.42 | 1.78 | 28.51 | 1.78 | 28.32 | 1.77 |
| Education | 5.46 | 2.00 | 5.23 | 2.00 | 5.68 | 1.97 |
| Depressive Symptoms | 2.13 | 2.25 | 1.97 | 2.05 | 2.29 | 2.39 |
| Neuroticism | 10.40 | 2.76 | 9.84 | 2.63 | 10.95 | 2.76 |
| Categorical Variables | ||||||
| Unweigh.N | Weigh.% | Unweigh.N | Weigh. % | Unweigh.N | Weight. % | |
| Variables Used in Main Models | ||||||
| Medication use | 3908 | 30 | 1614 | 26 | 2294 | 33 |
| Never smoked [ref] | 7292 | 51 | 3084 | 48 | 4208 | 54 |
| Past smoker | 2979 | 24 | 1429 | 24 | 1550 | 23 |
| Current smoker | 2896 | 25 | 1502 | 28 | 1394 | 22 |
| Variables Used in Sensitivity Analyses | ||||||
| Hispanic | 2091 | 12 | 970 | 12 | 1121 | 12 |
| Black | 2787 | 15 | 1151 | 15 | 1636 | 16 |
| Asian | 830 | 3 | 418 | 3 | 412 | 3 |
| American Indian | 241 | 2 | 117 | 2 | 124 | 2 |
| Other race | 110 | 1 | 53 | 1 | 57 | 1 |
| White [ref] | 7177 | 66 | 3346 | 67 | 3831 | 66 |
| Married | 5823 | 45 | 2453 | 41 | 3370 | 49 |
| Pregnant | . | . | . | . | 438 | 6 |
| Oral contraceptives | . | . | . | . | 2119 | 30 |
| Insomnia | 1961 | 15 | 740 | 13 | 1221 | 18 |
Weighted means and standard deviations are reported for continuous measures. Unweighted Ns and weighted percentages are reported for categorical measures.
Physical activity is a continuous measure based on the sum of two questions: “In the past seven days, how many times did you participate in individual sports such as running, wrestling, swimming, cross-country skiing, cycle racing, or martial arts?” and “In the past seven days, how many times did you participate in gymnastics, weight lifting, or strength training?” Responses for the individual items ranged from 0= not at all to 7= 7 or more times.
Drinking is a continuous measure based on the question “During the past 12 months, on how many days did you drink alcohol?” with values ranging from 0= never to 6= almost every day.
Analytic Strategy
We estimated six nested regression models predicting hs-CRP with SRH and increasingly stringent sets of controls. Model 1 included only self-rated health as a main effect. Model 2 entered the main effect of sex. Model 3 added the interaction between sex and SRH, which tested whether SRH-CRP associations differed in males and females. Model 4 added the health conditions and medication use variables as main effects. Model 5 added health behaviors (physical activity, drinking, current smoking, past smoking) as main effects. Finally, Model 6 added measures of health-related adiposity (BMI, BMI2, and waist circumference) as main effects, and also a BMI by sex interaction considering that BMI-CRP associations may differ in young adult females and males (Shanahan et al., 2013). All models were weighted and adjusted the standard errors of the coefficients for the sample design of Add Health. Sensitivity analyses tested whether the SRH findings from Model 6 would remain the same when adjusting for age and sociodemographic (race, marital status, education levels), psychological (depressive symptoms, neuroticism) factors, insomnia, and also when using hs-CRP < 10 mg/L only.
The analysis sample consisted of cases with valid sample weights (N=14,800), non-missing for hs-CRP (N=13,247), and non-missing for sex and race (N=13,236). We constructed 5 complete data sets via multiple imputation with chained equations to address the small amount of missing data in the remaining covariates. We also conducted all analyses using listwise deletion and obtained the same substantive pattern of results.
