Abstract
Purpose
Active reduced dose tetanus-diphtheria-acellular pertussis (Tdap) vaccination for adolescents and adults is necessary because waning immunity after primary diphtheria-tetanus-pertussis vaccination is related to the recent emergence of pertussis. This study was conducted to compare the immunogenicity and protection efficacy against Bordetella pertussis between a new GCC Tdap vaccine and a commercially available Tdap vaccine in a murine model.
Materials and Methods
BALB/c mice were immunized with two doses of diphtheria-tetanus-acellular pertussis (DTaP) vaccine for priming and a subsequent Tdap booster vaccination. According to the type of booster vaccine, mice were divided into four groups: commercially available Tdap vaccine in group 1 and GCC Tdap vaccines of different combinations of pertussis antigens in groups 2 to 4. Humoral and cell-mediated immune responses and protection efficacy using a murine intranasal challenge model after booster vaccination were compared among the four groups.
Results
Every group showed significant increases in antibody titers against pertussis antigens such as pertussis toxin, filamentous hemagglutinin, and pertactin after booster vaccination. Spleen cells showed both Th1 and Th2 cell-mediated immune responses stimulated by pertussis antigens in all groups without any significant difference. In the intranasal B. pertussis infection model, bacteria were eradicated in all groups five days after challenge infection.
Conclusion
This preliminary study did not show significantly different immunogenicity or protection efficacy of the new GCC Tdap vaccines compared to the commercially available Tdap vaccine, although a more extensive study is necessary to assess the differing efficacies of the new GCC Tdap vaccines.
Keywords: Diphtheria-tetanus-acellular pertussis vaccine, Immunogenicity, Efficacy, Mice, Republic of Korea
Introduction
Pertussis is caused by Bordetella pertussis, which exclusively infects humans, and is a highly contagious respiratory disease. Pertussis had decreased with the introduction of the diphtheria-tetanus-pertussis (DTP) vaccine, although it has since re-emerged in the 1990s, even in countries where the DTP vaccination coverage rate is greater than 90% [1,2,3]. Infants who have not received the primary series of DTP vaccination are particularly vulnerable to pertussis infection [1,2,3], and adolescent and adult patients with mild or no symptoms act as a source of transmission. This re-emergence of pertussis is assumed to be caused by waning immunity after primary DTP vaccination during childhood, increase in the diagnoses of pertussis patients with the improvement of diagnostic tests, increased attention to pertussis in the community, and the emergence of vaccine-resistant B. pertussis strains. To overcome these epidemiological changes in pertussis, reduced dose tetanus-diphtheria-acellular pertussis (Tdap) vaccination for adolescents was introduced, and new vaccines against the emerging vaccine-resistant strains are necessary [4,5,6,7].
In Korea, the number of pertussis patients has been increasing since the late 2000s: nine patients in 2008, 66 in 2009, 27 in 2010, and 97 in 2011, as well as local outbreaks reported in 2012 [8,9]. Hence, the use of Tdap boosting vaccinations for adolescents and adults should be emphasized in Korea. However, no domestic pharmaceutical company produced Tdap vaccines until recently, which raises the concern of possible vaccine shortages. The Green Cross Corporation (GCC, Yongin, Korea), a Korean domestic pharmaceutical company, has developed a new Tdap vaccine, the GCC Tdap vaccine. This study was conducted to compare the immunogenicity among a commercially available Tdap vaccine (Boostirx, GlaxoSmithKline, Rixensart, Belgium) and the GCC Tdap vaccines containing different combinations of pertussis antigens. Furthermore, protective efficacy against B. pertussis in a murine respiratory challenge model was compared between the two vaccines.
