Abstract
We studied links between human malnutrition and wild meat availability within the Rainforest Biotic Zone in central Africa. We distinguished two distinct hunted mammalian diversity distributions, one in the rainforest areas (Deep Rainforest Diversity, DRD) containing taxa of lower hunting sustainability, the other in the northern rainforest-savanna mosaic, with species of greater hunting potential (Marginal Rainforest Diversity, MRD). Wild meat availability, assessed by standing crop mammalian biomass, was greater in MRD than in DRD areas. Predicted bushmeat extraction was also higher in MRD areas. Despite this, stunting of children, a measure of human malnutrition, was greater in MRD areas. Structural equation modeling identified that, in MRD areas, mammal diversity fell away from urban areas, but proximity to these positively influenced higher stunting incidence. In DRD areas, remoteness and distance from dense human settlements and infrastructures explained lower stunting levels. Moreover, stunting was higher away from protected areas. Our results suggest that in MRD areas, forest wildlife rational use for better human nutrition is possible. By contrast, the relatively low human populations in DRD areas currently offer abundant opportunities for the continued protection of more vulnerable mammals and allow dietary needs of local populations to be met.
Electronic supplementary material
The online version of this article (doi:10.1038/srep08168) contains supplementary material, which is available to authorized users.
Subject terms: Sustainability, Tropical ecology
Introduction
In Africa's Congo Basin, people eat an estimated five million tons of bushmeat per year1,2 and there is evidence that bushmeat is an important source of many nutrients (especially protein, B vitamins, iron and zinc) for both rural and urban households throughout Africa2. However, the magnitude of exploitation and consumption, varies between countries and regions, determined primarily by its availability and influenced by such factors as governmental controls on hunting, socio-economic status and cultural prohibitions. In areas where wildlife still exists people collect, hunt, purchase and eat bushmeat. Some people depend on bushmeat because they have no other source of meat or cannot afford alternative sources; others eat bushmeat as a matter of preference or as a luxury item/delicacy for special occasions. The reality in central Africa is that, for the greater majority of rural people, bushmeat represents a vital dietary item for reasons dictated by lack of alternate sources, financial limitations, preferences and cultural values. For such people, wild animals constitute a valuable food resource, which cannot be easily withdrawn or replaced without causing wide-ranging socio-economic imbalances.
There is strong empirical evidence for the view that wildlife is being depleted on an unprecedented scale3 with a major transition in the scale of offtake in recent years. This drawdown is perceived by some as likely to have negative consequences for future generations3,4. Yet, conservation practitioners and planners often perceive hunting of wild animals as a drain to ecosystems5,6, in contrast to those involved with development issues who give greater emphasis to biodiversity as a resource to support human needs. Thus, to date, bushmeat has rarely figured seriously in international development strategies3, but has been a strong banner for the conservation lobby7,8. One reason for this may be that a strong relationship between use of wild meat and human health has not yet been fully confirmed.
Investigations of the role of wildlife on human health in central Africa are limited, most often restricted to isolated studies2 or based on estimated country-level production data from the Food Balance Sheets4 produced by the Food and Agriculture Organization of the United Nations (FAO). However, there is some evidence that indicates a strong causal link between bushmeat supply and human nutrition. For example, a study of children under 12 y of age in rural northeastern Madagascar showed that lack of access to wild meat causes a 29% increase in the numbers of children suffering from iron deficiency anemia and a tripling of anemia cases among children in the poorest households9. Thus, if consumption of sufficient amounts of nutrients to meet the body's needs are limited, including those contained in meats, chronic malnutrition will occur over time and will result in growth retardation in children (stunting) and eventually ill health in later life10.
In the absence of direct measures of nutritional status of human populations at a subnational level, stunting prevalence to the lowest administrative unit can be employed as a useful indicator of chronic malnutrition in Africa11. Stunting can then be used to correlate with the availability of different food items e.g. meats, even though various factors may affect retention of nutrients (e.g. disease12,13,14). Notwithstanding, in this paper we studied whether potential availability of wild meats was linked to stunting in children in central Africa. We base our analyses on the backdrop of the distribution of mammalian species assemblages, which we classify according to their hunting potential and in which we estimate wild meat biomass likely to be at the disposal of humans. Given the strong associations that appear between mammalian diversity areas and stunting, we then statistically test three plausible hypotheses to examine the association between stunting and huntable mammalian diversity as proxies of wild meat availability:
H1: Mammalian diversity patterns directly influence malnutrition in humans.
H2:Mammalian diversity patterns influence human population levels and their impacts and these are correlated with malnutrition in humans.
H3:Human population levels and their impacts influence both mammalian diversity areas and malnutrition in humans.
H0:There is no relationship between mammalian diversity patterns and human malnutrition.
We contend that if a strong correlation between bushmeat availability and malnutrition in humans is established, coalescing strategies that deal with conservation of wildlife, as well as human livelihoods, becomes imperative.
