Abstract
Nicotinamide adenine dinucleotide phosphate oxidase (NADPH-oxidase; NOX) is a complex enzyme responsible for increased levels of reactive oxygen species (ROS), superoxide (O2.−). NOX derived O2.− is a key player in oxidative stress and inflammation mediated multiple secondary injury cascades (SIC) following traumatic brain injury (TBI). The O2.− reacts with nitric oxide (NO), produces various reactive nitrogen species (RNS), and contributes to apoptotic cell death. Following a unilateral cortical contusion, young adult rats were killed at various times post injury (1, 3, 6, 12, 24, 48, 72, and 96 h). Fresh tissue from the hippocampus was analyzed for NOX activity, and level of O2.−. In addition we evaluated the translocation of cytosolic NOX proteins (p67Phox, p47Phox and p40Phox) to the membrane, along with total NO and the activation (phosphorylation) of endothelial nitric oxide synthase (p-eNOS). Results show that both enzymes and levels of O2.− and NO have time dependent injury effects in the hippocampus. Translocation of cytosolic NOX proteins into membrane, NOX activity and O2.− were also increased in a time dependent fashion. Both, NOX activity and O2.− were increased at 6 h. Levels of p-eNOS increased within 1 h, with significant elevation of NO at 12 h post TBI. Levels of NO failed to show a significant association with p-eNOS, but did associate with O2.−. NOX up-regulation strongly associated with both the levels of O2.− and also total NO. The initial 12 hours post TBI are very important as a possible window of opportunity to interrupt SIC. It may be important to selectively target the translocation of cytosolic subunits for the modulation of NOX function.
Keywords: NADPH-oxidase, free radicals, secondary injury cascades, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is associated with costly health problems and mortality in the population of all age. Millions of individuals suffer TBI each year resulting in long term disabilities, with many requiring hospitalization [1]. A majority of TBI survivors have chronic neurobehavioral problems including cognitive deficits. Considering that the hippocampus is an important brain region involved in learning and memory, it is important to investigate its involvement after TBI. Following a contusion or concussion, multiple secondary injury cascades/mechanisms (SIC) initiate delayed pathology that includes cytoskeletal damage and altered cell signal transduction [2,3] resulting in neuronal dysfunction, and/or death. Various cellular and molecular changes play a key role in these cascades and include ionic imbalance [4], mitochondrial dysfunction [5-8], excitotoxicity [9], oxidative stress [10-12], and inflammation [13-16].
Oxidative stress, the imbalance between the level of free radicals and antioxidants, consists an increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and decreased antioxidant defense. These ROS/RNS have been linked with the pathogenesis after TBI [10-12,17]. High content of poly-unsaturated fatty acids in the brain and reduced levels of antioxidants after neurotrauma [11,12,15] makes it more vulnerable to free radical attacks. Increased ROS/RNS causes oxidative/nitrosative damage [18] in lipids, proteins, and nucleic acids [11,12,19] are believed to initiate neurodegenerative processes in the brain [20,21].
Several studies have shown that NADPH-oxidase (NOX) is up-regulated following TBI [22-25] and coincides with the excessive production of superoxide (O2.−) [26-31]. Increased ROS in the brain following TBI appears to be linked to NOX up-regulation. Increased O2.− can intensify oxidative stress, participate in the inflammation [28], and increase further ROS/RNS production [14,26,32,33]. Elevated ROS/RNS can cause mitochondrial dysfunction and ultimately neuronal degeneration [34-36]. The ROS producing enzyme, NOX, is expressed in the various different types of cells, i.e., phagocytic cells, endothelial cells, vascular smooth muscles, and fibroblasts [37,38]. A growing body of evidence suggests that the NOX is expressed in microglia [39-43] and neurons [44,45], and plays a key role in CNS pathophysiology [46-49]. NOX is a multi-subunit enzyme composed of cytosolic (p-67Phox, p-47Phox, and p-40Phox) and membrane (p-91Phox, and p-22Phox) [50] subunits coupled with the GTPase protein Rac1 [51-53]. NOX is up-regulated with the translocation/interaction of its cytosolic proteins to the membrane subunit [29,31,50,54] and subsequently participates in various pathophysiological mechanisms [48,50,54-58]. Recent studies have demonstrated time dependent changes in NOX function after TBI [25]. NOX derived ROS after brain injury has been linked to the neuronal death and its inhibition has been shown to be neuroprotective [22,24,59,60]. Direct or indirect inhibition of NOX function appears to be neuroprotective after brain injury regardless if the inhibitor/modulator is administered pre- or post-injury [23-25,40].