Materials and Methods
Animals and immunization
BALB/c female mice (4-week-old) were used in this study and were housed in filter-top cages under standard pathogen-free conditions with food and water available ad libitum. The mice were divided into four groups according to the type of booster vaccination received, and each group included 16 mice. All mice received two doses of primary diphtheria-tetanus-acellular pertussis (DTaP) vaccine with Infanrix (GlaxoSmithKline, Rixensart, Belgium) at three-week intervals. Booster vaccines were given at six weeks after the second dose of primary vaccination. Group 1 was a positive control group, in which Boostirx was given as the booster vaccine. Groups 2 to 4 were study groups, and the doses of pertactin (PRN) differed among the three study groups. Group 2 received a standard GCC Tdap vaccine containing the same three pertussis antigens as in Boostrix: pertussis toxin (PT) 8 µg, filamentous hemagglutinin (FHA) 8 µg, and PRN 2.5 µg. Groups 3 and 4 received a moderate dose GCC Tdap vaccine (PT 8 µg, FHA 8 µg, PRN 4.0 µg) and a high dose GCC Tdap vaccine (PT 8 µg, FHA 8 µg, PRN 8.0 µg), respectively. All vaccines were administered intraperitoneally at one-fourth the human dose. This animal study was approved by the Institutional Animal Care and Use Committee (IACUC) in School of Medicine, The Catholic University of Korea (approval number: CUMC-2013-0029-02).
Assessment of humoral immune responses
Blood samples to evaluate the humoral immune response to vaccination were collected before vaccination, two weeks after the first dose of primary vaccination, one week before booster vaccination, and three weeks after booster vaccination. Blood was withdrawn from the retro-bulbar venous plexuses of 10 mice in each group at each scheduled time. Antibody titers against three pertussis antigens (PT, FHA, and PRN) were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (Alpha Diagnostic International Inc., San Antonio, TX, USA). At each scheduled time, antibody titers against each antigen were compared among the four study and control groups.
Assessment of cell-mediated immune responses
Cell-mediated immune response (CMI) was determined by measuring cytokine levels secreted by spleen cells after stimulation by pertussis antigens. Four mice in each group were sacrificed and spleens were taken at three weeks after booster vaccination. The removed spleens were homogenized to form a single cell suspension of spleen cells (2.0×106 cells/mL in RPMI 1640 medium, Life Technologies, Rockville, MD, USA). Each spleen cell suspension was stimulated with PT (5 µg/mL), FHA (5 µg/mL), PRN (5 µg/mL), and a mixture of the three pertussis antigens and then duplicate cell cultures were incubated in a 37℃, 5% CO2 environment for 72 hours. After incubation, the supernatants were extracted and stored at -70℃. The stored supernatants were then thawed, and concentrations of Th1 cytokines, such as interferon-γ (IFN-γ) and interleukin (IL)-2, and Th2 cytokines, such as IL-4, IL-5, and IL-10, were measured using commercial ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA). The levels of each cytokine were compared among the four groups.
Intranasal infection with B. pertussis
Mice were intranasally challenged three weeks after booster vaccination. A B. pertussis strain obtained from an adult pertussis patient was grown on a Regan-Lowe agar plate, and the grown B. pertussis strains were diluted with phosphate-buffered saline (PBS) to form a suspension of 1.2×108 colony forming units (CFUs)/mL. Fifty microliters of the suspension were administered into the nostrils of mice using a micropipette under peritoneal anesthesia with Zoletil/xylazine. Three mice from each group were sacrificed at 2 hours, 5 days, and 10 days after intranasal infection, and their lungs were removed. The removed lungs were homogenized in 10 mL of PBS, and the homogenates were diluted to 10-1, 10-3, and 10-5 concentrations. The diluted homogenates were inoculated on Regan-Lowe agar plates, and duplicate cultures were incubated at 36℃ for four days. The mean numbers of CFUs in each group at each time were compared.
Statistical analysis
The mean levels of antibody titers against each pertussis antigen were compared among the four groups using a Kruskal Wallis test at each sampling time. The mean cytokine concentrations in the CMIs were compared among the four groups and the mean CFUs in the intranasal infection model were also compared among the four groups at each time using a Kruskal Wallis test. Statistical analysis was performed with SPSS Statistics version 17.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was defined as a two-tailed p<0.05.