Geographical focus
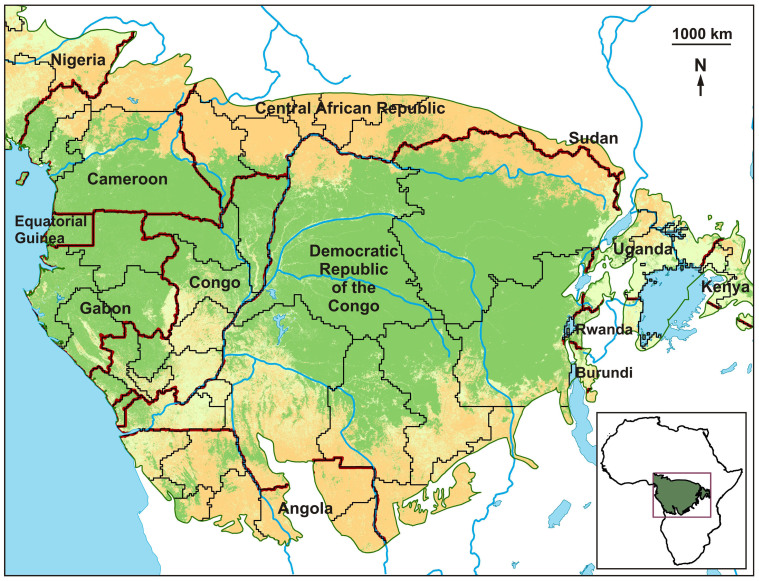
Our study area was limited to the Rainforest Biotic Zone (RBZ) of central Africa. The RBZ, defined by Kingdon et al. (2013)15, following White (1983)16 encompasses six main countries (the Democratic Republic of the Congo, the Republic of the Congo, Central African Republic, Cameroon, Gabon and Equatorial Guinea), as well as parts of another three (Angola, Burundi and Rwanda) (Fig. 1). The main vegetation type in the region is Guineo-Congolian lowland rain forest, concentrated in the Congo basin, corresponding to the second largest (close to 2 million km2) and the least degraded area of contiguous moist tropical forest in the world17. Away from the central regions of the RBZ, the dominant vegetation includes woody savannas, as well as areas of cropland-natural vegetation mosaic18.
Figure 1.
Study area.
Green areas are rainforests and warm pink areas are woody savannas, taken from the Collection 5 MODIS Global Land Cover Type product (www.landcover.org). Coarse red lines are country borders and slim black lines are limits of subnational units considered by FAO for data on children stunting20. Maps were generated using ArcGIS.
Datasets
Huntable mammal species
From a previous study19 in which we derived predicted distribution maps for all hunted terrestrial mammal species occurring within the RBZ, we delimited mammalian diversity areas for species of a lesser or greater hunting potential (see Methods). A total of 141 monotypic species and 24 others, including 67 subspecies, belonging to 11 Orders, were included in our analyses (see Methods and Appendix S1).
Child stunting
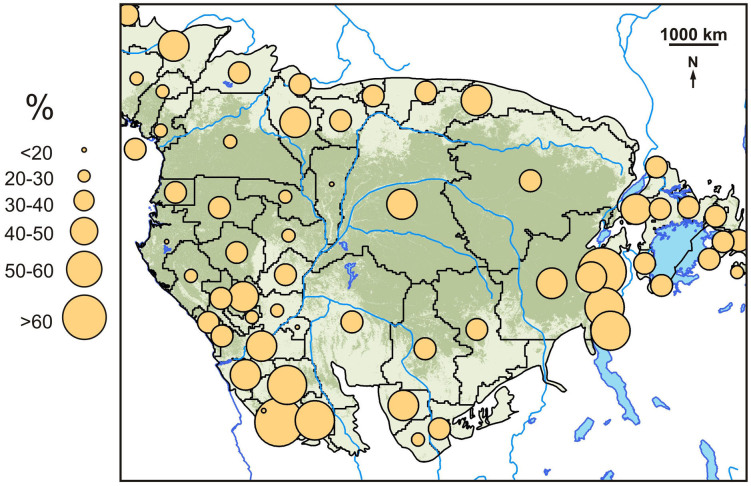
We used a global map of the distribution of chronic undernutrition at national and subnational levels depicting stunting in growth among children under five years of age20 (Fig. 2). This map, generated by the FAO, employs stunting as a measure of prevalence of chronic undernutrition. Stunting here is defined as height-for-age below minus two standard deviations from the international growth reference standard (National Center for Health Statistics/World Health Organization). This indicator reflects long-term cumulative effects of inadequate food intake and poor health conditions as a result of lack of hygiene and recurrent illness in poor and unhealthy environments.
Figure 2.
Prevalence of stunting among children under five20.
Circles are located at the centroids of subnational units providing data. Circle size indicates prevalence. Map was generated using ArcGIS.
Results
Huntable mammalian diversity and standing crop biomass
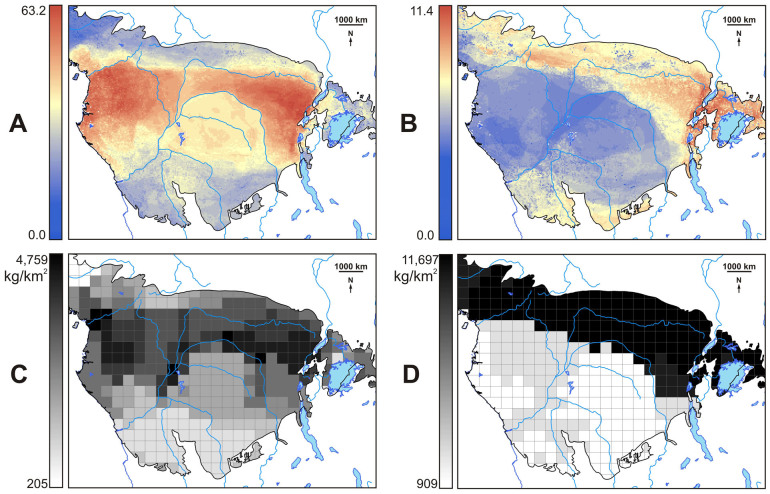
We distinguished two separate mammalian assemblages: (1) a Deep Rainforest Diversity (DRD), largely composed of low hunting-resilient species i.e. large-bodied, slow reproducing taxa, mostly found within wet Guinea-Congolian lowland rainforest in the center of the RBZ (Fig. 3A) and (2) a Marginal Rainforest Diversity (MRD), comprised of high hunting-resilient taxa, i.e. smaller-bodied, fast-reproducing mammals inhabiting the woody savanna/grasslands in the northern, eastern and southern RBZ21 (Fig. 3B).
Figure 3.
Diversity and standing biomass of mammals in central Africa.
(A) Deep Rainforest Diversity, DRD. (B) Marginal Rainforest Diversity, MRD. DRD and MRD are the accumulated favorability values, weighted by hunting sustainability values, of all hunted mammals (Appendix S1) found within the Rainforest Biotic Zone. (C) Potential standing biomass in DRD mammals. (D) Potential standing biomass in MRD mammals. Maps were generated using ArcGIS.