Results
Descriptive Statistics and Bivariate Models
Table 2 shows that approximately 56% of young adults in the analytic sample rated their health as “very good” or above; the remainder rated their health as “good” or below. Percentages in each category of SRH were similar for males and females. Figure 1 shows the percentage of people with low (< 3 mg/L), elevated (3 to < 10 mg/L), and very high (≥10 mg/L) hs-CRP for each category of self-rated health. Associations between SRH and elevated hs-CRP were graded: As ratings of self-rated health decreased from excellent to poor, the percentage of subjects with hs-CRP < 3 mg/L also decreased and the percentage of subjects with hs-CRP ≥ 3 mg/L increased. Notably, the shape of the associations between SRH and CRP 3 to < 10 mg/L, and SRH and CRP ≥ 10 mg/L were remarkably similar. Therefore, and also because evidence is accumulating that cases with CRP > 10 score highest on many indicators of chronic diseases risk (e.g., Ishii et al., 2012), cases with hs-CRP ≥ 10 mg/L were included in all subsequent analyses. All subsequent analyses use the log-transformed hs-CRP variable as the outcome.
Table 2.
Unweighted N and weighted percentage of young adults in each category of self-rated health.
| Overall N=13,236 |
Male N=6,055 |
Female N=7,181 |
||||
|---|---|---|---|---|---|---|
| Unweighted N | Weighted % | Unweighted N | Weighted % | Unweighted N | Weighted % | |
| [1] Excellent | 2435 | 18 | 1191 | 20 | 1244 | 17 |
| [2] Very good | 5024 | 38 | 2336 | 38 | 2688 | 38 |
| [3] Good | 4495 | 34 | 2000 | 33 | 2495 | 35 |
| [4] Average | 1133 | 8 | 475 | 8 | 658 | 8 |
| [5] Poor | 149 | 1 | 53 | 1 | 96 | 2 |
Bivariate Associations
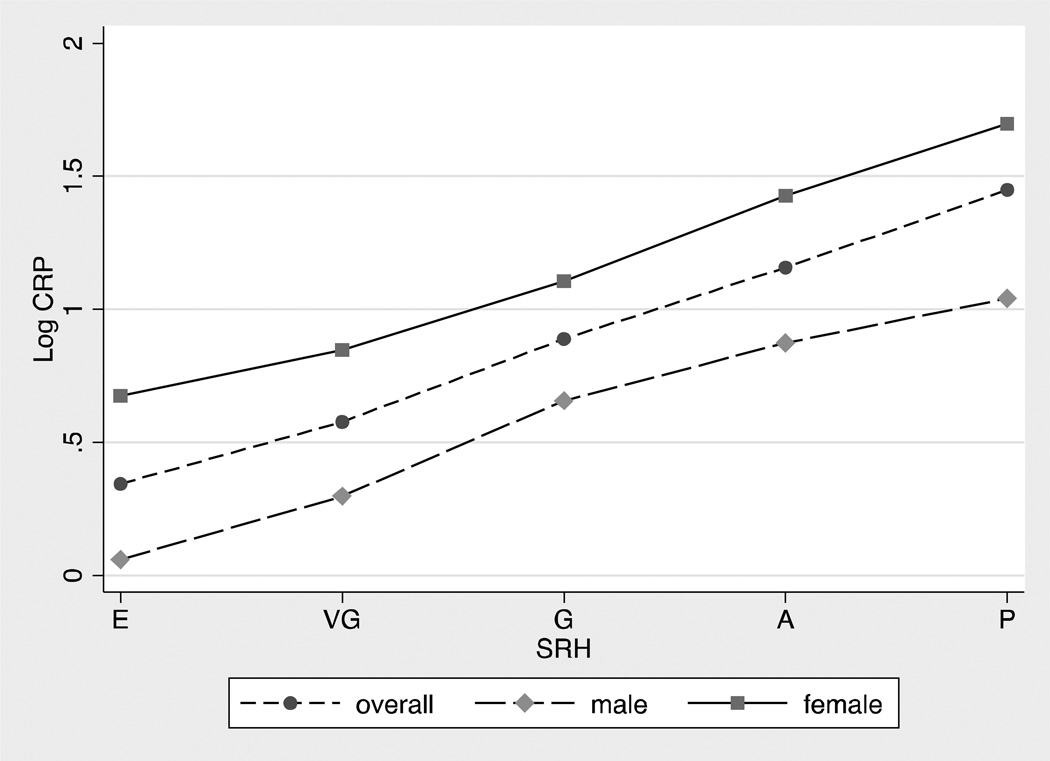
Figure 2 shows bivariate associations between self-rated health and the continuous logCRP variable in the overall sample, males, and females (b=0.28, p=0.000; b=0.29, p=0.000; b=0.25, p=0.000, respectively). Figure 2 further illustrates the graded bivariate association between SRH and hs-CRP, and also shows that, consistent with other work on young adults (Shanahan, 2013; Ford, 2003), hs-CRP levels were significantly higher in females than in males.