Results
Humoral immune responses
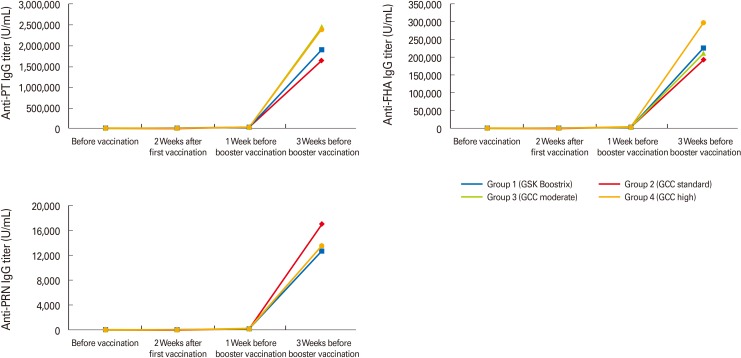
The mean titers of anti-PT IgG increased to more than 2,500 U/mL, while mean titers of anti-FHA IgG increased to more than 20 U/mL after the first dose of primary DTaP vaccination in all groups. These titers further increased after the second dose of primary DTaP vaccination in all groups, increasing much more sharply with Tdap booster vaccination (Table 1, Fig. 1). The final anti-PT IgG and anti-FHA IgG titers after booster vaccination showed no significant difference among the four groups (Fig. 1).
Table 1.
Results of humoral immune responses against pertussis toxin (PT), filamentous hemagglutinin (FHA), and pertactin (PRN)
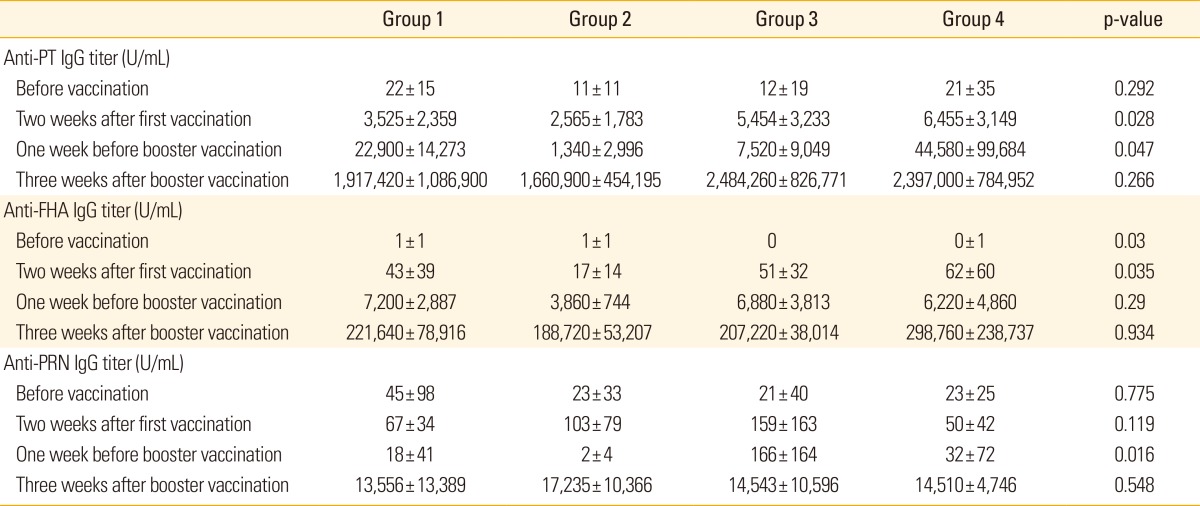
Values are presented as means±standard deviations.
Fig. 1.
The mean titers of anti-PT, anti-FHA, and anti-PRN IgGs sharply increased with Tdap booster vaccination in all groups. PT, pertussis toxin; FHA, filamentous hemagglutinin; PRN, pertactin; Tdap, reduced dose tetanus-diphtheria-acellular pertussis.