Total standing crop mammalian biomass within each mammalian assemblage correlated significantly and positively with both DRD (n = 367 grid cells; r = 0.167; P < 0.001) and MRD areas (n = 367 grid cells; r = 0.595; P < 0.001). However, potential standing biomass of mammal species of low hunting potential19 was significantly and positively correlated with DRD areas (n = 367 grid cells; r = 0.652; P < 0.001). Likewise, the potential standing biomass of mammal species of high hunting potential19 was significantly and positively correlated with MRD areas (n = 367 grid cells; r = 0.773; P < 0.001).
Using standing biomass as a surrogate of potential wild meat resources available to humans, we showed that higher mammalian biomass was typical of MRD but not of DRD areas, despite the latter areas having six times more diversity than MRD areas (Figs. 3A and 3B). Potential standing biomass in DRD areas (Fig. 3C) was lower (mean ± SE = 1,805 ± 1,074 kg/km2, median = 1,535 kg/km2, range = 205–4,759 kg/km2) than that in MRD areas (Fig. 3D) (mean ± SE = 5,618 ± 4,296 kg/km2, median = 2,461 kg/km2, range = 909–11,697 kg/km2).
Bushmeat extraction patterns
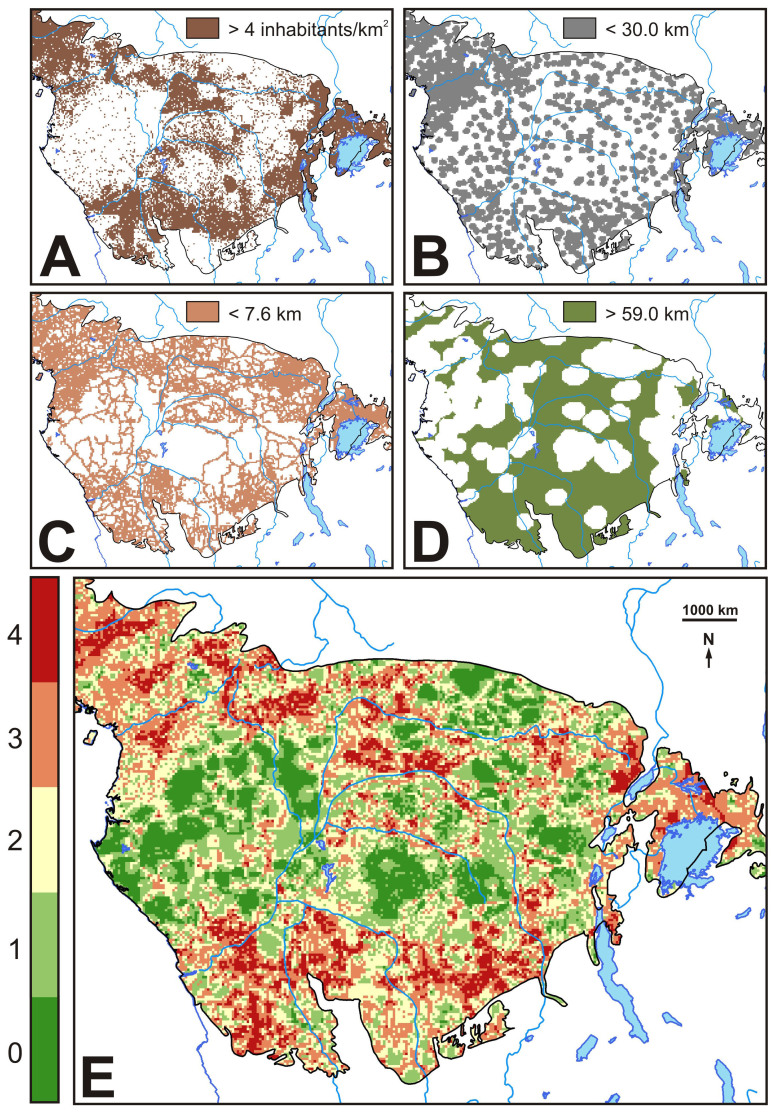
The overlay map of urban, road networks, protected areas and densely populated rural areas within the RBZ (see Methods) (Fig. 4) indicated that potential hunting intensity was higher in the MRD areas but lower in the DRD areas.
Figure 4.
Anthropogenic pressures.
(A) Brown: above median areas of rural human population density. (B) Grey: below median areas of distance to urban areas. (C) Pink: below median areas of distance to roads. (D) Green: above median areas of distance to protected areas. (E) Bushmeat extraction patterns emerging from the overlay of urban areas, road networks, protected areas and densely populated rural areas (areas with a total score of 4 had the highest bushmeat extraction potential, whereas areas with a total score of a 0 had the lowest). Maps were generated using ArcGIS.
Stunting, mammalian diversity and standing biomass
Stunting was unevenly distributed throughout the study region with more stunting occurring away from the central DRD areas (Fig. 2). Stunting was negatively correlated with mammalian diversity in DRD areas (n = 60; r = −0.288; P = 0.027) but positively associated to MRD areas (n = 60; r = 0.325; P = 0.012). Bushmeat extraction values were positively correlated with the prevalence of child stunting (n = 60; r = 0.373; P < 0.005) and with mammalian diversity in the MRD areas (n = 60; r = 0.484; P < 0.001). Extraction was negatively correlated with mammalian diversity in the DRD areas (n = 60; r = −0.469; P < 0.001).