Figure 2.
Weighted mean levels of log hs-CRP for the overall sample, male sample, and female sample across levels of self-rated health.
E = Excellent, VG = Very Good, G = Good, A = Average, P = Poor
Note: Log hs-CRP includes hs-CRP ≥ 10 mg/L cases.
Multivariate Models
Next, we tested nested regression models with increasingly stringent controls in the prediction of hs-CRP (described above). We began with Model 0, which tested bivariate associations. Results showed that, with the exception of smoking status, all variables were bivariately associated with hs-CRP. In Model 1 (main effect of SRH), poorer SRH ratings were associated with higher levels of hs-CRP. In Model 2 (main effect of sex), female sex predicted higher hs-CRP. In Model 3 (SRH by sex interaction), the SRH by sex interaction was not significant. In Model 4 (illness/medication use), higher numbers of subclinical symptoms, other diseases, and medication use were associated with higher hs-CRP. Importantly, even after accounting for these measures of health status and medication use, SRH independently contributed to the prediction of hs-CRP. In fact, the reduction of the size of the SRH coefficient with the inclusion of these health status and medication variables was negligible.
In Model 5 (health behaviors), physical activity and alcohol use were associated with lower levels of hs-CRP. Finally, in Model 6 (BMI/health-related adiposity), all indicators of health-related adiposity (BMI, BMI2, and waist circumference) were associated with hs-CRP, as was the BMI by sex interaction. Young adults with high BMI and high waist circumference were more likely to have higher levels of hs-CRP. BMI was also more strongly associated with hs- CRP in females (b=0.18, p < .001) than in males (b=0.10, p < .001). Importantly, once these variables were entered into the model, the SRH by sex interaction became significant, but the main effect of SRH was much smaller. The R2 of this final model was .30. Supplement 2 shows all models for males and females separately, revealing that the inclusion of BMI-related variables completely attenuated SRH-CRP associations in females (b=-.02, p=0.32), but not in males (b=.06, p=.01). Supplement 2 also shows that BMI was more strongly associated with hs-CRP in females than in males.
Sensitivity analyses were conducted in order to test the robustness of the sex by SRH interaction observed in Model 6. This interaction remained significant when 1) adjusting for age, and sociodemographic (race, marital status, education level) factors, psychological (neuroticism, depressive symptoms) factors, and also insomnia (see Supplement 3 for results), 2) deleting cases with hs-CRP ≥ 10 mg/L (see Supplement 4 for results), 3) deleting cases that reported ≥1 acute condition (e.g., fever, night sweats, vomiting etc.), 4) deleting cases that reported ≥2 acute conditions, 5) adjusting for variables that coded pregnancy and use of oral contraceptives and/or deleting females who endorsed these variables, 6) testing interactions between sex and the other health variables and also medication use (which were non-significant), and 7) testing interactions between SRH and obesity in the prediction of hs-CRP (which were non-significant).
We also tested whether entering BMI alone (without additional adiposity-related terms) would result in the emergence of the SRH by sex interaction—and this was the case. Finally, in an effort to better understand the SRH by sex interaction, we tested whether the BMI-SRH association was stronger in females than in males. SRH and BMI were correlated at r=.32, p<.001 in females and r=.29, p<.001 in males, but these correlations were not significantly different in size.
Assessing the size of the self-rated health effect in males
In order to gauge the effect size of the SRH-CRP association in Model 6 for males, we calculated standardized coefficients for SRH and other health conditions. Betas were at 0.05 for SRH, 0.11 for subclinical symptoms, and 0.06 for medication use. Thus, the size of the SRH-CRP association in males was relatively small, but comparable to that of associations between both subclinical symptoms and medication use with hs-CRP.
