Abstract
Background
The course of depressive symptoms during and after breast cancer treatment is not well understood.
Purpose
We sought to identify patient subgroups based on distinct trajectories of depressive symptoms using growth mixture modeling and determine whether subgroups could be distinguished by demographic and clinical characteristics and coping strategies.
Methods
Women with early stage breast cancer completed the Center for Epidemiologic Studies–Depression Scale on three occasions designed to reflect clinically meaningful events and on three occasions during the post-treatment period. The Illness Management Questionnaire was completed prior to treatment.
Results
A three-class mixture model provided the best fit to the data. In univariate analyses, subgroup membership was significantly (ps < .05) associated with marital status, history of depression and focusing on symptoms. In multivariate analysis, marital status and focusing on symptoms remained significant (ps < .05) predictors of subgroup membership.
Conclusions
Distinct trajectories of depression can be identified during and after adjuvant therapy for breast cancer. Predictors of these trajectories have implications for addressing depressive symptoms in this clinical population and for future research.
Keywords: Breast cancer, depression, trajectory
Introduction
Studies suggest that as many as 25% to 30% of women with breast cancer report depressive symptoms at some point in the cancer care trajectory (1, 2). While depressive symptoms are relatively common, the course of these symptoms during and after treatment is not well understood. This is because many of the previous studies have been cross-sectional in design and have focused either on the period of active treatment (3-5) or the post-treatment period (6, 7). Among the few longitudinal studies encompassing both the active and post-treatment periods, most studies consider depressive symptom scores only in the aggregate and do not examine whether there may be subgroups of women who differ in their experience of symptoms (8-12). Using aggregate (that is, sample mean) scores may mask meaningful differences among patients in the severity, course, and predictors of depressive symptoms.
Theory and research in the general population (13, 14) and in medically ill populations (15, 16), including women with breast cancer (17, 18), suggest there are distinct trajectories of mental health and psychological distress, including depressive symptoms. To date, the question of whether there are distinct trajectories of depressive symptoms in women with breast cancer has been addressed in just two studies (1, 19). Deshields et al. (1) used a case-based approach, grouping the patients on the basis of whether scores on the Center for Epidemiologic Studies-Depression Scale (CES-D) (20) were above or below the established cut-off of 16 in the first six months following radiation treatment. This study involved a relatively small sample of breast cancer patients and did not include an assessment of depressive symptoms prior to the end of treatment.
A more recent study by Dunn et al. (19) identified four distinct trajectories of depressive symptoms in the first six months after breast cancer surgery using growth mixture modeling, a statistical approach capable of detecting not only whether a class of persons changes on some outcome measure, but whether there are individual differences in the rates of change among class members (21). The assessments of depressive symptoms were tied to time since surgery. Although there was a pre-surgical baseline assessment of depressive symptoms, 20% of the total sample had received neoadjuvant chemotherapy and thus could rightly be considered already in active treatment at the pre-surgical assessment. In addition, there was considerable heterogeneity in the nature of the post-operative treatment received. Approximately 33% of the sample received adjuvant chemotherapy and 56% received radiation at any point in the study. Whether a significant number of the women were still in active treatment at the end of the six month period is not clear, but two treatment variables, having an axillary lymph node dissection and receiving post-operative chemotherapy, distinguished the subgroups, suggesting the importance of tying symptom assessments to clinically relevant time points in the care trajectory.
With respect to nonclinical factors that may distinguish subgroups of breast cancer patients with distinct depressive symptom trajectories, Deshields et al. (1) found that subgroups were distinguished by self-reported quality of life, with never depressed patients reporting better quality of life than the other subgroups; a finding that suggests those who experience distress do so globally (1). Anxiety also was a significant predictor of subgroup membership. Patients who were never depressed and patients who recovered reported lower anxiety levels. Dunn et al. (19) found that age, education, self-reported performance status, a current problem with depression, and, consistent with Deshields et al. (1), anxiety, were predictive of subgroup membership. Generally, these findings are consistent with research demonstrating the likelihood of anxious and depressive symptoms co-occurring (11).
To date, the scope and nature of the factors examined in relation to distinct patterns of depressive symptoms have been fairly limited. Studies conducted by us and others have examined the effect of past history of major depressive disorder on quality of life in breast cancer (10, 22). No studies have examined the relation between a past history of major depressive disorder and the trajectory of depressive symptomatology in these patients, however. This is a significant oversight given the overwhelming evidence that the experience of a major depressive episode predisposes an individual to future depressive episodes (23-27).
There also is considerable evidence that coping – the cognitive and behavioral strategies that individuals use to manage the effects of a significant stressor in their lives – can influence depressive symptoms (23, 24, 28-30). Research by Ray et al. (1993) has given rise to a conceptualization of coping that may be particularly useful in the current context. The researchers identified four coping strategies that they believe represent a problem-focused approach to illness: maintaining activity, which is characterized by an attempt to ignore symptoms and carry on with normal activities; focusing on symptoms, which is marked by a preoccupation with illness symptoms and an appraisal of one's life as dominated by the illness; accommodating to the illness which involves making lifestyle adjustments and managing stress; and information seeking, which involves searching for information and an openness to different treatments.
In general, the use of coping strategies that reflect a problem-focused approach to illness has been shown to be associated with more positive outcomes in early stage breast cancer (31-33). Research using the Illness Management Questionnaire (34) (IMQ) to measure the four forms of problem-focused coping described above has yielded contrasting patterns of results, however. In a study of quality of life after treatment for early stage breast cancer (35), for example, researchers found that greater focusing on symptoms was associated with less improvement in mental and physical quality of life six months after treatment completion. Greater information seeking was associated with greater improvement in physical, but not mental quality of life, while neither maintaining activity nor accommodating to illness was associated with changes in physical or mental quality of life. In other studies of early stage breast cancer patients (36, 37), a tendency to cope by accommodating to one's illness has been associated with reduced risk for developing cancer-related fatigue while focusing attention on one's symptoms has been linked to greater risk for cancer-related fatigue in the immediate and longer-term post-treatment period. Perhaps not surprisingly, given the evidence for relationships between coping strategies and both positive and negative outcomes in early stage breast cancer, no studies to date have examined whether individual differences in the use of coping strategies as measured by the IMQ predict distinct trajectories of depressive symptoms in women with breast cancer.