Hypothesis testing
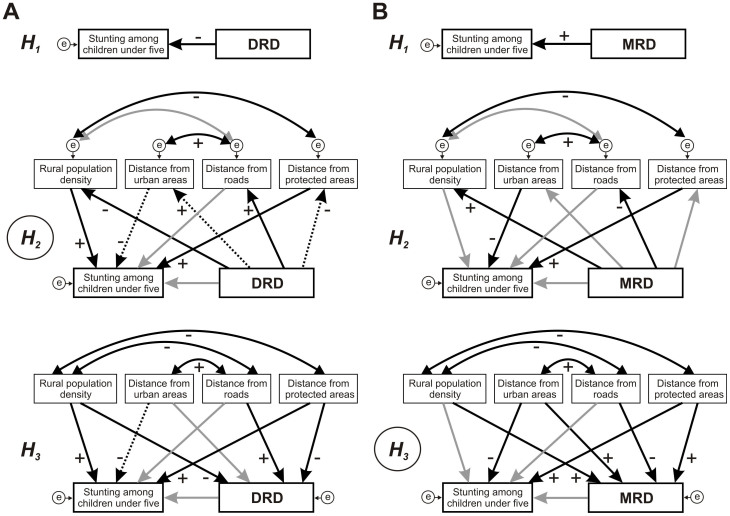
We found no evidence to support H1 for both DRD and MRD areas. Any direct relationship lost statistical significance when other factors were included in the models (Fig. 5; Table 1). Domestic meat was excluded from all models because it neither showed significant relationships with stunting among children nor influenced the rest of relationships among variables (compare Table 1 and Fig. 5 with Table S1 and Fig. S1 in Appendix S2). The inclusion of domestic meats enlarged the differences between observed and expected covariance matrices (see χ2 in Table S2, Appendix S2).
Figure 5.
Path diagrams representing relationships between diversity and stunting among children.
Three hypotheses are tested: H1 (direct relationship); H2 (diversity influences human variables and these influence stunting); H3 (human variables influence both diversity and stunting). (A) Models for Deep Rainforest Diversity (DRD). (B) Models for Marginal Rainforest Diversity (MRD). Circles enclosing "e": error terms associated to dependent variables. Solid black arrows: significant relationships (n = 60; P < 0.05); dashed black arrows: 0.05 < P < 0.07; grey arrows: non-significant relationships (P > 0.07); double arrows: covariance between variables, which are considered in the diagrams when significant correlations were identified within the study area (P < 0.05). +: Positive relationship; - : Negative relationship. Encircled hypotheses (H2 in A and H3 B) indicate the best fitted models.
Table 1.
Standardized weights (SW) and statistical significance (P) of regressions. Hypotheses tested for the relationship between mammal diversity and stunting: H1 (direct relationship); H2 (diversity influences human variables and these influence stunting); H3 (human variables influence both diversity and stunting). DRD: Deep Rainforest Diversity; MRD: Marginal Rainforest Diversity. To identify dependent and independent variables, see Fig. 5
| Diversity | Stunting prevalence | |||
|---|---|---|---|---|
| SW | P | SW | P | |
| H1 - DRD | ||||
| DRD | −0.288 | 0.022 | ||
| H2 -DRD | ||||
| DRD | −0.024 | 0.869 | ||
| Rural population density | −0.394 | 0.001 | 0.342 | 0.016 |
| Distance from urban areas | 0.235 | 0.066 | −0.264 | 0.058 |
| Distance from roads | 0.509 | <0.001 | 0.012 | 0.939 |
| Distance from protected areas | −0.234 | 0.067 | 0.323 | 0.014 |
| H3 - DRD | ||||
| DRD | −0.024 | 0.870 | ||
| Rural population density | −0.358 | 0.003 | 0.351 | 0.015 |
| Distance from urban areas | −0.098 | 0.427 | −0.268 | 0.054 |
| Distance from roads | 0.391 | 0.003 | 0.012 | 0.940 |
| Distance from protected areas | −0.364 | <0.001 | 0.328 | 0.015 |
| H1 - MRD | ||||
| MRD | 0.325 | 0.009 | ||
| H2 - MRD | ||||
| MRD | 0.145 | 0.376 | ||
| Rural population density | 0.643 | <0.001 | 0.261 | 0.117 |
| Distance from urban areas | −0.103 | 0.432 | −0.297 | 0.035 |
| Distance from roads | −0.444 | <0.001 | 0.044 | 0.781 |
| Distance from protected areas | 0.019 | 0.886 | 0.298 | 0.019 |
| H3 - MRD | ||||
| MRD | 0.149 | 0.378 | ||
| Rural population density | 0.643 | <0.001 | 0.263 | 0.125 |
| Distance from urban areas | 0.231 | 0.030 | −0.300 | 0.036 |
| Distance from roads | −0.269 | 0.018 | 0.043 | 0.781 |
| Distance from protected areas | 0.241 | 0.011 | 0.301 | 0.020 |
H2 was more consistent than H3 for DRD areas, (Fig. 5A; Table 2). Arrow signs linking DRD areas with the four main human variables were opposite to those linking these variables to bushmeat extraction. This suggests that remoteness to human agglomerations and infrastructures was linked to lower levels of bushmeat offtake. Moreover, child stunting was higher in rural and urban areas of higher human concentrations, but lower in those areas closer to protected areas.
Table 2.
Fit summary for models relating mammal diversity, stunting and human pressure. χ2: test of differences between observed and expected covariance matrices; P: statistical significance of χ2; TLI: Tucker-Lewis Index; CFI: Comparative Fit Index; NFI: Normed Fit Index; RMSEA: Root Mean Square Error of Approximation; AIC: Akaike Information Criterion. Hypotheses tested for the relationship between diversity and stunting: H2 (diversity influences human variables and these influence stunting); H3 (human variables influence both diversity and stunting). DRD: Deep Rainforest Diversity; MRD: Marginal Rainforest Diversity. All statistics show best fit of H2 for DRD and of H3 for MRD
| χ2 | P [d.f. = 3] = 3] | TLI | CFI | NFI | RMSEA | AIC | |
|---|---|---|---|---|---|---|---|
| H2 - DRD | 3.572 | 0.312 | 0.964 | 0.993 | 0.962 | 0.057 | 51.572 |
| H3 - DRD | 4.573 | 0.206 | 0.900 | 0.980 | 0.951 | 0.095 | 52.573 |
| H2 - MRD | 4.905 | 0.179 | 0.898 | 0.980 | 0.955 | 0.105 | 52.905. |
| H3 - MRD | 4.573 | 0.206 | 0.916 | 0.983 | 0.958 | 0.095 | 52.573 |
In MRD areas, H3 was better supported than H2 (Fig. 5B; Table 2). In this model, arrow signs linking the human variables with MRD were the same as those linking human variables to bushmeat extraction, with the exception of a positive relationship with distance to urban areas.