Discussion
Subjective perceptions of the body’s functioning—including SRH—are associated with inflammatory markers even after adjusting for health behaviors, chronic disease risk indicators and/or measures of actual chronic or other illness in samples of mostly middle and older adults (e.g., Christian et al., 2011; Cohen et al., 1997; Jylhä et al., 2006; Lekander et al., 2004; Tanno et al., 2012; Unden et al., 2007). Although many young adults have not yet been diagnosed with chronic illness, almost half of the young adults in the nationally representative sample studied here rated their health as less than “very good,” and we tested whether SRH is associated with elevated hs-CRP during young adulthood and whether these linkages differ by sex.
Bivariate results identified graded SRH-CRP associations. As subjective ratings of health declined, the proportion of individuals with hs-CRP > 3 mg/L and mean levels of hs-CRP increased—even when health conditions, medication use, health behaviors and psychological dispositions (in the sensitivity analyses) had been taken into account. Research on SRH has argued that subjective ratings reflect, in part, subtle bodily feelings that would be missed by health checklists or other “objective” health assessments (Christian et al., 2011; Craig, 2002; Jylhä et al., 2006). It is possible that SRH ratings, in part, capture such subtle aspects of health during young adulthood.
BMI and Sex Differentiated SRH-CRP Associations in Young Adults
Fundamental changes in the SRH-CRP association were observed when measures of health-related adiposity, assessed by trained interviewers, were taken into account. In both females and males, the size of the SRH-CRP association decreased with the inclusion of BMI. In females, the SRH-CRP coefficient was fully attenuated; in males, independent associations between SRH and hs-CRP remained. What could explain this sex-difference in attenuation?
In young adult females, BMI may be a more powerful “surrogate” or summary measure of pro-inflammatory influences than in males. BMI is typically a better indicator of pro-inflammatory body fat in young adult females than in males (Gallagher et al., 1996; Wozniak et al., 2009); this notion was supported by our finding that BMI was more closely associated with hs-CRP in females than in males. Furthermore, BMI in young adult females could capture additional pro-inflammatory conditions, including childbirth and pregnancy-related physical and metabolic changes (Stuebe and Rich-Edwards, 2009)—that would not necessarily be reflected in subjective health ratings.
In males, SRH-CRP associations remained significant even after the inclusion of BMI. One simple question assessing subjective perceptions of health could uniquely contribute to identifying young adult males at increased risk for morbidity/mortality from chronic disease. BMI may not be an ideal surrogate indicator of health in young males, because it could reflect high amounts of body fat but also of lean muscle mass (Gallagher et al., 1996). To address this concern, we had also included waist circumference as a predictor of hs-CRP, but this measure also did not fully attenuate SRH-CRP associations. Nevertheless, the size of the SRH-CRP association in males’ final multivariate models was relatively small, and Figure 1 also suggests that the specificity of prediction is not high. Both SRH and hs-CRP are global, systemic, nonspecific indicators. That is, SRH could be rated as less than “very good” for a multitude of reasons. Similarly, the actual physiological origins of elevated systemic inflammatory proteins could be wide-ranging. Thus, perhaps it would be unrealistic to expect large associations between these two relatively global indicators of perceived and biologically manifested health.
Our sex-differentiated findings are consistent with several studies and reviews reporting greater SRH-mortality associations in males than females (Benjamins et al., 2004; Benyamini and Idler, 1999; Hays et al., 1996; Idler and Benyamini, 1997). Indeed, one study reported that among individuals with less than “very good” self-rated health, men were more likely to die from cancer and also heart disease than women (Benjamins et al., 2004)—and both of these conditions are also associated with elevated hs-CRP (e.g., Ko et al., 2012; Ridker, 2007; Shah et al., 2009).
We should also note, however, that our sex-differentiated findings were inconsistent with some studies from middle and older adulthood that reported that SRH-inflammatory marker associations were present in females even after accounting for health-related adiposity (Janszky et al., 2005; Unden et al., 2007), or that these association were stronger in females than in males (Lekander et al., 2004; Tanno et al., 2012). Jylhä (2009) proposed that sex differences in associations between SRH and its correlates and outcomes could change with age. It is plausible, for example, that young adult males face fewer “competing” pro-inflammatory influences compared to older males—who experience decreasing levels of testosterone, more central adiposity, and also clinical stages of chronic disease (Eskes and Haanen, 2007; Kelly and Jones, 2013; Laughlin et al., 2008; Pugh et al., 2000). In turn, as we detailed above, young adult females in their reproductive years face a variety of pro-inflammatory factors (Shanahan et al., 2013), but fewer during at least some parts of older adulthood, before they reach clinical endpoints of chronic disease (Eskes and Haanen, 2007; Pérez-López et al., 2010). Thus, the SRH-CRP signal may be most “noisy” for older adult males and young adult females, contributing to changing sex differences in this association across the life course.