The current study had two specific aims. The first aim was to determine whether we could identify subgroups of patients based on their distinct trajectory or pattern of depressive symptoms before, during, and after adjuvant treatment using growth mixture modeling. The second aim was to examine whether the subgroups could be distinguished based on demographic characteristics, clinical characteristics, and coping strategies; we were specifically interested in whether problem-focused coping strategies assessed at the start of adjuvant treatment could distinguish subgroups of patients based on their pattern of depressive symptoms over time. Based on prior research described above, we hypothesized that greater reliance on accommodating to illness and on information seeking would be associated with less clinically significant (i.e., more benign) trajectories of depressive symptoms. In contrast, we hypothesized that greater reliance on maintaining activity and focusing on symptoms would be associated with more clinically significant (i.e., less benign) trajectories of depressive symptoms.
Similar to Dunn et al. (19), we used growth mixture modeling to examine longitudinal changes in depressive symptoms from before the start of adjuvant treatment for breast cancer to six months after its completion, as well as to determine whether meaningful subgroups or classes of women exist that differ in their experience of depressive symptoms. Conventional mixed model approaches to data analysis acknowledge the heterogeneity in change over time, but assume that the individuals who are varying are also derived from the same population. By contrast, growth mixture modeling does not require this assumption be made, and, in fact, allows for the identification of classes of individuals through the application of a latent class membership variable (38, 39). To some extent, this approach evaluates whether there is sufficient homogeneity in the heterogeneity of change over time to allow the identification of classes of individuals who differ in terms of their initial starting points and/or rates of change over time.
Methods
Participants
Participants were women with early stage breast cancer scheduled to be treated with chemotherapy followed by radiotherapy at the Moffitt Cancer Center or the Lucille Parker Markey Cancer Center at the University of Kentucky. Eligibility criteria were that participants: a) be at least 18 years of age, b) have no documented or observable psychiatric or neurological disorders that would interfere with study participation (e.g., dementia or psychosis), c) report no history of a condition in which fatigue is a prominent symptom (e.g., multiple sclerosis or chronic fatigue syndrome, d) be able to speak and read standard English, e) have no history of cancer other than basal cell skin carcinoma, f) be diagnosed with stage 0, I or II breast cancer, g) have been treated surgically with lumpectomy or mastectomy, h) be scheduled to receive a minimum of 4 cycles of chemotherapy followed by radiotherapy, i) have no prior history of treatment with chemotherapy or radiotherapy, j) provide written informed consent.
Procedure
Participants were recruited as part of a larger study evaluating fatigue and other aspects of quality of life during and after treatment for early stage breast cancer (22, 40, 41). The larger study (N = 335) includes women scheduled to receive chemotherapy followed by radiotherapy and women scheduled to receive radiation only for stage 0, I or II breast cancer. As noted previously, the current study includes only those women who received chemotherapy followed by radiotherapy. Eligibility was determined by chart review and consultation with the attending physician. Eligible patients were recruited and informed consent was obtained during an outpatient clinic visit prior to the start of chemotherapy. Those women who provided informed consent completed a questionnaire assessing demographic characteristics prior to beginning treatment. Depressive symptoms were assessed on three occasions chosen to reflect clinically meaningful events following surgery: just before the start of adjuvant treatment, mid-treatment (after chemotherapy and prior to radiotherapy), at the end of adjuvant treatment; and then an additional three times in the post-treatment period: two, four, and six months after completing active treatment. All other assessment instruments described below were administered just before the start of adjuvant treatment. The assessment timeline varied by the number of chemotherapy and radiotherapy cycles received by individual participants. Different approaches were used to collect data at different points in the cancer care trajectory. Assessments 1, 2, and 3 were completed in person in clinic. Assessments 4 and 5 were completed by mail and assessment 6 was completed in person in clinic. Participants were mailed reminders of upcoming assessments throughout their participation. Of the 147 women included in the analyses, 100% completed at least 3 of 6 assessments, 98% completed at least 4 assessments, 90% completed at least 5 assessments, and 74% completed all 6 assessments.
Measures
Demographic data
Demographic data were obtained prior to treatment via self-report and included age, race/ethnicity, marital status, annual household income, and educational level.
Clinical data
Variables assessed via chart review before treatment included disease stage and type of breast surgery. Height and weight were assessed and used to calculate body mass index. Comorbid medical conditions were also assessed at this time using a self-report version of the Charlson Comorbidity Index (42). Number of chemotherapy cycles, number of radiotherapy cycles and cumulative radiation doses, and hormone therapy status were recorded following active treatment via chart review.
Coping Strategies
The Illness Management Questionnaire (IMQ) was developed originally to assess cognitive and behavioral coping in individuals with chronic fatigue syndrome (34). As per the standard instructions, respondents were asked to indicate on a six-point scale (1 = never; 6 = always) the extent to which they used each of 55 coping strategies “in relation to your illness” in the past week; breast cancer was not specifically mentioned. The IMQ yields four empirically-derived subscales that represent a problem-focused approach to coping with illness: maintaining activity, accommodating to illness, information seeking, and focusing on symptoms. In the current study, these subscales demonstrated high internal consistency (alpha = .85 - .93). Intercorrelations of the subscales were as follows: maintaining activity – accommodating to illness = .02, – information seeking = .02, – focusing on symptoms = -.26; accommodating to illness – information seeking = .13, – focusing on symptoms = .13; information seeking – focusing on symptoms = .13. In general, the IMQ has been shown to be a valid measure of illness-related coping (34, 43, 44).
History of Major Depression
Participants were administered the Mood, Anxiety, and Adjustment Disorders modules from the Structured Clinical Interview for the DSM-IV, Research Version (45). These modules include questions that can be used to rule out forms of disorders that are secondary to a general medical condition or substance use. SCID diagnoses reported in this study represent primary major depression and not secondary forms. All interviews were conducted by doctoral students in clinical psychology trained in structured clinical interviewing. Training involved review of diagnostic criteria, conduct of practice interviews, and review of audiotaped interviews. Analyses for this report focus on evaluation of the presence or absence of a past history of major depressive disorder.