Discussion
There is growing evidence that forest cover and dietary diversity are correlated in Africa22. Forest foods help maintain household nutrition in many communities, especially during lean seasons (complementing, for example, the seasonality of staple agricultural crops), in times of low agricultural production, during periods of climate-induced vulnerability and when gaps in the availability of food occur due to other cyclical events. Animal source food consumption, however, was not related to tree cover23, perhaps because wildlife is the main source of nutrients in many tropical forest and non-forest regions2,24. A significant proportion of the wildlife biomass hunted by humans for food across the tropics, especially large-bodied primates, ungulates and rodents (average weight greater than 1 kg), is found in tropical rainforests, with ungulates and sometimes rodents dominating the biomass in more open habitats25. Animal-based foods supply many important micronutrients in much higher amounts or with higher bioavailability than most plant-based foods26 and as attested in Golden et al.'s (2011)9 study on the importance of wild meat in reducing iron deficiency anemia in children.
There are growing concerns that any decline in the availability of wild meat will threaten the food security and livelihoods of forest communities27, especially those in which home consumption is more common than wild-meat trading. However, the relationship between wild meat availability and human nutrition may vary according to habitat type and region. In our study, we show, for the first time, that the relationship between hunted mammalian diversity, which in turn is linked to wild meat availability and human pressure, are correlated with children malnutrition levels. We show that the more remote forest areas within central African rainforests seem capable of adequately supporting existing human populations at a reasonable level of health. This contrasts with the more highly populated woody savannas/grasslands along the northern, eastern and southern RBZ that are under much higher anthropogenic pressures i.e. more bushmeat extraction. Hence, this spatial disparity in human needs and bushmeat supply present challenges for development and conservation.
Although our analyses are based on inferences made from correlations between interacting environmental and nutrition variables, our results correspond with others on the state of conservation of habitats and fauna in the Congo Basin. For example, our data points to the importance of the central rainforest blocks as significant regions of continued forest protection28. Such ‘deep forest’ faunas, though also under substantial threats28, are currently under less anthropogenic pressures than the ecotonal regions along the margins of the RBZ. Human activity in these more open habitats, primarily burning and land clearing for cultivation is intense29. Moreover, anthropogenic disturbance around cities has led to significant decreases in faunal diversity30. In our MRD model, proximity to urban areas is the only human-pressure variable significantly explaining stunting, but closer to protected areas stunting is less.
An immediate consequence of our study should be to rouse producer governments to put appropriate management regimes in place to integrate the bushmeat issue into the discussion on assessment of environmental assets. This is not new7,8, but here we advance the debate by presenting a more complex scenario, in which deep rainforest wildlife may still support food security of hunter-gatherers and others on condition that human concentrations are kept low. Instead, along the RBZ margins, composed of more sustainable wildlife and more productive in terms of wild meat, higher population densities here explain the observed levels of malnutrition. More specifically, adequate human nutrition is likely in rural landscapes, but as our analyses show, collapses around urban areas, where child malnutrition is more prevalent. Although our results require further empirical tests and more work on the ground to investigate how the different drivers affect malnutrition and the role wildlife plays, the strong correlations we confirm between wild meat and malnutrition are noteworthy. We thus argue that this is not a spurious effect, but one that powerfully points to the significance of wild meat in sustaining human populations in central Africa. However, our results should be considered of heuristic value and this stage not to be used to propose unfettered access rights for the poor nor draconian conservation schemes. What it does underline, rather, is the need to consider a wider political agenda for developing practical policies that benefit both people and biodiversity. Emerging strategies from this framework would increase public recognition of bushmeat's economic value and the need to regulate and plan its use, but it would also emphasize the need for adequate and accessible alternative food sources to overturn the malnutrition levels seen along the marginal RBZ habitats. All this would raise an interesting set of questions about (for example) the relationship between natural resource use and economic growth, or between effective conservation and resilient development.
Methods
Mapping mammalian assemblages
We distinguished two separate mammalian assemblages, DRD and MRD, in central Africa based on Fa et al.'s (2014)19 analysis of differing capacity for hunting sustainability of each species. For this, we employed two indices based on accumulating favorability values obtained through distribution modeling, for every species in every locality19,31. Favorability models define to which degree environmental conditions at each locality favor the species' presence, independently of the species' prevalence32,33. We built favorability models for 165 species (see Datasets section and Appendix S1) using 1° × 1°-resolution presences and absences derived from IUCN's (2014)34 range maps. At this spatial resolution, models based on extent-of-occurrence maps are still meaningful35. We trained the models using 27 variables describing climate, topo-hydrography, land cover/use and other anthropogenic forces (see Fa et al. 201419 for more details). We then used the "direct downscaling approach" to project all models to a 0.1° × 0.1° resolution grid36. Only favorability values where species are known to occur according to the IUCN (2014)34 were retained. Here, favorability values for every subspecies were considered separately.
MRD values corresponded to the "Sustainable Accumulated Favorability" (SAFj) in Fa et al. (2014)19. This index was calculated by adding up the favorability value (Fi) of all i taxa in each j cell in the study area, after each taxon's favorability was weighted according to the taxon's potential resilience to hunting (Potential Hunting Sustainability, PHS, see the "restrictive" approach in Fa et al. 201419). PHS was measured according to four ecological traits that are linked with extinction proneness19,37: population density, habitat breadth, rarity and vulnerability. SAFj (and so MRD) was finally computed as follows:
DRD values corresponded, instead, to the "Unustainable Accumulated Favorability" (UAFj) in Fa et al. (2014)19, which was computed as follows:
Mapping SAFj and UAFj revealed the existence of two partially disjoint mammalian assemblages, respectively located in the northern, eastern and southern margins of the rainforest region (hence MRD) and in the Guinea-Congolian rainforest blocks (hence DRD).