The mean titers of anti-PRN IgG also increased to greater than 50 U/mL in all groups after the first dose of primary vaccination and then increased sharply with booster vaccination (Table 1, Fig. 1). However, unlike the anti-PT and anti-FHA IgG titers, anti-PRN IgG titers were lower at one week before booster vaccination compared with those at two weeks after the first dose of primary DTaP vaccination in all groups (Table 1).
Th1 CMIs (IFN-γ and IL-2)
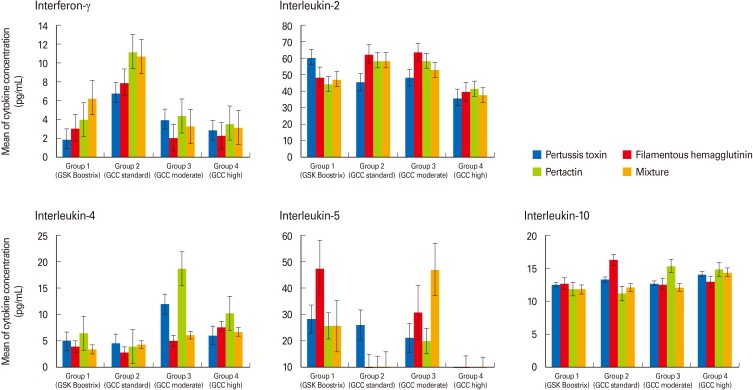
The IFN-γ levels after stimulation by PT, FHA, PRN, and mixed antigen were not significantly different among the four groups, and most IFN-γ levels were less than 10 pg/mL (Fig. 2). The IL-2 levels of the four groups were also not significantly different, although most levels were greater than 40 pg/mL (Fig. 2).
Fig. 2.
The concentrations of Th1 cytokines (interferon-γ, interleukin-2) and Th2 cytokines (interleukin-4, -5, and -10) which were secreted by spleen cells after stimulation by pertussis antigens were not significantly different among four groups. Error bars indicate standard errors.
Th2 CMIs (IL-4, -5, and -10)
After stimulation by PT, FHA, PRN, and mixed antigen, most IL-4 levels were less than 10 pg/mL, most IL-5 levels were between 10 and 30 pg/mL, and IL-10 levels were near 10 pg/mL (Fig. 2). Although the IL-5 levels in groups 1 and 3 were higher than those of the other groups, there was no significant difference in the IL-4, IL-5, or IL-10 levels among the four groups (Fig. 2).
Protection efficacy against B. pertussis in the intranasal infection model
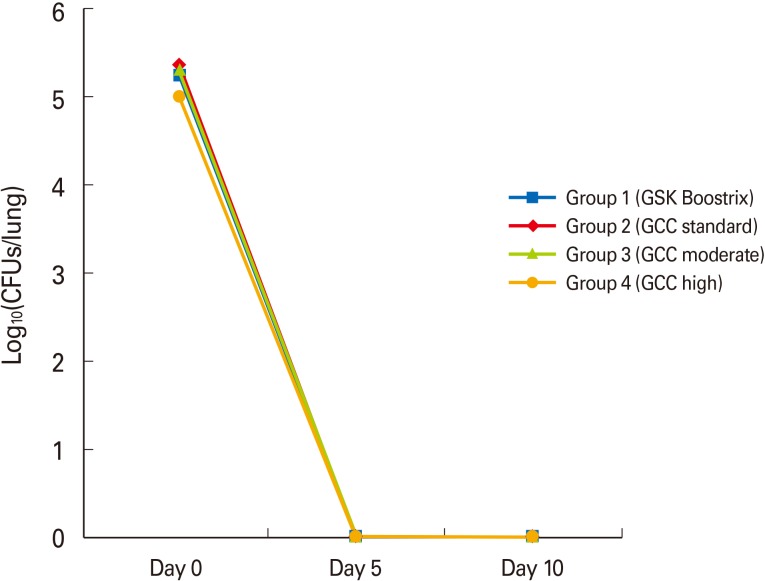
The mean CFUs of groups 1 to 4 were 177,000, 233,667, 212,000, and 103,667 CFUs/lung, at two hours after intranasal infection, respectively, and showed no significant difference (p=0.335). At five days after intranasal infection, B. pertussis was completely eradicated in all groups (Fig. 3).
