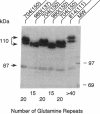
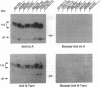
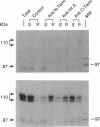
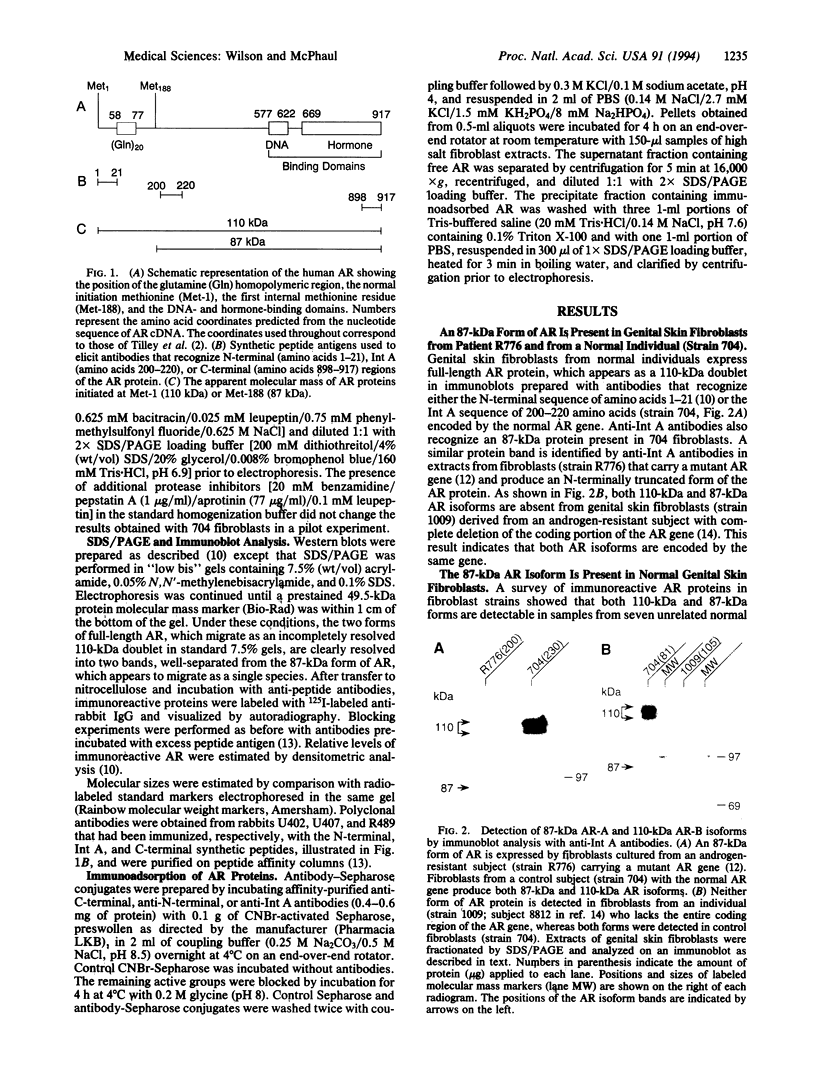
Abstract
Two forms of the androgen receptor (AR) protein (apparent molecular masses, approximately 110 kDa and approximately 87 kDa) are present in human genital skin fibroblasts. The 87-kDa isoform (AR-A) contains an intact C terminus but lacks the normal N terminus found in the 110-kDa isoform (AR-B). AR-A is the same size as the mutant form of AR produced in fibroblasts from an androgen-resistant individual (R776) by initiation of AR synthesis at the internal Met-188 residue of AR-B. The ratio of AR-B to AR-A in fibroblasts derived from normal individuals is approximately 10:1. The AR isoforms detected in these experiments resemble the A and B forms of the human progesterone receptor, which also are encoded by a single gene and differ by the absence or presence of an N-terminal segment. The A and B forms of the human progesterone receptor differ in their ability to activate target genes and are regulated differently in various types of cells. The identification of similar forms of human AR raises the possibility that AR-A and AR-B also subserve different functions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely O. M., Kettelberger D. M., Tsai M. J., Schrader W. T., O'Malley B. W. The chicken progesterone receptor A and B isoforms are products of an alternate translation initiation event. J Biol Chem. 1989 Aug 25;264(24):14062–14064. [PubMed] [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992 Feb;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeyer H., Meyer M. E., Bocquel M. T., Kastner P., Turcotte B., Chambon P. Progestin receptors: isoforms and antihormone action. J Steroid Biochem Mol Biol. 1991;40(1-3):271–278. doi: 10.1016/0960-0760(91)90192-8. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Alexander P. S. In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology. 1983 Dec;113(6):2195–2201. doi: 10.1210/endo-113-6-2195. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. The molecular biology of RU486. Is there a role for antiprogestins in the treatment of breast cancer? Endocr Rev. 1992 May;13(2):146–163. doi: 10.1210/edrv-13-2-146. [DOI] [PubMed] [Google Scholar]
- Husmann D. A., Wilson C. M., McPhaul M. J., Tilley W. D., Wilson J. D. Antipeptide antibodies to two distinct regions of the androgen receptor localize the receptor protein to the nuclei of target cells in the rat and human prostate. Endocrinology. 1990 May;126(5):2359–2368. doi: 10.1210/endo-126-5-2359. [DOI] [PubMed] [Google Scholar]
- Ilenchuk T. T., Walters M. R. Rat uterine progesterone receptor analyzed by [3H]R5020 photoaffinity labeling: evidence that the A and B subunits are not equimolar. Endocrinology. 1987 Apr;120(4):1449–1456. doi: 10.1210/endo-120-4-1449. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G. G., de Ruiter P. E., Brinkmann A. O. Androgen receptor heterogeneity in LNCaP cells is caused by a hormone independent phosphorylation step. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):697–700. doi: 10.1016/0960-0760(92)90407-a. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Loosfelt H., Logeat F., Vu Hai M. T., Milgrom E. The rabbit progesterone receptor. Evidence for a single steroid-binding subunit and characterization of receptor mRNA. J Biol Chem. 1984 Nov 25;259(22):14196–14202. [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sar M., Tan J., Higgs H. N., Larson R. E., French F. S., Wilson E. M. The human androgen receptor: complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol. 1988 Dec;2(12):1265–1275. doi: 10.1210/mend-2-12-1265. [DOI] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius C. A., Tung L., Takimoto G. S., Horwitz K. B. Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem. 1993 May 5;268(13):9262–9266. [PubMed] [Google Scholar]
- Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct. IV. Characterization of purified subunits. J Biol Chem. 1972 Jan 10;247(1):51–59. [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L., Gronemeyer H., Turcotte B., Gaub M. P., Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988 May 12;333(6169):185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Trapman J., Klaassen P., Kuiper G. G., van der Korput J. A., Faber P. W., van Rooij H. C., Geurts van Kessel A., Voorhorst M. M., Mulder E., Brinkmann A. O. Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem Biophys Res Commun. 1988 May 31;153(1):241–248. doi: 10.1016/s0006-291x(88)81214-2. [DOI] [PubMed] [Google Scholar]
- Trifiro M., Gottlieb B., Pinsky L., Kaufman M., Prior L., Belsham D. D., Wrogemann K., Brown C. J., Willard H. F., Trapman J. The 56/58 kDa androgen-binding protein in male genital skin fibroblasts with a deleted androgen receptor gene. Mol Cell Endocrinol. 1991 Jan;75(1):37–47. doi: 10.1016/0303-7207(91)90243-l. [DOI] [PubMed] [Google Scholar]
- Tung L., Mohamed M. K., Hoeffler J. P., Takimoto G. S., Horwitz K. B. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993 Oct;7(10):1256–1265. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- Vegeto E., Shahbaz M. M., Wen D. X., Goldman M. E., O'Malley B. W., McDonnell D. P. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993 Oct;7(10):1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- Wahli W., Martinez E. Superfamily of steroid nuclear receptors: positive and negative regulators of gene expression. FASEB J. 1991 Jun;5(9):2243–2249. doi: 10.1096/fasebj.5.9.1860615. [DOI] [PubMed] [Google Scholar]
- Wilson C. M., Griffin J. E., Wilson J. D., Marcelli M., Zoppi S., McPhaul M. J. Immunoreactive androgen receptor expression in subjects with androgen resistance. J Clin Endocrinol Metab. 1992 Dec;75(6):1474–1478. doi: 10.1210/jcem.75.6.1464650. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. Syndromes of androgen resistance. Biol Reprod. 1992 Feb;46(2):168–173. doi: 10.1095/biolreprod46.2.168. [DOI] [PubMed] [Google Scholar]
- Zoppi S., Wilson C. M., Harbison M. D., Griffin J. E., Wilson J. D., McPhaul M. J., Marcelli M. Complete testicular feminization caused by an amino-terminal truncation of the androgen receptor with downstream initiation. J Clin Invest. 1993 Mar;91(3):1105–1112. doi: 10.1172/JCI116269. [DOI] [PMC free article] [PubMed] [Google Scholar]