Limitations
First, this study was cross-sectional, with only one assessment of hs-CRP. Therefore, we could not test whether decreases in self-rated health predicted increases in systemic inflammation or vice versa. Second, although the Add Health study has quite extensive health and medication assessments considering its field-based, large-scale nature, the health status measures were nevertheless self-reported. Future research should also assess physician-verified diagnoses when possible. Third, the Add Health study currently has data on one inflammatory marker only. Previous studies with older samples had also reported associations between SRH with other markers of immune function, including IL-6 (Christian et al., 2011), TNF-alpha, IL-1 (Lekander et al., 2004), and white blood cell counts (Jylhä et al., 2006). These additional biomarkers also should be tested in younger samples.
Despite these limitations the Add Health data presented a rare opportunity to test SRH-hsCRP associations in a nationally representative sample of young adults. Our bivariate analyses showed that assessing this non-invasive one-item measure could be a simple first step in identifying both females and males at risk for heightened systemic inflammatory processes. Especially in young males, SRH may be a useful independent indicator of disease risk even beyond BMI.
Supplementary Material
Table 3.
Regression coefficients and 95% confidence intervals (in brackets) from analyses that regressed log hs-CRP on self-rated health, sex, health conditions, medication use, and health behaviors. N=13,236.
| (0) Bivariate |
(1) SRH |
(2) Sex |
(3) SRH by Sex |
(4) Medical |
(5) Health Behaviors |
(6) BMI |
|
|---|---|---|---|---|---|---|---|
| SRH | 0.279*** [0.243,0.315] | 0.279*** [0.243,0.315] | 0.268*** [0.233,0.303] | 0.288*** [0.239,0.337] | 0.248*** [0.199,0.296] | 0.218*** [0.167,0.269] | 0.079** [0.031,0.128] |
| Female | 0.546*** [0.483, 0.609] | 0.528*** [0.466,0.589] | 0.622*** [0.439,0.804] | 0.621*** [0.440,0.801] | 0.528*** [0.351,0.704] | 0.461*** [0.261,0.662] | |
| Female X SRH | --- | −0.040 [−0.115,0.035] | −0.055 [−0.129,0.020] | −0.045 [−0.118,0.029] | −0.144*** [−0.208,−0.080] | ||
| Subclinical | 0.253*** [0.208, 0.298] | 0.153*** [0.104,0.202] | 0.152*** [0.104,0.201] | 0.181*** [0.139,0.224] | |||
| Infect/Inflam. Illness | 0.115*** [0.072, 0.158] | −0.004 [−0.050,0.041] | 0.002 [−0.044,0.047] | 0.003 [−0.035,0.042] | |||
| Other Illness | 0.256*** [0.211, 0.301] | 0.123*** [0.077,0.170] | 0.117*** [0.070,0.164] | 0.010 [−0.029,0.048] | |||
| Medication Use | 0.234*** [0.164, 0.306] | 0.098** [0.030,0.167] | 0.100** [0.032,0.168] | 0.124*** [0.065,0.183] | |||
| Physical Activity | −0.078*** [−0.090, −0.066] | −0.044*** [−0.056, −0.032] | −0.025*** [−0.036, −0.015] | ||||
| Alcohol Use | −0.084*** [−0.102, −0.065] | −0.040*** [−0.058, −0.022] | −0.007 [−0.023,0.009] | ||||
| Past Smoker | 0.010 [−0.062, 0.081] | 0.020 [−0.053,0.094] | 0.055 [−0.007,0.117] | ||||
| Current Smoker | 0.004 [−0.082, 0.090] | −0.064 [−0.157,0.029] | 0.086* [0.005,0.168] | ||||
| Body Mass Index | 0.167*** [0.149, 0.184] | 0.134*** [0.112,0.155] | |||||
| BMI2 | −0.001*** [−0.001, −0.001] | −0.001*** [−0.001, −0.001] | |||||
| Waist circumference | 0.035*** [0.033, 0.037] | 0.012*** [0.008,0.016] | |||||
| Female by BMI | --- | 0.014*** [0.007,0.021] |
p < 0.05
p < 0.01
p < 0.001
Highlights.