Fig. 3.
The mean colony forming units (CFUs) of groups 1 to 4 were between 5.02 to 5.37 log10(CFUs/lung) after intranasal infection without any significant difference among the four groups (p=0.335). Bordetella pertussis was completely eradicated in all groups at five days after intranasal infection.
Discussion
Although humans are the only natural host of B. pertussis, murine models have been accepted as a useful animal study model evaluating pertussis vaccine immunogenicity [10,11,12,13,14]. However, animal studies have some limitations arising from the differences between animals and humans in vaccination route, the number and dose of vaccinations, the intervals between vaccination and blood sampling, and intrinsic immune systems. In the era that used killed whole cell pertussis vaccine, the intracerebral murine challenge model (Kendrick test) was principally used to determine vaccine potency [15,16], although the protective immune mechanisms in the intracerebral challenge model were not clearly defined [17,18,19,20]. Because of the high incidence of side effects with the whole cell pertussis vaccine, acellular pertussis (aP) vaccines have been used in most countries since the mid-1990s, and murine respiratory challenge models and humoral and cell-mediated immunity tests have been performed to determine aP vaccine potency and efficacy [10,11,21,22]. However, seed bacteria and purification as well as the detoxification methods of the pertussis antigens in each aP vaccine are different among the DTaP vaccines, and the protective immune mechanisms against pertussis have not been fully defined. Therefore, a generally accepted animal study model to assess aP vaccines has not been established. In addition, human studies to determine the clinical efficacy of Tdap vaccine have also not been standardized, although the incidence of pertussis has been increasing, especially in adolescents and adults [18]. Nevertheless, measurement of humoral and cellular immune responses against B. pertussis infection has been widely used to assess DTaP and Tdap vaccines because humoral immune responses and CMIs have shown a complementary role in protecting against pertussis [10,12,23]. Therefore, this study compared the new GCC Tdap vaccine and a commercially available Tdap vaccine with regard to immunogenicity based on antibody titers against pertussis antigens included in Tdap vaccines (humoral immune responses) and cytokine assays in splenocytes stimulated by pertussis antigens (CMI), as well as their protection efficacy based on an intranasal infection model. In addition, we determined an appropriate combination of pertussis antigens by comparing three study groups with different combinations of pertussis antigens.
Natural pertussis infection and diphtheria-tetanus-whole cell pertussis (DTwP) vaccination tend to activate Th1 CMI against pertussis [24], and repeat exposures to natural pertussis infection enhance the Th1 CMI [25]. However, DTaP vaccination tends to activate Th2 CMI [26,27]. In the case of primary DTwP and subsequent Tdap booster vaccination, both Th1 and Th2 CMIs were activated [28], whereas Th2 CMI was activated in the case of primary DTaP and subsequent DTaP booster vaccination [29,30]. This indicates that different CMI may be evoked according to the type of primary vaccination. Reynolds et al. [31] reported that Tdap booster vaccination following two doses of primary DTwP vaccination in mice and humans did not activate Th2 CMI, but did activate Th1 CMI, a result that was thought to be principally caused by the primary DTwP vaccination. In the present study where the Tdap booster vaccination followed two doses of primary DTaP vaccination, both Th1 and Th2 CMIs were activated despite the injection of aP. However, there was no significant difference in either Th1 or Th2 cytokine level among the four groups. Two doses of primary DTaP vaccination and a relatively short interval between the primary DTaP and booster Tdap vaccination may have reduced the effects of different combinations of pertussis antigens for Tdap booster vaccination in groups 2 to 4. Therefore, the interval between the primary and booster vaccination should be extended, and selective stimulation of T cells rather than whole splenocytes should be evaluated for comparison of CMI in future studies.