Depressive Symptoms
The Center for Epidemiological Studies–Depression Scale (CES-D) is a 20-item measure of depressive symptoms (20). Respondents rate how frequently they have experienced each symptom in the past week on a four-point scale (0=rarely or none of the time; 3=most or all of the time). Items are summed to produce scores ranging from 0 to 60; a cutoff score of 16 or greater is commonly used to indicate clinically significant depressive symptoms. The CES-D has good internal consistency with alphas of .85 for the general population and .90 for a psychiatric population (7). The validity of the CES-D has been demonstrated with a wide range of populations, including cancer patients (46, 47).
Statistical Analysis
Modeling of longitudinal changes in depressive symptoms followed a three-stage procedure. First we examined changes in depressive symptoms across the study period using random effects models (48). In these models we examined whether changes in symptoms were present across the study period, as well as whether they followed a linear pattern of change and/or a non-linear pattern of change (i.e., a quadratic change component). Second, to examine potential subgroups or classes in the data, we applied growth mixture modeling (39, 49) using Mplus. This approach involves iteratively extracting different classes of participants from the data and examining measures of model fit. In the current study, we assessed fit using several different statistical criteria. The -2 Log Likelihood (-2LL) ratio test, the Akaike information criteria (AIC), the Bayesian information criteria (BIC), and the Lo-Mendell-Rubin likelihood ratio test (50). We also calculated Entropy (51), which is a statistic that ranges from 0 to 1 as implemented in Mplus and provides an estimate of the confidence with which individuals have been classified as belonging to one class or another. Values above .80 are thought to represent adequate separation of the classes (39, 52).
Once the best fitting and most theoretically relevant model was obtained, univariate multinominal logistic regression analyses were conducted to examine differences in class membership as a function of demographic characteristics, clinical characteristics, and use of coping strategies. Finally, multivariate multinomial logistic regression analysis was conducted to investigate the combined potential of these variables to predict class membership. Only variables that were statistically significant (p < .05) in univariate analyses were included in the multivariate model.
Results
Participant Characteristics
Table 1 presents the demographic and clinical characteristics of participants. The mean age of the sample was 52 years of age. Approximately 90% of the women were Caucasian and nearly half had at least some college. Seventy-six percent were married. With respect to clinical characteristics 72% of women had stage II breast cancer and 84% underwent a lumpectomy. Mean time in treatment and in the post-treatment period was 5.6 ± 1.4 months (range = 2.1 to 10.8) and 6.7 ± 1.8 months (range = 3.7 to 17.4), respectively.
Table 1.
Sample Description and Univariate Predictors of Class Membership
| Depression Class | ||||||
|---|---|---|---|---|---|---|
| Predictor | Total Sample (N = 147) n(%) | Class 1 (n = 39) n(%) | Class 2 (n = 70) n(%) | Class 3 (n = 38) n(%) | X2 | p value |
| Age (Mn ± SD) | 51.63 ± 9.03 | 52.11 ± 8.87 | 50.99 ± 8.34 | 52.34 ± 10.50 | .70 | .70 |
| Race/Ethnicity | 3.16 | .21 | ||||
| White/Caucasian | 132 (89.8%) | 32 (82.1%) | 65 (92.9%) | 35 (92.1%) | ||
| Other | 15 (10.2%) | 7 (17.9%) | 5 (7.1%) | 3 (7.9%) | ||
| Education | 1.00 | .61 | ||||
| ≥ College Degree | 64 (43.5%) | 17 (43.6%) | 28 (40.0%) | 19 (50.0%) | ||
| ≤ High School Degree | 83 (56.5%) | 22 (56.4%) | 42 (60.0%) | 19 (50.0%) | ||
| Marital Status | 8.57 | .01 | ||||
| Not Marriedb | 35 (23.8%) | 15 (38.5%) | 16 (22.9%) | 4 (10.5%) | ||
| Married | 112 (76.2%) | 24 (61.5%) | 54 (77.1%) | 34 (89.5%) | ||
| Household Income | 3.92 | .14 | ||||
| ≤ $40,000/year | 42 (30.2%) | 16 (43.2%) | 17 (25.8%) | 9 (25.0%) | ||
| ≥ $40,000/year | 97 (69.8%) | 21 (56.8%) | 49 (74.2%) | 27 (75.0%) | ||
| Menopausal Status | 1.21 | .55 | ||||
| Pre-menopausal | 57 (38.8%) | 12 (30.8%) | 30 (42.9%) | 15 (39.5%) | ||
| Peri- or post-menopausal | 79 (53.7%) | 23 (59.0%) | 36 (51.4%) | 20 (52.6%) | ||
| Body Mass Index (Mn ± SD) | 27.65 ± 6.73 | 28.57 ± 5.78 | 28.01 ± 7.97 | 26.10 ± 4.71 | 3.27 | .20 |
| History of Depression | 6.91 | .03 | ||||
| Yesb | 27 (18.5%) | 12 (30.8%) | 12 (17.4%) | 3 (7.9%) | ||
| No | 119 (81.5%) | 29 (69.2%) | 57 (82.6%) | 35 (92.1%) | ||
| Charlson Comorbidity Index (Mn ± SD) | 2.31 ± 0.64 | 2.33 ± 0.58 | 2.30 ± 0.64 | 2.32 ± 0.70 | 0.07 | .97 |
| Surgery | .91 | .64 | ||||
| Lumpectomy | 124 (84.4%) | 32 (82.1%) | 59 (84.3%) | 33 (86.8%) | ||
| Mastectomy | 18 (12.2%) | 5 (12.8%) | 10 (14.3%) | 3 (7.9%) | ||
| Disease Stagea | .52 | .77 | ||||
| 0 – 1 | 41 (28.1%) | 9 (23.7%) | 21 (30.0%) | 11 (28.9%) | ||
| 2 | 105 (71.9%) | 29 (76.3%) | 49 (70.0%) | 27 (71.1%) | ||
| Number of Chemotherapy Cycles | 1.63 | .44 | ||||
| 4 | 80 (54.4%) | 18 (46.2%) | 40 (57.1%) | 22 (57.9%) | ||
| 6 – 9 | 59 (40.1%) | 19 (48.7%) | 26 (37.1%) | 14 (36.8%) | ||
| Number of Radiation Cycles | 31.05 ± 4.29 | 31.41 ± 3.75 | 30.48 ± 4.96 | 31.75 ± 3.30 | 2.51 | .29 |
| Cumulative radiation dose in cGy (Mn ± SD) | 5977.79 ± 620.82 | 5964.05 ± 618.66 | 5941.79 ± 609.34 | 6058.89 ± 653.73 | .92 | .63 |
| Hormonal Therapy | .58 | .75 | ||||
| No | 124 (84.4%) | 31 (79.5%) | 61 (87.1%) | 32 (84.2%) | ||
| Yes | 8 (5.4%) | 2 (5.1%) | 3 (4.3%) | 3 (7.9%) | ||
| Illness Management Questionnaire (Mn ± SD) | ||||||
| Focusing on symptomsb | 3.61 ± 0.68 | 3.00 ± 0.8 | 2.86 ± 0.7 | 2.50 ± 0.6 | 12.50 | .002 |
| Accommodating to illness | 3.79 ± 0.79 | 3.68 ± 0.7 | 3.87 ± 0.8 | 3.80 ± 0.9 | 1.52 | .47 |
| Maintaining activity | 2.80 ± 0.73 | 3.70 ± 0.8 | 3.58 ± 0.6 | 3.60 ± 0.7 | .77 | .68 |
| Information seeking | 4.06 ± 0.87 | 3.83 ± 0.8 | 4.16 ± 0.9 | 4.10 ± 0.8 | 3.77 | .15 |
These data were incomplete; thus, their totals do not add up to n = 147; a total of 3 patients had a diagnosis of stage 0 disease and so were combined with stage I disease
Group differences by post-hoc test at p < .05: for unmarried status 1 > 3, for history of depression 1 > 3, for Focusing on Symptoms 1 > 2 > 3.