Mammalian standing biomass
We assessed wild meat availability by estimating the standing crop mammalian biomass existing in a 1° × 1°-resolution grid of the study region. Standing biomass was estimated as a function of the number of occurring species (>1 kg in weight and known to be hunted19), the mean population density of every species and the mean body size of each species' individuals. Species occurrences were taken from IUCN (2014)34, body sizes from Kingdon et al. (2013)15 and population densities derived from various sources. Mean density data for 53 (32%) species were taken from the PanTHERIA world mammal database38; 15 (9%) from Fa & Purvis (1997)39; and for 97 (59%) other taxa we derived expected values from the linear regression of log population density on log body mass. This regression, of high statistical significance (n = 949 species; r = 0.574; P < 0.001), was performed using data contained in PanTHERIA38.
To calculate the potential mammal standing biomass of a given 1° × 1° grid cell, we first multiplied, for every species occurring in the grid, its mean population density and mean body size. We then summed the products of these multiplications. Four species [savanna elephant (Loxodonta africana), forest elephant (L. cyclotis), hippopotamus (Hippopotamus amphibius) and forest buffalo (Syncerus caffer nanus)] were excluded from our calculations because, although hunted for meat40, are only occasional prey and thus do not represent an important source of wild meat.
Potential standing biomass in mammals of low hunting potential was calculated considering only species with Potential Hunting Sustainability (PHS) < (mean PHS - standard error, SE) (i.e. PHS < 0.06 in Appendix S1). Likewise, to calculate the potential standing biomass of mammal species of high hunting potential we considered all species with PHS > (mean PHS + SE) (i.e. PHS > 0.09 in Appendix S1).
Bushmeat extraction patterns
The concentration of human populations, their accessibility to hunting areas, as well as the presence of protected areas have been reported as significant predictors of bushmeat extraction intensity in the Congo Basin41. From this, we considered four relevant anthropogenic variables in our models that could determine potential bushmeat extraction levels in our study area: (1) rural human population density— assumed to be the population fraction engaged in hunting42—, (2) proximity to urban areas—representing non-subsistence bushmeat demanding areas43—, (3) proximity to roads— as a measure of access to hunting areas—and (4) distance to protected areas — often reservoirs areas for many species (for variable sources, see Supplementary Information). We estimated the spatial distribution of potential bushmeat extraction throughout the RBZ, by first classifying each of the four variables in 0.1° × 0.1° resolution maps with a 1 if above the median and with a 0 if below the median. Resulting maps for each variable were finally summed, so that areas with a total score of 4 had the highest bushmeat extraction potential, whereas areas in with a total score of a 0 had the lowest. We assessed the suitability of our proxy by testing the correlation with Ziegler et al.'s41 (in press) model in the Congo Basin, using average values for both estimations on 1° × 1° grids. Our extraction model and Ziegler's et al. were highly correlated (n = 60; r = 0.803; P < 0.001).
Statistical methods
The consistence of the above-listed hypotheses was tested using Structural Equation Modelling44. A set of interrelated variables were linked to each-other according to a priori models following the working hypotheses (Appendices 3 and 4), which were designed as diagrams describing a system of possible relationships among response and predictor variables (Fig. 5 and Fig. S1 in Appendix S1). These variables were DRD, MRD, prevalence of stunting among children, rural human population density, distance to urban areas, distance to roads, distance to protected areas and domestic meat (for variable sources, see Supplementary Information). Structural Equation Modelling, basically an extension of Path Analysis45 allowing for model comparison, was used to assess the diagrams (hypothesis-testing studies using this approach46,47,48). Cause-and-effect relationships were depicted by one-headed arrows and every arrow was given a path coefficient that can be either significant or not. This coefficient is a standard partial regression coefficient45 and measures the strength of a relationship as a proportion of the total standard deviation (Table 1). Thus, variables that, in isolation, are highly correlated can be given low path coefficients as a result of indirect relationships between third variables. Covariances between independent variables were considered in the diagrams when significant correlations were identified within the study area (n = 60; P < 0.05). We used 60 sub-national administrative units as the basis for the analysis (Fig. 1), because the original data of stunting among children were only available on this geographical support20. We, thus, used average values of the rest of variables, referred to the 60 units of reference.
The goodness of fit of each structural equation model to data was assessed using five parameters (table S2): (1) a χ2 statistic test of the differences between observed and expected covariance matrices, quantified by a likelihood function49; (2) the Tucker-Lewis Index (TLI)50; (3) the Comparative Fit Index (CFI)50; (4) the Normed Fit Index (NFI)51,52; (5) the Root Mean Square Error of Approximation (RMSEA)53,54; the Akaike Information Criterion (AIC)55. Accepting a model requires χ2 being non-significant and as small as possible; TLI, CFI and NFI values close to one indicate a very good fit; RMSEA should be lower than 0.1 and as small as possible. The best model should minimize AIC as well.
Electronic supplementary material
Supplementary Information
Supplementary Information
Acknowledgements
This work was funded by the KnowFor (International Forestry Knowledge) initiative of the UK Department for International Development (UKAID). We are also grateful to USAID and the CGIAR programme on Forests, Trees and Agroforestry for support. The paper is a product of the CIFOR Bushmeat Research Initiative.
Author Contributions
J.E.F. and J.O. designed the project. J.E.F. and J.O. wrote the main manuscript text. J.O., R.R., M.A.F., A.L.M. and J.E.F. analyzed the data and prepared the maps and figures. D.B., J.M.V., B.M. and R.N. provided contextual data. All authors reviewed and commented on the manuscript.