A form of RNS, nitric oxide (NO), production is also increased following TBI [2,61-64] and participates in neurodegenerative cascades [2,27,65]. NO can be derived from different NO-synthase among which is endothelial NOS (eNOS) [66]. Although, expression of eNOS has been shown to be increased after TBI [61,62], it is unclear whether eNOS up-regulation following head trauma contributes to oxidative stress via increasing NO. Several studies have implicated eNOS in the variety of physiological functions [67-69], and it’s up-regulation might be beneficial after TBI [61,63]. The activation of eNOS by phosphorylation (p-eNOS) has been linked to several cell signaling processes [64,70]. The bioavailability of eNOS derived NO depends on the rate of reaction with O2− (NO + O2.− → ONOO−) that may be linked to NOX up-regulation. Most researchers adhere to the idea that modulation of NOX function can suppress ROS/RNS formation [22-24,30,59,60] and reduces pathology after head trauma [22,24,60].
Characterizing the time course of early changes in mechanisms responsible for both ROS/RNS, will help determine the window of opportunity for maximizing a more favorable outcome following TBI. This study evaluated possible time-dependent changes in NOX activity with including translocation of their cytosolic subunits and production of O2.−. The analysis also focused on the time course of NO production and its correlation with eNOS activation.
Materials and Methods
Chemicals
Mouse monoclonal anti β-actin (sc-47778), p-47Phox (sc-17845), p-40Phox (sc-48376), p-eNOS (sc-136519), GAPDH (sc-365062), rabbit polyclonal anti p-67Phox (sc-15342), and Na+/K+-ATPase (sc-28800) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Alkaline phosphatase conjugated anti-mouse and anti-rabbit secondary antibodies, and all other chemicals/reagents were purchased from Sigma (St Louis, MO, USA). Materials, and chemicals used in electrophoresis were purchased from Bio-Rad (Hercules, CA, USA) unless stated otherwise.
Animals and surgical procedures
Adult male Sprague-Dawley rats (250-275g) (Harlan Laboratories, Indianapolis, IN) were used in this study. Animal protocol and procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Animals were housed 2/cage on a 12 h light/dark cycle and provided access to food and water ad libitum. Rats were subjected to a moderate unilateral controlled cortical impact (CCI) utilizing an electronic controlled pneumatic impact device (ECPI) (TBI0310, Precision System & Instrumentation, Fairfax Station, VA) as previously described [10,12]. Each animal was anesthetized with 2% isofluorane and placed in a Kopf stereotaxic frame (Tujunga, CA) with the incisor bar set at −5. Body temperature of each rat were monitored and maintained at 36 °C with a heating pad. Following a midline incision and retraction of the skin, a 6-mm-diameter craniotomy was made approximately midway between bregma and lamda with a Michele hand trephine (Miltex, NY). The skull disk was removed without disturbing the dura membrane. The open brain was injured using the ECPI; as a 5-mm-diameter cortex compressed for a depth of 2.0 mm at 3.5 m/s. All the surgical procedure was completed in 15-20 min. Animals were divided into nine groups (n=6/group) and allowed to survive for 1, 3, 6, 12, 24, 48, 72 and 96 h post TBI for biochemical analysis. Sham animals (n=2/group) received the craniotomy without TBI and killed at 6, 12, and 48h. Since our previous studies failed to demonstrate a significant change in oxidative stress following sham injury [10,12], tissue from all three sham groups were combined for analysis.