Longitudinal Changes in Depressive Symptoms
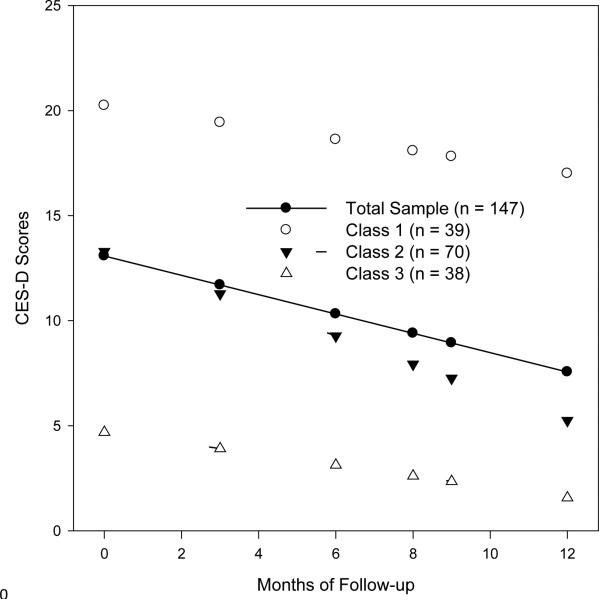
Evaluation of linear and quadratic random effects models revealed that changes in depressive symptoms over time were best described by a linear model. Mean CES-D scores for the total sample over the course of the study period are shown in Figure 1. At baseline, prior to adjuvant treatment, the score on the CES-D for the sample was approximately 13. This score declined by almost half a point per month over the course of the study period. Next, we examined the possibility of different classes of change in CES-D scores using growth mixture modeling. Fit statistics for the four models tested are shown in Table 2. Results indicated that the inclusion of additional classes resulted in statistically significant increments to model fit, up until the four-class model. In the case of four classes, despite the fact that the AIC and BIC values continued to decline, the improvement in model fit was not statistically significant, relative to the number of additional parameters that were added, as illustrated by the Δ-2LL and LMR fit statistics. Moreover, the fourth class had only 8 members, representing only 3.2% of the sample. As a result, the three-class solution was selected as the final model (see Footnote 1). These classes are shown graphically in Figure 1.
Figure 1.
Mean depressive symptom trajectories for patients in each class and the mean CES-D scores for the total sample
Table 2.
Summary of Statistical Fit Indices for Growth Mixture Models
| Classes | AIC | BIC | -2LL | df | Δ-2LL | LMR | Entropy |
|---|---|---|---|---|---|---|---|
| 1 | 5534.20 | 5552.15 | 2761.10 | 6 | -- | -- | -- |
| 2 | 5311.69 | 5350.57 | 2642.85 | 13 | 118.25*** | 229.92*** | .82 |
| 3 | 5257.81 | 5311.64 | 2610.90 | 18 | 31.95*** | 67.09* | .80 |
| 4 | 5249.41 | 5330.15 | 2597.71 | 27 | 13.19 | 20.69 | .88 |
Note: LL = loglikelihood; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; LMR = Lo-Mendell-Rubin likelihood ratio.
p < .05
** p < .01
p < .001
Parameter estimates for the three-class solution are presented in Table 3. In this table, the fixed effects represent the class average for the intercept and the linear change over time. The variance components indicate whether statistically significant variation, indicating individual differences, was present for the intercept or linear change. Finally, the covariance parameter is the relationship between the intercept and linear change. The three classes differed significantly in CES-D scores prior to adjuvant treatment as evidenced by the non-overlapping 95% confidence intervals for the fixed model estimates of the intercepts. With regard to the linear changes over time, class 1 did not exhibit statistically significant declines over time; classes 2 and 3 did. In the case of class 2, the decline was approximately two-thirds of a point per month, whereas class 3 declined by approximately one-quarter of a point per month. These effects were significantly different from one another; this is evidenced by the fact that the parameter estimates for one class are not included in the confidence intervals of another. At the end of the six month-follow up, the mean CES-D score for class 1 was 17.0 (95% CI = 8.9 – 25.1). The mean CES-D score for class 2 was 5.2 (95% CI = 3.8 – 6.6) and 1.5 (95% CI = 0.18 – 2.9) for class 3. The three classes differed significantly in CES-D scores at the last assessment as evidenced by the non-overlapping 95% confidence intervals for the class mean scores.
Table 3.