Reynolds et al. [31] also reported increases in antibody titers against pertussis antigens including PT, FHA, PRN, and fimbriae after Tdap booster vaccination in mice, and the degree of increase in anti-PT IgG titers was associated with protection against pertussis in an aerosol challenge model. The present study also demonstrated significant increases in antibody titers against pertussis antigens after Tdap booster vaccination, and the antibody titers were not significantly different among the positive control group and three study groups. In particular, anti-PRN IgG titers significantly increased with Tdap booster vaccination, although they had decreased since primary DTaP vaccination. These boosting effects of the Tdap vaccine, which includes lower doses of pertussis antigens compared with the DTaP vaccine, may be triggered by memory T cells established after primary DTaP vaccination. However, anti-PRN IgG titers were not significantly different among the study groups receiving different doses of PRN.
In the intranasal infection model, infected bacterial counts were not significantly different in the four groups, and B. pertussis was completely eradicated five days after intranasal infection in all of the four groups. From a previous study, the unvaccinated mice showed an increase of intrapulmonary bacterial count 10 days after intranasal infection, and then decreased [32]. The intrapulmonary bacterial count in DTP-vaccinated mice decreased without the early increasing phase after intranasal infection; however, a complete eradication of the intrapulmonary bacteria within eight days after infection was very rarely reported [32,33,34,35]. Considering the early eradication of infected B. pertussis in the intranasal infection and the lack of significance of different antibody titers against pertussis antigens after Tdap booster vaccination in groups receiving different combinations of antigens in the present study, primary DTaP vaccination may interfere with the Tdap booster vaccination effect. Accordingly, future studies should be performed with one dose of primary DTaP vaccination and a more prolonged interval between primary and booster vaccination.
This study has some limitations. First, as mentioned above, two doses of primary DTaP vaccination provided such a prolonged protective immunity that complete protection against intranasal B. pertussis infection was achieved after booster vaccination in all of the four groups, and therefore we could not differentiate the effects of the three study vaccines. In addition, an appropriate positive control group using universal mitogen-stimulating splenocytes and a negative control group using no antigen were not established when assessing CMI. By assessment of the humoral immune responses and bacterial eradication in the intranasal challenge model, negative control groups including exclusively unvaccinated mice and booster-vaccinated mice without primary DTaP vaccination were not established, and therefore the immunogenicity of the new Tdap vaccine could not be properly determined. Future studies including appropriate negative control groups should be performed.
In conclusion, the immunogenicity and protection efficacy against B. pertussis of the newly developed GCC Tdap vaccine were not significantly different from those of a commercially available Tdap vaccine in a murine model. However, antibody titer levels after booster vaccination were much higher than those after the first DTaP vaccination, and there was no significant difference in antibody titer levels among groups receiving different doses of included pertussis antigens. These results are assumed to be due to the two doses of primary DTaP vaccination and the relatively short interval between primary and booster vaccination. Therefore, future studies should include one dose of primary vaccination and a longer interval between primary and booster vaccination. In addition, selective separation of T cells from the spleen cell suspension should be performed in order to determine the exact cytokine levels after stimulation by pertussis antigens, reflecting the CMI against B. pertussis.
Footnotes
This trial was supported by the Green Cross Corporation (Grant number: 5-2013-D0083-00001). We thank the volunteers who participated in this study and the staff of the Vaccine Bio Research Institute of the Catholic University of Korea, who supported and helped with the practical organization of this study.
