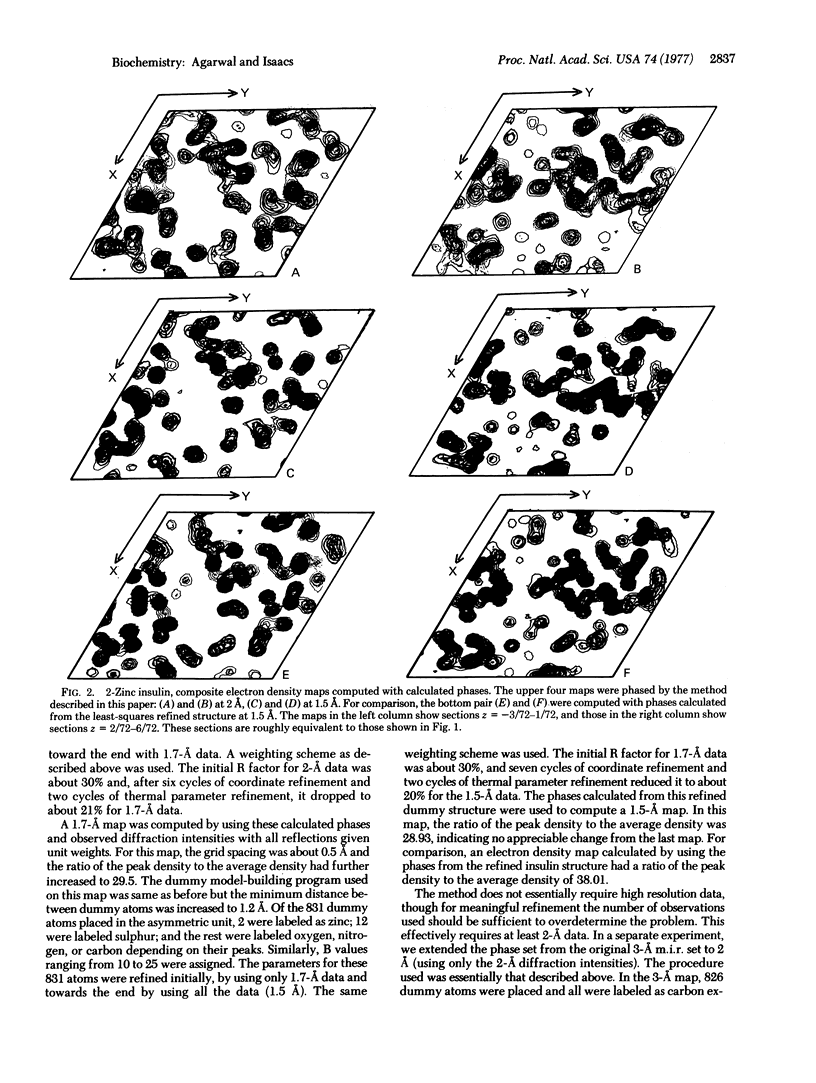
Abstract
A method is described for estimating the phases of high resolution single-crystal diffraction data from proteins, by using as a starting point a set of low resolution phases (about 3 A) derived by multiple isomorphous replacement (or other) methods. The method consists in refining by least-squares the positions and thermal parameters of a set of dummy atoms placed in the initial low resolution electron density map, so as to minimize the discrepancy between the calculated scattering intensities and the scattering intensities observed in the high resolution data set. Phases calculated from these refined atomic positions are used to extend the resolution and to improve the quality of the electron density map. The success of the method depends on a new least-squares algorithm that has a radius of convergence of about 0.75 A. This large radius of convergence, together with the severe restrictions placed on the initial positions of the dummy atoms by the requirement that they lie within limited regions of the isomorphous electron density map, and the constraint imposed by the polymeric nature of a polypeptide chain account for the success of the method. The method has been successfully used to phase the structure factors of 2-zinc insulin at a resolution of 2 A and 1.5 A, starting from a set of isomorphous phases at 3-A resolution.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins D. M., Cotton F. A., Hazen E. E., Jr, Meyer E. F., Jr, Morimoto C. N. Protein crystal structures: quicker, cheaper approaches. Science. 1975 Dec 12;190(4219):1047–1053. doi: 10.1126/science.1188383. [DOI] [PubMed] [Google Scholar]