Tissue processing
Animals were euthanized with Fatal-Plus (Med-Vet International, Mettawa, IL) and hippocampi isolated from both the ipsilateral and contralateral hemispheres. Tissue samples were immediately placed in dry ice and then stored at −80°C until used for analysis. Tissue was homogenized (10%; w/v) on ice in 0.1M, PBS (pH 7.4) containing protease inhibitor cocktail (Millipore Inc.) and centrifuged at 1000g for 10 min/4°C to remove the cell debris pellet (P1). Collected supernatants were further centrifuged at 15,000g for 10 min/4°C and then both the pellet-2 (P2) and post mitochondrial supernatant (PMS) was collected. PMS of all samples were used for enzymatic and non-enzymatic biochemical analyses. These assays were completed in 96-well plates and analyzed with a SpectraMax® micro-plate reader (Molecular Devices, Sunnyvale, CA). Total protein concentration was determined using the Pierce BCA method (Sigma, St. Louis, MO).
Estimation of NOX activity and its derived superoxide (O2.−)
NOX activity and it’s derived O2.− was quantified as previously described using lucigenin-enhanced chemiluminescence [50]. Reaction mixture containing HEPES buffer (pH 7.4), protease inhibitor cocktail, L-NAME (1 mmol/L), triethylenetetramine (1.0 mmol/L), SDS (100 μmol/L) lucigenin (20 μmol/L), and 20 μl PMS were aliquoted in 96-well plates and luminescence values were recorded. Blank values were subtracted from sample readings and chemiluminescence/mg protein of each sample was calculated as percent change versus sham operates. Chemiluminescence was also monitored in the presence of diphenyliodonium and quinacrine (100 μM and 1.0 mM), oxypurinol (100 μM), rotenone (100 μM), or indomethacin (10 μM) as a corresponding inhibitor of NOX, xanthine oxidase, mitochondrial respiration, and cyclooxygenase function for O2.− production.
NOX activity was evaluated in 96-well plates. In a dark area of work, plates were placed in the luminometer (SpectraMax®)) to calculate basal luminescence, then after addition of NADPH (0.2 mM), kinetic readings were taken for 15 min. Blank readings were subtracted from the PMS-added well’s results of each sample. The changes in chemiluminescence/min/mg protein in each sample were calculated for percent changes versus sham operated rats (% control). Samples were also analyzed in the presence of NOX inhibitor diphenyliodonium and quinacrine (100 μM and 1.0 mM, respectively) and readings were subtracted from the values in the absence of inhibitors. Subsequently, measured chemiluminescence was due to O2.− produced with NOX.
Estimation of NO in hippocampus
Assessment of total NO was completed as the analysis of total nitrite/nitrate present in samples according to the method described earlier [71]. In brief, 100 μl of nitrate standards (in serial dilution) and samples were added to 400 μl of carbonate buffer in test tubes, followed by a small amount of (~0.15 g) cadmium beads. The tubes were then incubated at room temperature for 1h with thorough shaking. The reaction was stopped by the addition of 100 μl of NaOH (0.35 M), and vortexed with the addition of 400 μl of ZnSO4 (120 mM). The tubes were allowed to stand for 10 min and centrifuged at 4000g for 10 min. Clear supernatant (150 μl aliquots) was transferred into the 96 well plates in triplicate. Griess reagent (equal volumes of 1% naphthalene diamine dihydrochloride in distilled water and a mixture of 10% sulfanilamide and 50% concentrated H3PO4) (150 μl ) was added with gentle mixing. After 10 min, the absorbance was taken at 545nm against blank containing reaction mixture but no biological sample or nitrate standard. The absorbance values of each sample calculated first for the nmol-NO/mg protein and then results converted into percent change versus sham operates.