References
- 1.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Melker HE, Schellekens JF, Neppelenbroek SE, Mooi FR, Rümke HC, Conyn-van Spaendonck MA. Reemergence of pertussis in the highly vaccinated population of the Netherlands: observations on surveillance data. Emerg Infect Dis. 2000;6:348–357. doi: 10.3201/eid0604.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowcroft NS, Britto J. Whooping cough: a continuing problem. BMJ. 2002;324:1537–1538. doi: 10.1136/bmj.324.7353.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg DP. Pertussis in adolescents: increasing incidence brings attention to the need for booster immunization of adolescents. Pediatr Infect Dis J. 2005;24:721–728. doi: 10.1097/01.inf.0000172905.08606.a3. [DOI] [PubMed] [Google Scholar]
- 5.Packard ER, Parton R, Coote JG, Fry NK. Sequence variation and conservation in virulence-related genes of Bordetella pertussis isolates from the UK. J Med Microbiol. 2004;53(Pt 5):355–365. doi: 10.1099/jmm.0.05515-0. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Updated recommendations for the use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:13–15. [PubMed] [Google Scholar]
- 7.Wei SC, Tatti K, Cushing K, et al. Effectiveness of adolescent and adult tetanus, reduced-dose diphtheria, and acellular pertussis vaccine against pertussis. Clin Infect Dis. 2010;51:315–321. doi: 10.1086/653938. [DOI] [PubMed] [Google Scholar]
- 8.Korea Centers for Disease Control and Prevention. Increasing incidence of pertussis in Korea, 2009. Public Health Wkly Rep. 2009;2:709. [Google Scholar]
- 9.Report of Yeongam pertussis epidemiological investigation in Korea. Public Health Wkly Rep. 2012;5:510–512. [Google Scholar]
- 10.Mills KH, Ryan M, Ryan E, Mahon BP. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect Immun. 1998;66:594–602. doi: 10.1128/iai.66.2.594-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guiso N, Capiau C, Carletti G, Poolman J, Hauser P. Intranasal murine model of Bordetella pertussis infection. I. Prediction of protection in human infants by acellular vaccines. Vaccine. 1999;17:2366–2376. doi: 10.1016/s0264-410x(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 12.Mills KH. Immunity to Bordetella pertussis. Microbes Infect. 2001;3:655–677. doi: 10.1016/s1286-4579(01)01421-6. [DOI] [PubMed] [Google Scholar]
- 13.Canthaboo C, Williams L, Xing DK, Corbel MJ. Investigation of cellular and humoral immune responses to whole cell and acellular pertussis vaccines. Vaccine. 2000;19:637–643. doi: 10.1016/s0264-410x(00)00253-x. [DOI] [PubMed] [Google Scholar]
- 14.Mills KH, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993;61:399–410. doi: 10.1128/iai.61.2.399-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Expert Committee on Biological Standardization. WHO technical report series 800. Geneva: World Health Organization; 1990. [PubMed] [Google Scholar]
- 16.European Directorate for the Quality of Medicines. European pharmacopoeia. 4th ed. Strasbourg: Council of Europe; 2002. [Google Scholar]
- 17.Corbel MJ, Xing DK, Kreeftenberg JG. Informal consultation with manufacturers and WHO Ad hoc working group on mouse protection models for acellular pertussis vaccines national institute for biological standards, 12 november 1998. Biologicals. 1999;27:189–193. doi: 10.1006/biol.1999.0209. [DOI] [PubMed] [Google Scholar]
- 18.Corbel MJ, Mastrantonio P, Kreeftenberg JG. Ad hoc Working Group on acellular pertussis vaccines, World Health Organisation, Geneva, 27-28 July 2000. Vaccine. 2001;20:288–291. doi: 10.1016/s0264-410x(01)00343-7. [DOI] [PubMed] [Google Scholar]
- 19.Corbel MJ, Kreeftenberg JG, Knezevic I. WHO Working Group on the standardisation and control of pertussis vaccines-report of a meeting held on 6-7 May 2003, Ferney Voltaire, France. Vaccine. 2004;22:293–300. doi: 10.1016/j.vaccine.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Corbel MJ, Xing DK. Toxicity and potency evaluation of pertussis vaccines. Expert Rev Vaccines. 2004;3:89–101. doi: 10.1586/14760584.3.1.89. [DOI] [PubMed] [Google Scholar]