Competing interests
The authors declare no competing financial interests.
References
- Fa JE, Peres CA, Meeuwig J. Bushmeat exploitation in tropical forests: an intercontinental comparison. Conserv Biol. 2002;16:232–237. doi: 10.1046/j.1523-1739.2002.00275.x. [DOI] [PubMed] [Google Scholar]
- Nasi R, Taber A, Van Vliet N. Empty forests, empty stomachs? Bushmeat and livelihoods in the Congo and Amazon Basins. Int Forest Rev. 2011;13:355–368. doi: 10.1505/146554811798293872. [DOI] [Google Scholar]
- Fa JE, Currie D, Meeuwig J. Bushmeat and food security in the Congo basin: linkages between wildlife and people's future. Environ Conserv. 2003;30:71–78. doi: 10.1017/S0376892903000067. [DOI] [Google Scholar]
- Ziegler S. Application of food balance sheets to assess the scale of the bushmeat trade in Central Africa. TRAFFIC Bull. 2011;22:1–12. [Google Scholar]
- Brashares JS, et al. Economic and geographic drivers of wildlife consumption in rural Africa. P Natl Acad Sci USA. 2011;108:13931–13936. doi: 10.1073/pnas.1011526108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E, et al. Hunting for consensus: reconciling bushmeat harvest, conservation and development policy in west and central Africa. Conserv Biol. 2007;21:884–887. doi: 10.1111/j.1523-1739.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- Brown D, Williams A. The case for bushmeat as a component of development policy: Issues and challenges. Int Forest Rev. 2003;5:148–155. doi: 10.1505/IFOR.5.2.148.17414. [DOI] [Google Scholar]
- Brown, D., Fa, J. E. & Gordon, L. Assessment of Recent Bushmeat Research and Recommendations to Her Majesty's Government (DEFRA, Bristol, 2006).
- Golden C, et al. Benefits of wildlife consumption to child nutrition in a biodiversity hotspot. P Natl Acad Sci USA. 2011;108:19653–19656. doi: 10.1073/pnas.1112586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard, I. & Wijayaratne, K. B. S. The Use of Stunting and Wasting as Indicators for Food Insecurity and Poverty (Integrated Food Security Programme, Sri Lanka, 2002).
- Wondimagegn ZT. Magnitude and determinants of stunting among children in Africa: a systematic review. Curr Res Nutr Food Sci J. 2014;2.2:88–93. doi: 10.12944/CRNFSJ.2.2.05. [DOI] [Google Scholar]
- Custodio E, et al. Nutritional and socio-economic factors associated with Plasmodium falciparum infection in children from Equatorial Guinea: results from a nationally representative survey. Malaria J. 2009;8:225. doi: 10.1186/1475-2875-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner S, et al. Influence of helminth infections on childhood nutritional status in lowland Bolivia. Am J Hum Biol. 2009;21:651–656. doi: 10.1002/ajhb.20944. [DOI] [PubMed] [Google Scholar]
- Caulfield, L. E. et al. Chapter 28: Stunting, wasting and micronutrient deficiency disorders. In: Jamison, D. T., Breman, J. G. & Measham, A. R. et al. (Eds.), Disease Control Priorities in Developing Countries. 2nd edition. [551–567] (Washington (DC), World Bank, 2006).
- Kingdon, J. et al. (Eds)Mammals of Africa: 6 Vols. (Bloomsbury Publishing London, 2013).
- White, F. The vegetation of Africa: A descriptive memoir to accompany the UNESCO/AETFAT/UNS Vegetation Map of Africa. (UNESCO, Paris, 1983).
- De Wasseige, C. et al. Les Forêts du Bassin du Congo – Etat des Forêts 2010. (Office des Publications de l'Union Européenne, Luxembourg., 2012).
- Friedl MA, et al. MODIS Collection 5 global land cover: Algorithm refinements and characterization of new datasets. Remote Sens Environ. 2010;114:168–182. doi: 10.1016/j.rse.2009.08.016. [DOI] [Google Scholar]
- Fa Julia E., Olivero Jesús, Farfán Miguel Ángel, Márquez Ana Luz, Vargas Juan Mario, Real Raimundo, Nasi Robert. Integrating Sustainable Hunting in Biodiversity Protection in Central Africa: Hot Spots, Weak Spots, and Strong Spots. PLoS ONE. 2014;9(11):e112367. doi: 10.1371/journal.pone.0112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Chronic Undernutrition among Children: An Indicator of Poverty (FAO/SDRN & ESNA, Rome, 2003).
- Happold, D. & Lock, J. M. The biotic zones of Africa In: Kingdon, J., Happold, D., Hoffmann, M., Butynski, T., Happold, M. & Kalina, J. (Eds.). The Mammals of Africa: Volume 1. Introductory Chapters and Afrotheria. [57–74] (Bloomsbury Press, London, 2013).
- Colfer, C. J. P. Human Health and Forests: Global Overview of Issues, Practice and Policy. (London, Earthscan, 2008).
- Ickowitz A, Powell B, Salim MA, Sunderland TCH. Dietary quality and tree cover in Africa. Global Environ. Chang. 2014;24:287–294. doi: 10.1016/j.gloenvcha.2013.12.001. [DOI] [Google Scholar]
- Arnold M, Powell B, Shanley P, Sundernald TCH. Forests, biodiversity and food security. Int Forest Rev. 2011;13:259–264. doi: 10.1505/146554811798293962. [DOI] [Google Scholar]
- Robinson JG, Bennett EL. Having your wildlife and eating it too: an analysis of hunting sustainability across tropical ecosystems. Anim Conserv. 2004;7:397–408. doi: 10.1017/S1367943004001532. [DOI] [Google Scholar]
- Murphy SP, Allen LA. Nutritional importance of animal source foods. J Nutr. 2003;133:3932S–35S. doi: 10.1093/jn/133.11.3932S. [DOI] [PubMed] [Google Scholar]
- Heywood, V. Overview of agricultural biodiversity and its contribution to nutrition and health. In: Fanzo, J., Hunter, D., Borelli, T. & Mattei, F. (Eds.), Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health Issues in Agricultural Biodiversity [35–67] (London, Earthscan, 2013).