Western-blot analysis for eNOS and NOX proteins
NOX associated key proteins (p67Phox, p47Phox, and p-p40Phox) and p-eNOS was analyzed as previously described using the Western-blot method [50,56]. In the present study, β-actin, GAPDH, and Na+/K+-ATPase were used as a corresponding standard protein markers of cytoskeleton, cytosol, and cell membrane. Each P2 fraction (mentioned above) was mixed with corresponding PMS and diluted 1:1 (v/v) in lysis buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1% Triton X-100, 10% glycerol, and protease inhibitor cocktail). All the samples were centrifuged at 13,000g for 10 min at 4°C and then collected supernatants further centrifuged for 1h at 100,000g at 4°C. The obtained pellets and supernatants were designated as the membrane and cytosolic fraction [48]. Equal amounts of protein (50μg) were loaded with the appropriate marker (Bio-Rad) on a gradient gel (4-20% Tris-HCl), followed by transfer to a nitrocellulose membrane using a semi-dry transfer system (Bio-Rad) in transfer buffer (25 mM Tris, 150 mM glycine, 20% MeOH) for 2 h at 15 volt. NOX protein analyzing membranes were blocked with 5% milk, and p-eNOS assessing blots were blocked with 3% bovine serum albumin (BSA), in Tris/saline buffer-Tween (TBST). Primary antibodies of p-67Phox, p-47Phox, p-40Phox, p-eNOS β-actin, GAPDH, and Na+/K+-ATPase were added at a concentration of 1:1000 and incubated overnight at 4°C. The blots were washed three times in TBST and incubated for 1 h with alkaline phosphatase conjugated secondary antibodies in a 1:8000 dilution. All the membranes were washed three times in TBST for 10 min and developed in Sigma Fast tablets (BCIP/NBT substrate). Blots were then dried, scanned with Adobe Photoshop, and quantified with Scion Image (PC version of Macintosh-compatible NIH Image).
Statistical analysis
Time-dependent changes in enzymatic and non-enzymatic oxidative/nitrosative markers, NOX proteins, and p-eNOS are reported as mean ± SD. The values of each variable normalized first with the results in contralateral sample (own control) and then compared with sham operated animals. Possible differences between group means were evaluated with a one-way ANOVA (Graph Pad Prizm) followed by Dunnett’s test. Association between both the enzyme’s function, O2.− and NO production, and translocation of the NOX subunits were examined using Spearman correlation. For significant differences, alpha was set at 0.05.
Results
Time course of NOX activity and O2.− levels
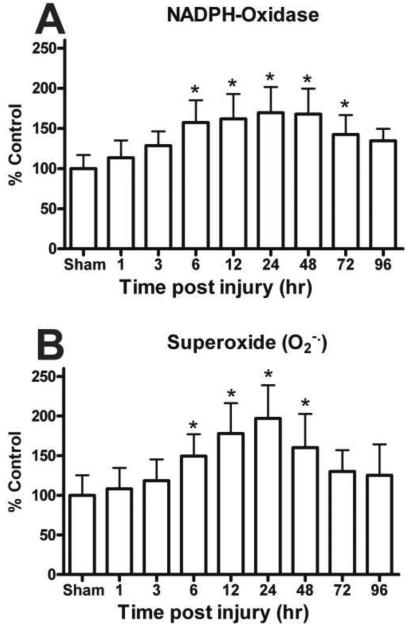
NOX produces O2.− radicals during oxidative metabolism of NADPH as a cell function. The time course analysis of NOX function revealed a significant time-dependent up-regulation in the hippocampus following TBI [F (8, 45) = 5.903, p < 0.0001] (Fig. 1A). The earliest significant NOX up-regulation was observed at 6 h (160%) after injury. This increased activation continued until at least 72h post TBI. At 96 h post injury, although NOX activity remained elevated it was no longer statistically different from sham operated animals.