Parameter estimates for growth mixture models
| Parameter estimates | Class 1 n = 39 (27%) | Class 2 n = 70 (48%) | Class 3 n = 38 (26%) | |
|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | ||
| Fixed | ||||
| Intercept (SE) | 20.24 (1.23)*** | 13.29 (2.34)*** | 4.69 (.57)*** | |
| 95% CI | 18.21 – 22.27 | 9.45 – 17.14 | 3.75 – 5.63 | |
| Slope (SE) | −.27 (.43) | −.67 (.17)*** | −.26 (.08)*** | |
| 95% CI | −.98 - .44 | −.95 - −.39 | −.39 - −.13 | |
| Variance Components | ||||
| Intercept (SE) | 26.82 (8.27)** | 17.30 (16.92) | 4.56 (2.06)* | |
| Slope (SE) | 0a | .23 (.10)* | .04 (.03) | |
| Covariance | 0a | −1.80 (1.07) | −.39 (.22) |
Note: SE = standard error;
Parameter fixed at zero;
p < .05
p < .01
p < .001
Predictors of Class Membership
The results of univariate analyses comparing the three classes with respect to demographic and clinical characteristics, including history of depression, and the use of problem-focused coping strategies for the entire sample and for the three classes are presented in Table 1. Marital status and history of depression were significantly associated with class membership. Compared to women in class 3, women in class 1 were significantly more likely to be unmarried (p = .007). Women in class 1 also were significantly more likely than women in class 3 to report symptoms indicative of a history of major depression (p = .02). There was also a difference among classes on the focusing on symptoms subscale of the IMQ. Women in class 3 were less likely to be preoccupied with symptoms in managing their illness than women in class 1 (p = .002) and women in class 2 (p = .006). Based on this pattern of findings, marital status, history of depression, and focusing on symptoms were entered simultaneously into a multivariate multinomial logistic regression model predicting class membership. The Nagelkerke pseudo R2 was .18, indicating that the model explained 18% of the variance in trajectory class membership. Marital status (χ2 (2, N = 147) = 7.44, p = .024) remained a significant predictor of class membership, as did focusing on symptoms (χ2 (2, N = 147) = 13.61, p = .001), but not history of depression (χ2 (2, N = 147) = 2.66, p = .265).
Discussion
The first aim of the current study was to determine whether we could identify subgroups of patients based on their pattern of depressive symptoms before, during, and after adjuvant treatment for breast cancer. Results indicated that three subgroups of patients could be identified. Based on a CES-D cut-off score of 16 or greater as indicative of clinically significant depressive symptoms, one subgroup (class 1) reported clinically significant symptoms of depression prior to treatment that declined only slightly over time, remaining at a clinically significant level six months after completing treatment. A second subgroup (class 2) reported subclinical depressive symptoms prior to treatment that declined significantly over time to a minimal level of symptomatology. Lastly, a third subgroup (class 3) reported minimal symptoms of depression prior to treatment that declined significantly to a still lower level six months after completing treatment.
These results are similar to the findings in the study by Dunn et al. (19) that also used growth mixture modeling to examine the course of depressive symptoms in women with a history of breast cancer. Both Dunn et al. (19) and the current study found that some women with breast cancer may experience minimal depressive symptoms over time while others experience depressive symptoms that increase or persist. However, the current study identified three subgroups of breast cancer patients with distinct trajectories of depressive symptoms while Dunn et al. (19) described four distinct subgroups. One plausible explanation for this discrepancy is that the current study tied the assessment of depressive symptoms to occasions chosen to reflect clinically meaningful events during adjuvant therapy as well as to two-month intervals in the first six months following completion of active treatment; in contrast, assessments in the Dunn et al. study (19) occurred prior to surgery and then once every month for six months following surgery. Whereas, in the current study, patient treatment status was similar at each assessment, there was considerable heterogeneity in the patients’ treatment status at any one assessment in the Dunn et al. study (19); 20% of the total sample had received chemotherapy prior to surgery and more than half of the patients, but not all, were in active treatment over the course of the study.
Results of the current study also are similar to those from previous studies of cancer patients that used growth mixture modeling to determine the trajectory of symptoms similar in nature to depressive symptoms. For example, Helgeson et al. (17) identified distinct trajectories of mental functioning as measured by the Medical Outcomes Study Short Form-36 based on seven assessments over a four-year period following a diagnosis of breast cancer. Like the Dunn et al.(19) study, this study did not take into account how the timing of assessments differed across patients depending on the type of adjuvant treatment received. Some of the patients were in active treatment at particular assessments while others were not. The current study's results also are generally consistent with a study by Henselmans et al. (18) which identified distinct trajectories of psychological distress as measured by the 12-item General Health Questionnaire over five specific illness-related phases in the first year after a breast cancer diagnosis: shortly after diagnosis, after surgery, after completion of adjuvant therapy, and two and six months after treatment completion in patients who went through all five phases. Although the timing and number of assessments and the number and shape of the trajectories identified differ across the current and previous studies, taken together, the results support the need to examine individual differences in women with breast cancer using newer methods of longitudinal data analysis.
The second aim of the current study was to examine whether the three subgroups identified could be distinguished based on demographic characteristics, clinical characteristics, and use of problem-focused coping strategies. With respect to demographic characteristics, the current study yielded a significant effect of marital status in distinguishing different trajectories of depressive symptoms. Compared to married women, unmarried women were more likely to be in the subgroup with clinically significant depressive symptoms that persisted over time. This finding is consistent with a recent longitudinal study of breast cancer patients that found that women who were not married reported higher levels of depressive symptoms during the first year following the diagnosis of breast cancer (12). The current study did not confirm previously reported findings that trajectories of symptoms may be distinguished by age (19) and education (9) although there was little variance in our sample with respect to these variables.