- Usongo L, Nagahuedi J. Participatory land-use planning for priority landscapes of the Congo Basin. Unasylva. 2008;59:17–24. [Google Scholar]
- Favier C, et al. Modelling forest-savanna mosaic dynamics in man-influenced environments: effects of fire, climate and soil heterogeneity. Ecol Model. 2004;171:85–102. doi: 10.1016/j.ecolmodel.2003.07.003. [DOI] [Google Scholar]
- Mertens B, Lambin EF. Land-cover-change trajectories in Southern Cameroon. Ann Assoc Am Geogr. 2000;90:467–494. doi: 10.1111/0004-5608.00205. [DOI] [Google Scholar]
- Estrada A, Real R, Vargas JM. Using crisp and fuzzy modelling to identify favourability hotspots useful to perform gap analysis. Biodiver Conserv. 2008;17:857–871. doi: 10.1007/s10531-008-9328-1. [DOI] [Google Scholar]
- Real R, et al. Obtaining environmental favourability functions from logistic regression. Environ Ecol Stat. 2006;13:237–245. doi: 10.1007/s10651-005-0003-3. [DOI] [Google Scholar]
- Acevedo P, Real R. Favourability: concept, distinctive characteristics and potential usefulness. Naturwissenschaften. 2012;99:515–522. doi: 10.1007/s00114-012-0926-0. [DOI] [PubMed] [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2012.1. Available at: http://www.iucnredlist.org. (Accessed: 11th November 2014).
- Hurlbert AH, Jetz W. Species richness, hotspots and the scale dependence of range maps in ecology and conservation. P Natl Acad Sci USA. 2007;104:13384–13389. doi: 10.1073/pnas.0704469104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombi P, d'Amen M. Scaling down distribution maps from atlas data: a test of different approaches with virtual species. J Biogeogr. 2012;39:640–651. doi: 10.1111/j.1365-2699.2011.02627.x. [DOI] [Google Scholar]
- Cardillo M, et al. Multiple causes of high extinction risk in large mammal species. Science. 2005;309:1239–1241. doi: 10.1126/science.1116030. [DOI] [PubMed] [Google Scholar]
- Jones KE, et al. PanTHERIA: a species-level database of life history, ecology and geography of extant and recently extinct mammals. Ecology. 2009;90:2648. doi: 10.1890/08-1494.1. [DOI] [Google Scholar]
- Fa JE, Purvis A. Body size, diet and population density in Afrotropical forest mammals: a comparison with neotropical species. J Anim Ecol. 1997;66:98–112. doi: 10.2307/5968. [DOI] [Google Scholar]
- Taylor G, et al. Synthesising bushmeat research effort in West and Central Africa: A new regional database. Biol Conserv. 2015;181:199–205. doi: 10.1016/j.biocon.2014.11.001. [DOI] [Google Scholar]
- Ziegler, S. et al. Mapping bushmeat extraction levels in central Africa. Biotropica (in press)
- Wilkie DS, Carpenter JF. Bushmeat hunting in the Congo Basin: an assessment of impacts and options for mitigation. Biodiver Conserv. 1999;8:927–955. doi: 10.1023/A:1008877309871. [DOI] [Google Scholar]
- Fa JE, Brown D. Impacts of hunting on mammals in African tropical moist forests: a review and synthesis. Mamm Rev. 2009;39:231–264. doi: 10.1111/j.1365-2907.2009.00149.x. [DOI] [Google Scholar]
- Mitchell RJ. Testing evolutionary and ecological hypotheses using path analysis and structural equation modeling. Funct Ecol. 1992;6:123–129. doi: 10.2307/2389745. [DOI] [Google Scholar]
- Sokal, R. R. & Rohlf, F. J. Biometry. (WH Freeman and Company, New York, 1981).
- Grace JB, Pugesek BH. A structural equation model of plant species richness and its application to a coastal wetland. Am Nat. 1997;149:436–460. doi: 10.1086/285999. [DOI] [Google Scholar]
- Márquez AL, Real R, Vargas JM. Dependence of broad-scale geographical variation in freshy-fruited plant species richness on disperser bird species richness. Global Ecol Biogeogr. 2004;13:295–304. doi: 10.1111/j.1466-822X.2004.00100.x. [DOI] [Google Scholar]
- Carvalho GH, et al. Are fire, soil fertility and toxicity, water availability, plant functional diversity and litter decomposition related in a Neotropical savanna. Oecologia. 2014;175:923–935. doi: 10.1007/s00442-014-2937-3. [DOI] [PubMed] [Google Scholar]
- Hayduk, L. A. Structural Equation Modeling with LISREL, Essentials and Advances. (The Johns Hopkins University Press, Baltimore, 1987).
- Hair, J. F., Anderson, R. E., Tatham, R. L. & Black, W. C. Multivariate Data Analysis. (Prentice Hall, Englewood Cliffs NJ., 1995).
- Byrne, B. M. Structural Equation Modeling with EQS and EQS/Windows. (Sage Publications, Thousand Oaks CA, 1994).
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88:588–606. doi: 10.1037/0033-2909.88.3.588. [DOI] [Google Scholar]
- Browne, M. W. & Cudeck, R. Alternative ways of assessing model fit, In: Bollen, K. A. & Long, J. S. (Eds.), Testing Structural Equation Models [36–162] (Sage, Newbury Park CA, 1993).
- Kaplan, D. Structural Equation Modeling: Foundation and Extensions. (Sage Publications, Thousand Oaks CA, 2000).
- Akaike, H. Information theory and an extension of the maximum likelihood principle, In: Petrov, B. N., Csaki, F. (Eds.), Proceedings of the Second International Symposium on Information Theory [267–281] (Akadémia Kiadó, Budapest, 1973).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information