- 21.Xing DK, Das RG, Williams L, Canthaboo C, Tremmil J, Corbel MJ. An aerosol challenge model of Bordetella pertussis infection as a potential bioassay for acellular pertussis vaccines. Vaccine. 1999;17:565–576. doi: 10.1016/s0264-410x(98)00235-7. [DOI] [PubMed] [Google Scholar]
- 22.Capiau C, Poolman J, Hoet B, Bogaerts H, Andre F. Development and clinical testing of multivalent vaccines based on a diphtheria-tetanus-acellular pertussis vaccine: difficulties encountered and lessons learned. Vaccine. 2003;21:2273–2287. doi: 10.1016/s0264-410x(03)00107-5. [DOI] [PubMed] [Google Scholar]
- 23.Zepp F, Knuf M, Habermehl P, et al. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun. 1996;64:4078–4084. doi: 10.1128/iai.64.10.4078-4084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esposito S, Agliardi T, Giammanco A, et al. Long-term pertussis-specific immunity after primary vaccination with a combined diphtheria, tetanus, tricomponent acellular pertussis, and hepatitis B vaccine in comparison with that after natural infection. Infect Immun. 2001;69:4516–4520. doi: 10.1128/IAI.69.7.4516-4520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Southern J, Andrews N, Burrage M, Miller E. Immunogenicity and reactogenicity of combined acellular pertussis/tetanus/low dose diphtheria vaccines given as a booster to UK teenagers. Vaccine. 2005;23:3829–3835. doi: 10.1016/j.vaccine.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 26.Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. Vaccine and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun. 1997;65:2168–2174. doi: 10.1128/iai.65.6.2168-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redhead K, Watkins J, Barnard A, Mills KH. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993;61:3190–3198. doi: 10.1128/iai.61.8.3190-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Q, Tran Minh NN, Edelman K, Viljanen MK, Arvilommi H, Mertsola J. Cytokine mRNA expression and proliferative responses induced by pertussis toxin, filamentous haemagglutinin, and pertactin of Bordetella pertussis in the peripheral blood mononuclear cells of infected and immunized schoolchildren and adults. Infect Immun. 1998;66:3796–3801. doi: 10.1128/iai.66.8.3796-3801.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan M, Murphy G, Ryan E, et al. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998;93:1–10. doi: 10.1046/j.1365-2567.1998.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan EJ, Nilsson L, Kjellman N, Gothefors L, Mills KH. Booster immunization of children with an acellular pertussis vaccine enhances Th2 cytokine production and serum IgE responses against pertussis toxin but not against common allergens. Clin Exp Immunol. 2000;121:193–200. doi: 10.1046/j.1365-2249.2000.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds E, Walker B, Xing D, et al. Laboratory investigation of immune responses to acellular pertussis vaccines when used for boosting adolescents after primary immunisation with whole cell pertussis vaccines: a comparison with data from clinical study. Vaccine. 2006;24:3248–3257. doi: 10.1016/j.vaccine.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Dolby JM, Thow DC, Standfast AF. The intranasal infection of mice with Bordetella pertussis. J Hyg. 1961;59:191–204. doi: 10.1017/s0022172400038857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denoel P, Godfroid F, Guiso N, Hallander H, Poolman J. Comparison of acellular pertussis vaccines-induced immunity against infection due to Bordetella pertussis variant isolates in a mouse model. Vaccine. 2005;23:5333–5341. doi: 10.1016/j.vaccine.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Bottero D, Gaillard ME, Fingermann M, et al. Pulsed-field gel electrophoresis, pertactin, pertussis toxin S1 subunit polymorphisms, and surfaceome analysis of vaccine and clinical Bordetella pertussis strains. Clin Vaccine Immunol. 2007;14:1490–1498. doi: 10.1128/CVI.00177-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komatsu E, Yamaguchi F, Abe A, Weiss AA, Watanabe M. Synergic effect of genotype changes in pertussis toxin and pertactin on adaptation to an acellular pertussis vaccine in the murine intranasal challenge model. Clin Vaccine Immunol. 2010;17:807–812. doi: 10.1128/CVI.00449-09. [DOI] [PMC free article] [PubMed] [Google Scholar]