With respect to clinical characteristics, previous research has demonstrated that trajectories of symptoms may be distinguished by performance status (6) surgical procedure (17, 19), chemotherapy (17, 19) and the total number of treatment-related complaints (18). In the current study, we assessed a broad range of clinical features, including body mass index, menopausal status prior to treatment, and hormonal therapy following adjuvant treatment, factors largely unexamined in previous research. We found that none of these clinical factors distinguished different trajectories of depressive symptoms. Given the considerable heterogeneity within study samples, how the timing of assessments differed across patients within studies (at a given assessment, some patients were in active treatment, while others were not), and the lack of clinical significance related to the timing of particular assessments in most studies, it was unlikely, based on the well-defined clinical characteristics of the current study sample and the related clinical significance of the assessments in the current study, that we would replicate previous results. It remains to be determined whether there are other clinical factors that distinguish patterns of depressive symptoms in women with breast cancer.
With respect to psychosocial factors and their relationships to trajectories, there is a large body of research demonstrating that the experience of a major depressive episode predisposes an individual to future depressive episodes (23-27, 53). Dunn et al. (19) found that problems with depression at study outset distinguished breast cancer patients with increasing depressive symptoms over time from those with decreasing depressive symptoms over time. Previous research suggests that a diagnosis of breast cancer may precipitate the onset of recurrent depressive symptoms in women with a history of a depressive disorder (3, 9). The current study is the first to examine the predictive effect of history of major depressive disorder on depressive symptom trajectories. In univariate analysis, compared to women with minimal depressive symptoms over the course of the study, women with clinically significant depressive symptoms throughout were more likely to have a history of depression. This effect did not remain significant in the final multivariate analysis, however.
The current study also examined whether trajectories could be distinguished based on specific problem-focused coping strategies as measured by the Illness Management Questionnaire. As conceptualized by Ray et al. (32), problem-focused coping may include non-instrumental strategies, such as focusing on symptoms, in which a principal problem becomes the focus of attention and thought in an attempt to better manage it. Indeed, we found that focusing on symptoms, but not maintaining activity, accommodating to illness, or information seeking, was a statistically significant predictor of subgroup membership in univariate analyses. A tendency to be preoccupied with symptoms, linked with an appraisal of one's life as dominated by the illness, was associated with membership in the subgroup characterized by persistent clinical levels of depressive symptoms over the course of the study period (focusing on symptoms was not associated with past history of depression, however). In the multivariate analysis, focusing on symptoms was one of two factors that remained a statistically significant predictor of subgroup membership. Thus, the current study extends existing research demonstrating that coping strategies are associated with depressive symptoms and recurrence of depression in the general population (see for example, Carragher et al.(13) and in primary care patients (see for example, Conradi and colleagues (28) to women with breast cancer.
The current study has several implications for clinical care and for future research. First, findings showing that more than a quarter of the sample had clinically significant depressive symptoms at the start of adjuvant therapy that did not decline over time suggest the importance of psychosocial screening early in the course of treatment. This observation is consistent with existing evidence-based recommendations (54) for the management of depression in adults with cancer which stress the importance of routinely screening all patients at treatment outset and at regular intervals thereafter. These findings also support the identified need for more research examining whether use of psychosocial screening as part of an integrated care model leads to better quality of life outcomes in people with cancer (55, 56).
Second, findings suggest the importance of evaluating history of major depression in addition to current levels of depressive symptomatology at treatment outset. At the same time, the vulnerability that appears to be conferred by history of major depression needs to be investigated further. One possibility is that it reflects an underlying biological vulnerability that may have a genetic component. Another not mutually exclusive possibility is that it reflects an enduring difficulty in coping effectively with major life stressors. The latter possibility is suggested by the patterns of results showing that history of major depression was no longer significant in a multivariate analysis that also included tendencies to cope with illness by focusing on symptoms. Similar conclusions can also be reached about findings showing that marital status was related to different trajectories of depressive symptomatology. Although useful clinically in identifying at-risk individuals, the mechanism(s) underlying this finding still needs to be elucidated. Does it reflect lack of social support from a marital partner or is it a reflection of broader lifestyle issues?
Finally, findings regarding focusing on symptoms suggest that decreasing reliance on this form of coping should be an important part of efforts to relieve depressive symptomatology in patients undergoing cancer treatment. This conclusion is buttressed by research with cancer patients that has demonstrated the benefits of modifying negative illness-related cognitions as part of a multi-component cognitive-behavioral intervention for patients who have completed cancer treatment (see for example, (57). At the same time, we are unaware of intervention research that has explicitly examined whether modifying the tendency to focus on illness-related symptoms has a beneficial effect on depression in patients undergoing cancer treatment. This is a topic deserving of further study.
Certain limitations of the current study should be noted. The women in our sample were predominantly Caucasian and married, and had annual household incomes over $40,000. In addition, a majority had stage 2 disease and underwent lumpectomy prior to adjuvant therapy. Whether our findings are generalizable to a more demographically and clinically diverse population of women with breast cancer is not known. Our design included multiple assessments in the post-treatment period; however, we did not assess depressive symptoms prior to surgery and we assessed depressive symptoms only until 6 months after treatment. Whether different patterns of depressive symptoms emerge earlier in the cancer care trajectory or later in the course of cancer survivorship is not clear. We did not find that accommodating to illness was related to more benign depressive symptom trajectories. This negative finding may be due to the generic nature of the IMQ as it assesses coping styles with respect to illness in general and not breast cancer in particular. Further, coping styles may have varied over the course of the study; however, we assessed coping styles only at the start of the study. Finally, there are likely to be other psychosocial and psychological factors that distinguish trajectories of depressive symptoms; ones that we did not examine in the current study. Such factors might include emotion-focused forms of coping, personality characteristics (e.g., neuroticism) and social support.
Despite its limitations, the current study demonstrates the value of growth mixture modeling for examining depressive symptomatology over time in individuals with a diagnosis of cancer. In addition to distinguishing three trajectories of symptomatology that vary with regard to their clinical meaningfulness, use of this analytic approach led to identification of a demographic characteristic (i.e., marital status) and two psychosocial characteristics (i.e., history of major depression and a tendency to focus on symptoms) that predict which trajectory a woman with breast cancer is likely to follow during and after adjuvant treatment for breast cancer. The findings with respect to psychosocial characteristics, which have not been examined in previous studies of cancer-related depressive symptom trajectories, lay the foundation for future research into mechanisms that underlie risk for prolonged depressive symptomatology in women undergoing treatment for breast cancer as well as for efforts to develop more effective ways of addressing the problem of depression in this patient population.