Self-rated health is associated with elevated C-reactive protein (CRP) in young adulthood, and it uniquely contributes to explaining elevated CRP levels in males.
Acknowledgements
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis. Support from this work also came from NICHD (R01 HD061622-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldasaro RE, Shanahan MJ, Bauer DJ. Psychometric properties of the mini-IPIP in a large, nationally representative sample of young adults. J. Pers. Assess. 2013;95:74–84. doi: 10.1080/00223891.2012.700466. [DOI] [PubMed] [Google Scholar]
- Bauldry S, Shanahan MJ, Boardman JD, Miech RA, Macmillan R. A life course model of self-rated health through adolescence and young adulthood. Soc. Sci. Med. 2012;75:1311–1320. doi: 10.1016/j.socscimed.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins MR, Hummer RA, Eberstein IW, Nam CB. Self-reported health and adult mortality risk: An analysis of cause-specific mortality. Soc. Sci. Med. 2004;59:1297–1306. doi: 10.1016/j.socscimed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- Benyamini Y. Why does self-rated health predict mortality? An update on current knowledge and a research agenda for psychologists. Psychology & health. 2011;26:1407–1413. doi: 10.1080/08870446.2011.621703. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, Idler EL. Community studies reporting association between self-rated health and mortality. Res. Aging. 1999;21:392–401. [Google Scholar]
- Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011;36:1495–1504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J. Gerontol. A. Biol. Sci. Med. Sci. 1997;52:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain. Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question: A meta-analysis. J. Gen. Intern. Med. 2006;21:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan MB, Oswald FL, Baird BM, Lucas RE. The mini-IPIP scales: Tiny-yet-effective measures of the Big Five factors of personality. Psychol. Assess. 2006;18:192–203. doi: 10.1037/1040-3590.18.2.192. [DOI] [PubMed] [Google Scholar]
- Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- Eskes T, Haanen C. Why do women live longer than men? Eur. J. Obstet. Gynecol. Reprod. Biol. 2007;133:126–133. doi: 10.1016/j.ejogrb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: Findings from the National Health and Nutrition Examination Survey 1999–2000. Clin. Chem. 2003;49:1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age sex, and ethnic groups? Am. J. Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- Harris KM, Halpern CT, Whitsel EA, Hussey JM, Tabor JW, Entzel PP, Udry JR. The National Longitudinal Study of Adolescent Health: Research Design. 2009 http://www.cpc.unc.edu/projects/addhealth/design. [Google Scholar]
- Harris KM, Udry JR. 2013 http://www.icpsr.umich.edu/icpsrweb/DSDR/studies/33443. [Google Scholar]
- Hays JC, Schoenfeld D, Blazer DG, Gold DT. Global self-ratings of health and mortality: Hazard in the North Carolina Piedmont. J. Clin. Epidemiol. 1996;49:969–979. doi: 10.1016/0895-4356(96)00138-2. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J. Health Soc. Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- Ishii S, Karlamangla AS, Bote M, Irwin MR, Jacobs DRJ, Cho HJ, Seeman TE. Gender, obesity and repeated elevation of C-reactive protein: Data from the CARDIA cohort. PLoS ONE. 2012;7:e36062. doi: 10.1371/journal.pone.0036062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain. Behav. Immun. 2005;19:555–563. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc. Sci. Med. 2009;69:307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Jylhä M, Volpato S, Guralnik JM. Self-rated health showed a graded association with frequently used biomarkers in a large population sample. J. Clin. Epidemiol. 2006;59:465–471. [Google Scholar]
- Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, Jilma B, Röggla G, Wolzt M, Widhalm K, Wagner OF. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler. Thromb. Vasc. Biol. 2006;26:2541–2546. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- Kelly DM, Jones TH. Testosterone: A metabolic hormone in health and disease. J. Endocrinol. Reprod. 2013;217:R25–R45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- Ko YJ, Kwon YM, Kim KH, Choi HC, Chun SH, Yoon HJ, Goh E, Cho B, Park M. High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol. Biomarkers Prev. 2012;21:2076–2086. doi: 10.1158/1055-9965.EPI-12-0611. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: Mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J. Clin. Endocrinol. Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekander M, Elofsson S, Neve IM, Hansson LO, Unden AL. Self-rated health is related to levels of circulating cytokines. Psychosom. Med. 2004;66:559–563. doi: 10.1097/01.psy.0000130491.95823.94. [DOI] [PubMed] [Google Scholar]