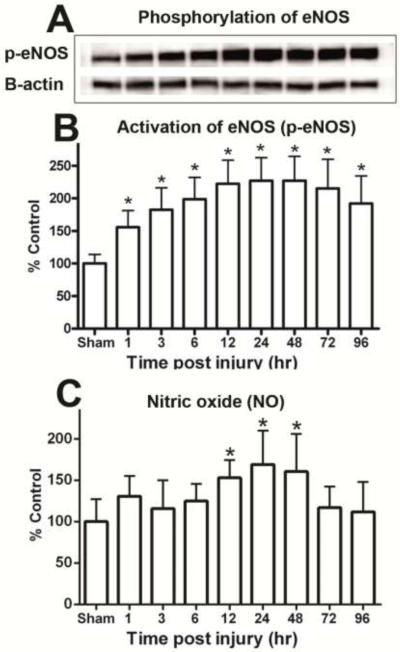
Figure 1.
Time course for the activity of NADPH Oxidase (NOX) and levels of superoxide (O2.−) in the ipsilateral hippocampus following a moderate cortical contusion. (A) A significant elevation in the NOX activity occurred at 6 h after injury, with the maximum NOX activity observed at 24 h. Levels of NOX derived O2.− also demonstrated a time-dependent increase with significant changes within 6 h post trauma and maximum increases at 24 h post injury. (B) Each bar represents the group mean ± SD of six animals/group. *p < 0.05 versus sham operates.
The level of O2.− also demonstrated a significant time-dependent injury effect in the hippocampus [F (8, 45) = 5.698, p < 0.0001] (Fig. 1B). The amount of O2.− was significantly increased by 6 h post trauma (149%) and remained significantly elevated at 48h, with peak levels observed at 24 h (197%) post TBI. The contralateral hippocampus failed to demonstrate any significant (p > 0.1) change in either NOX function or levels of O2.−.
Time course of NOX subunits
The cytosolic NOX subunits p-67Phox, p-47Phox, and p-40Phox were investigated in both the ipsilateral and contralateral hippocampus after a moderate TBI. Western-blot analysis of both the cytosolic and membrane fractions demonstrated a time-dependent translocation (Fig. 2A&B) of NOX subunits in the ipsilateral hippocampus. The contralateral hippocampus failed to demonstrate any significant change in any of these NOX proteins.
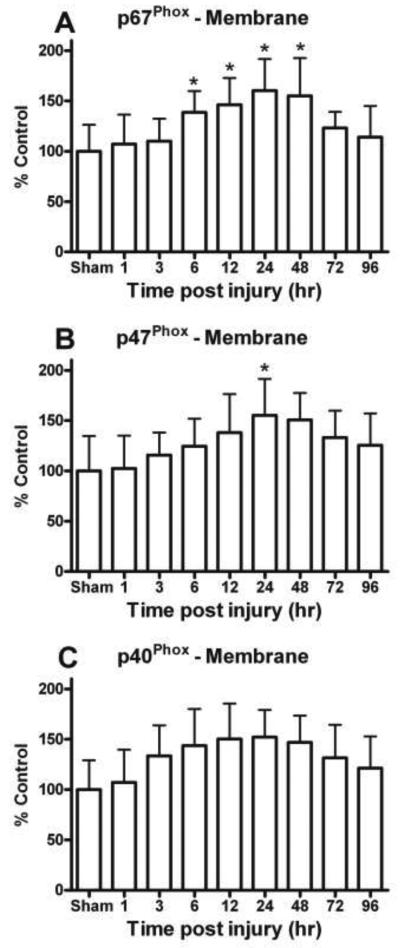
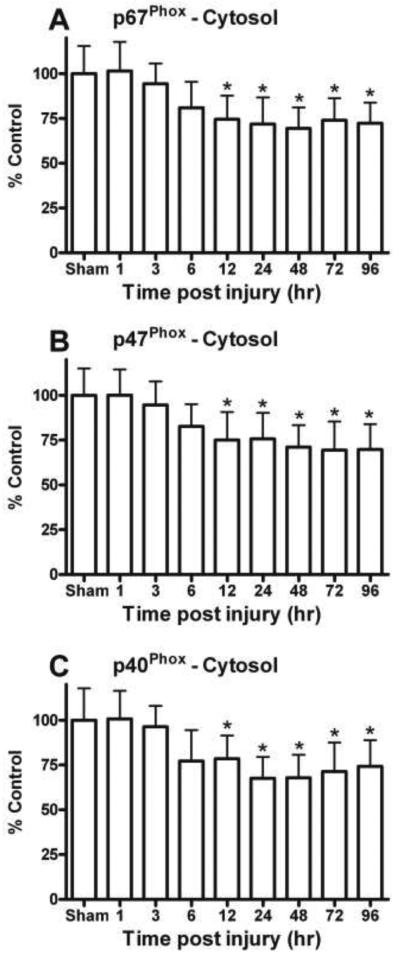
Figure 2.