Acknowledgments
Supported in part by National Cancer Institute Grant R01CA82822. Kristine A. Donovan was supported in part by American Cancer Society Grant MRSG-06-082-01-CPPB.
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to disclose.
Following a reviewer's suggestion, we reran the three class model constraining the intercept and slope covariance to zero for all three classes and allowing the intercept and slope variance in each class to be freely estimated. Unfortunately, the convergence problem was not remedied and the slope variance for the highest depressive symptom class was negative. Moreover, the fit of the model with the covariances constrained was not appreciably better than the three class model that we originally reported (-2ll = 2611.93, df = 17, AIC = 5257.85, BIC = 5308.69). As such, we have retained the three class model.
References
- 1.Deshields T, Tibbs T, Fan MY, Taylor M. Differences in patterns of depression after treatment for breast cancer. Psycho-Oncology. 2006;15:398–406. doi: 10.1002/pon.962. [DOI] [PubMed] [Google Scholar]
- 2.Ganz PA, Kwan L, Stanton AL, et al. Quality of life at the end of primary treatment of breast cancer: first results from the Moving Beyond Cancer randomized trial. JNCI. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 3.Christensen S, Zachariae R, Jensen AB, et al. Prevalence and risk of depressive symptoms 3-4 months post-surgery in a nationwide cohort study of Danish women treated for early stage breast-cancer. Breast Cancer Res Treat. 2009;113:339–355. doi: 10.1007/s10549-008-9920-9. [DOI] [PubMed] [Google Scholar]
- 4.Luutonen S, Vahlberg T, Eloranta S, Hyvari H, Salminen E. Breast cancer patients receiving postoperative radiotherapy: distress, depressive symptoms and unmet needs of psychosocial support. Rad Onc. 2011;100:299–303. doi: 10.1016/j.radonc.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Hopwood P, Howell A, Maguire P. Psychiatric morbidity in patients with advanced cancer of the breast; prevalence measured by two self-rating questionnaires. British J Cancer. 1991;64:349–352. doi: 10.1038/bjc.1991.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantarero-Villanueva I, Fernandez-Lao D, Fernandez-De-Las_penas C, et al. Associations among musculoskeletal impairments, depression, body image and fatigue in breast cancer survivors wtihin the first year after treatment. Eur J Cancer. 2011;20:632–639. doi: 10.1111/j.1365-2354.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 7.Meyer L, Aspergren K. Long-term psychological sequelae of mastectomy and breast conserving treatment for breast cancer. Acta Onc. 1989;28:13–18. doi: 10.3109/02841868909111174. [DOI] [PubMed] [Google Scholar]
- 8.Den Oudsten BL, Van Heck GL, Van der Steeg AF, Roukema JA, De Vries J. Predictors of depressive symptoms 12 months after surgical treatment of early-stage breast cancer. Psycho-Oncology. 2009;18:1230–1237. doi: 10.1002/pon.1518. [DOI] [PubMed] [Google Scholar]
- 9.Hill J, Holcombe C, Clark L, et al. Predictors of onset of depression and anxiety in the year after diagnosis of breast cancer. Psychol Med. 2011;41:1429–1436. doi: 10.1017/S0033291710001868. [DOI] [PubMed] [Google Scholar]
- 10.Costanzo ES, Lutgendorf SK, Mattes ML, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. British J Cancer. 2007;97:1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlegel RJ, Manning MA, Molix LA, Talley AE, Bettencourt BA. Predictors of depressive symptoms among breast cancer patients during the first year post diagnosis. Psychol Health. 2012;27:277–293. doi: 10.1080/08870446.2011.559232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carragher N, Adamson G, Bunting B, McCann S. Subtypes of depression in a nationally representative sample. J Affect Dis. 2009;113:88–99. doi: 10.1016/j.jad.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Colman I, Ploubidis GB, Wadsworth ME, Jones PB, Croudace TJ. A longitudinal typology of symptoms of depression and anxiety over the life course. Bio Psychiatry. 2007;62:1265–1271. doi: 10.1016/j.biopsych.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Norton S, Sacker A, Young A, Done J. Distinct psychological distress trajectories in rheumatoid arthritis: findings from an inception cohort. J Psychosom Res. 2011;71:290–295. doi: 10.1016/j.jpsychores.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Murphy BM, Elliott PC, Higgins RO, et al. Anxiety and depression after coronary artery bypass graft surgery: most get better, some get worse. Eur J Cardio PreventRehab. 2008;15:434–440. doi: 10.1097/HJR.0b013e3282fbc945. [DOI] [PubMed] [Google Scholar]
- 17.Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychol. 2004;23:3–15. doi: 10.1037/0278-6133.23.1.3. [DOI] [PubMed] [Google Scholar]
- 18.Henselmans I, Helgeson VS, Seltman H, et al. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010;29:160–168. doi: 10.1037/a0017806. [DOI] [PubMed] [Google Scholar]
- 19.Dunn LB, Cooper BA, Neuhaus J, et al. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol. 2011;30:683–692. doi: 10.1037/a0024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radloff LS. A self-report depression scale for research in the general population. App Psychol Measure. 1977;1:385–401. [Google Scholar]
- 21.Nesselroade JR, Baltes PB. Longitudinal Research in the Study of Behavior and Development. Academic Press; New York: 1979. [Google Scholar]
- 22.Jim HS, Small BJ, Minton S, Andrykowski M, Jacobsen PB. History of major depressive disorder prospectively predicts worse quality of life in women with breast cancer. Ann Behav Med. 2012;43:402–408. doi: 10.1007/s12160-011-9333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ten Doesschate MC, Bockting CL, Koeter MW, Schene AH. Prediction of recurrence in recurrent depression: a 5.5-year prospective study. J Clin Psychiatry. 2010;71:984–991. doi: 10.4088/JCP.08m04858blu. [DOI] [PubMed] [Google Scholar]
- 24.Bockting CL, Spinhoven P, Koeter MW, Wouters LF, Schene AH. Prediction of recurrence in recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. J Clin Psychiatry. 2006;67:747–755. [PubMed] [Google Scholar]