- McDade T, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin. Chem. 2004;50:652–654. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- Nguyen QC, Tabor JW, Entzel PP, Lau Y, Suchindran C, Hussey JM, Halpern CT, Harris KM, Whitsel EA. Discordance in national estimates of hypertension among young adults. Epidemiology. 2011;22:532–541. doi: 10.1097/EDE.0b013e31821c79d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain. Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW Centers for Disease Control and Prevention, American Heart Association. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pérez-López FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: Hormonal and biochemical influences. Reprod. Sci. 2010;17:511–531. doi: 10.1177/1933719110367829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreira KM, Deeb-Sossa N, Harris KM, Bollen KA. What are we measuring? An evaluation of the CES-D across race/ethnicity and immigrant generation. Soc. Forces. 2005;83:1567–1601. [Google Scholar]
- Pradhan AD, Manson JE, Rifai M, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA (J. Am. Med. Assoc.) 2002;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Pugh PJ, English KM, Jones TH, Channer KS. Testosterone: A natural tonic for the failing heart? Q. J. Med. 2000;93:689–694. doi: 10.1093/qjmed/93.10.689. [DOI] [PubMed] [Google Scholar]
- Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: Relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism. 2006;55:113–118. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: Moving an inflammatory hypothesis toward consensus. J. Am. Coll. Cardiol. 2007;29:2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- Shah T, Casas JP, Cooper JA, Tzoulaki I, Sofat R, McCormack V, Smeeth L, Deanfield JE, Lowe GD, Rumley A, Fowkes FGR, Humphries SE, Hingorani AD. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: New data and systematic review of 31 prospective cohorts. Int. J. Epidemiol. 2009;38:217–231. doi: 10.1093/ije/dyn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan L, Copeland WE, Worthman C, Erkanli A, Angold A, Costello EJ. Sex-differentiated changes in C-reactive protein from ages 9 to 21: The contributions of BMI and physical/sexual maturation. Psychoneuroendocrinology. 2013;13:2209–2217. doi: 10.1016/j.psyneuen.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Rich-Edwards JW. The reset hypothesis: Lactation and maternal metabolism. Am. J. Perinatol. 2009;26:81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno K, Ohsawa M, Onoda T, Itai K, Sakata K, Tanaka F, Makita S, Nakamura M, Omama S, Ogasawara K, Ogawa A, Ishibashi Y, Kuribayashi T, Koyama T, Okayama A. Poor self-rated health is significantly associated with elevated Creactive protein levels in women, but not in men in the Japanese general population. J. Psychosom. Res. 2012;73:225–231. doi: 10.1016/j.jpsychores.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Unden AL, Andreasson A, Elofsson S, Brismar K, Mathsson L, Ronnelid J, Lekander M. Inflammatory cytokines, behaviour and age as determinants of self-rated health in women. Clin. Sci. (Lond.) 2007;112:363–373. doi: 10.1042/CS20060128. [DOI] [PubMed] [Google Scholar]
- Whitsel EA, Cuthbertson CC, Tziakas DN, Power C, Wener MH, Killeya-Jones LA, Harris KM. Add Health Wave IV documentation: Measures of inflammation and immune function. 2013 http://www.cpc.unc.edu/projects/addhealth/data/guides/add-healthwave-iv-documentation-measures-of-inflammation-and-immune-function. [Google Scholar]
- Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- Wunsch R, de Sousa G, Toschke AM, Reinehr T. Intima-media thickness in obese children before and after weight loss. Pediatrics. 2006;118:2334–2340. doi: 10.1542/peds.2006-0302. [DOI] [PubMed] [Google Scholar]
- Yeh ET, Willerson JT. Coming of age of C-reactive protein: Using inflammation markers in cardiology. Circulation. 2003;107:370–371. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.