- 25.Solomon DA, Keller MB, Leon AC, et al. Multiple Recurrences of Major Depressive Disorder. AmerJ Psychiatry. 2000;157:229–233. doi: 10.1176/appi.ajp.157.2.229. [DOI] [PubMed] [Google Scholar]
- 26.Belsher G, Costello CG. Relapse after recovery from unipolar depression: a critical review. Psychol Bull. 1988;104:84–96. doi: 10.1037/0033-2909.104.1.84. [DOI] [PubMed] [Google Scholar]
- 27.Keller MB, Lavori PW, Lewis CE, Klerman GL. Predictors of relapse in major depressive disorder. JAMA. 1983;250:3299–3304. [PubMed] [Google Scholar]
- 28.Conradi HJ, de Jonge P, Ormel J. Prediction of the three-year course of recurrent depression in primary care patients: different risk factors for different outcomes. J Affect Disorders. 2008;105:267–271. doi: 10.1016/j.jad.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Kuehner C, Weber I. Responses to depression in unipolar depressed patients: an investigation of Nolen-Hoeksema's response styles theory. Psychol Med. 1999;29:1323–1333. doi: 10.1017/s0033291799001282. [DOI] [PubMed] [Google Scholar]
- 30.Blalock JA, Joiner TE., Jr. Interaction of cognitive avoidance coping and stress in predicting depression and anxiety: Gender differences. Cog Ther Res. 2000;24:47–65. [Google Scholar]
- 31.Kraemer LM, Stanton AL, Meyerowitz BE, Rowland JH, Ganz PA. A longitudinal examination fo couples' coping strategeis as predictors of adjustment to breast cancer. J Fam Psychol. 2011;25:963–972. doi: 10.1037/a0025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Low CA, Stanton AL, Thompson N, Kwan L, Ganz PA. Contextual life stress and coping strateiges as predictors of adjustment to breast cancer survivorship. Ann Beh Med. 2006;32:235–244. doi: 10.1207/s15324796abm3203_10. [DOI] [PubMed] [Google Scholar]
- 33.Stanton AL, Danoff-Burg S, Huggins ME. The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psycho-Oncology. 2002;11:93–102. doi: 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- 34.Ray C, Weir W, Stewart D, Miller P, Hyde G. Ways of coping with chronic fatigue syndrome: development of an illness management questionnaire. Soc Sci Med. 1993;37:385–391. doi: 10.1016/0277-9536(93)90268-9. [DOI] [PubMed] [Google Scholar]
- 35.Ransom S, Jacobsen PB, Schmidt JE, Andrykowski MA. Relationship of problem-focused coping strategies to changes in quality of life following treatment for early stage breast cancer. J Pain Symptom Manage. 2005;30:243–253. doi: 10.1016/j.jpainsymman.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors: a longitudinal, multi-site study. Cancer. 2010;116:5740–5748. doi: 10.1002/cncr.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identifying cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Onc. 2005;23:6613–6622. doi: 10.1200/JCO.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 39.Muthen BO. Latent variable analysis. Growth mixture modeling and related techniques. In: Kapland D, editor. Handbook of Quantitative Methodology for the Social Sciences. Sage; Newbury Park, CA: 2004. pp. 345–369. [Google Scholar]
- 40.Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol. 2007;26:464–472. doi: 10.1037/0278-6133.26.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobsen PB, Donovan KA, Small BJ, et al. Fatigue after treatment for early stage breast cancer: a controlled comparison. Cancer. 2007;110:1851–1859. doi: 10.1002/cncr.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Ray C, Jefferies S, Weir W. Coping with chronic fatigue syndrome: illness responses and their relationship with fatigue, functional impairment, and emotional status. Psychol Med. 1995;25:937–945. doi: 10.1017/s0033291700037429. [DOI] [PubMed] [Google Scholar]
- 44.Ray C, Jefferies S, Weir WR. Coping and other predictors of outcome in chronic fatigue syndrome: a 1-year follow-up. J Psychosom Res. 1997;43:405–415. doi: 10.1016/s0022-3999(97)00111-6. [DOI] [PubMed] [Google Scholar]
- 45.First MB, Gibbons M, Spitzer RL. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders: Research Version. Biometrics Research; New York: 1996. [Google Scholar]
- 46.Beeber LS, McCorkle R. The Center for Epidemiologic Studies Depression Scale as a measure of depressive symptoms in newly diagnosed patients. J Psychosoc Onc. 1998;16:1–20. [Google Scholar]
- 47.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 48.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2nd Ed. SAS Press; Cary, NC: 2006. [Google Scholar]
- 49.Ram N, Grimm KJ. Methods and Measures: Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. Internat J Behav Dev. 2009;33:565–576. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo Y, Mendell NRR DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 51.Jedidi K, Ramaswamy VV, Desarbo WS. A maximum likelihood method for latent class regression involving a censored dependent variable. Psychometrika. 1993;358:373–394. [Google Scholar]
- 52.Greenbaum PE, Del Boca FK, Darkes J, Wang CP, Goldman MS. Variation in the drinking trajectories of freshmen college students. J Consul Clin Psychol. 2005;73:229–238. doi: 10.1037/0022-006X.73.2.229. [DOI] [PubMed] [Google Scholar]
- 53.Burcusa SL. Iacono WG: Risk for recurrence in depression. Clin Psychol Rev. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dy SM, Lorenz KA, Naeim A, et al. Evidence-based recommendations for cancer fatigue, anorexia, depression, and dyspnea. J Clin Onc. 2008;26:3886–3895. doi: 10.1200/JCO.2007.15.9525. [DOI] [PubMed] [Google Scholar]
- 55.Jacobsen PB, Wagner LI. A new quality standard: the integration of psychosocial care into routine cancer care. J Clin Onc. 2012;30:1151–1153. doi: 10.1200/JCO.2011.39.5046. [DOI] [PubMed] [Google Scholar]
- 56.Velikova G. Patient benefits from psychosocial care: screening for distress and models of care. J Clin Onc. 2010;28:4871–4872. doi: 10.1200/JCO.2010.31.0136. [DOI] [PubMed] [Google Scholar]
- 57.Gielissen MFM, Verhagen S, Witjes F, Bleijenberg G. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Onc. 2006;24:4882–4887. doi: 10.1200/JCO.2006.06.8270. [DOI] [PubMed] [Google Scholar]