Key cytosolic NOX subunit proteins were quantitatively assessed in the hippocampus with immunobloting followed by Western-blot. (A) The levels of cytosolic p67Phox, p47Phox, and p40Phox were increased in membrane fractions and (B) declined in cytosolic fraction, demonstrating a substantial translocation of these subunits. Na+/K+-ATPase and GAPDH show equal protein used for membrane and cytosolic Western-blots.
Time dependent changes in different NOX proteins in ipsilateral hippocampus membrane fraction following a moderate cortical contusion. Cytosolic NOX protein, p67Phox (C), was significantly increased early (6 h) in the membrane fraction of the hippocampus following TBI. The maximum increase of p67Phox in the membrane fraction was at 24 h and continued at 96 h post TBI. In a similar fashion, other key cytosolic NOX protein, p47Phox (E), and p40Phox (G) were also increased in membrane fraction of ipsilateral hippocampus as compared sham animals. Each bar represents the group mean ± SD of six animals/group. *p < 0.05 versus sham operates.
Levels of NOX proteins, p67Phox (D), p47Phox (F), and p40Phox (H) significantly decreased in cytosolic fraction of the hippocampus following TBI. Result indicates there was a time dependently sifting/translocation of cytosolic component to the membrane for the up-regulation of NOX and increase production of O2− after TBI. Each bar represents the group mean ± SD of six animals/group. *p < 0.05 versus sham operated rats.
Quantitative analysis of NOX protein p-67Phox revealed a time-dependent injury effect in the hippocampus, suggesting translocation of this subunit. Levels of p-67Phox demonstrated a significant increase in membrane [F (8, 45) = 3.807, p < 0.005] as early as 6 h post injury (Fig. 2C) and time dependent decline in the cytosolic fraction [F (8, 45) = 5.419, p < 0.0001] (Fig. 2D). The decline in the cytosolic p-67Phox was apparent even at 96 h after head trauma. Similar changes were observed for the cytosolic NOX protein p-47Phox, which showed a time-dependent increase [F (8, 45) = 2.458, p < 0.05] in the membrane (Fig. 2E) and decrease [F (8, 45) = 4.900, p < 0.0005] in the cytosolic fraction (Fig. 2F). In the membrane fraction, both the p-67Phox and p-47Phox subunits were maximal increased at 24h post TBI.
The cytosolic phosphorprotein p-40Phox is required to assemble NOX subunits and participates in its function. In the membrane fraction, p-40Phox was also increased by 50% (overall variability prevented significance of the main effect) compared to sham controls (Fig. 2G) and demonstrated a significant decline in the cytosol fraction [F (8, 45) = 5.205, p < 0.0001] (Fig. 2H). Like p67Phox and p-47Phox, levels of p40Phox demonstrated an early post trauma translocation of this important subunit.
Time course of p-eNOS and the total NO
The p-eNOS activation is the catalyzed production of NO that participates in pathophysiological mechanisms after TBI. Including participation in SIC, p-eNOS derived NO might also work to maintain blood flow in the adjacent area, penumbra region, of the impact [68]. Quantitative analysis revealed that p-eNOS increases in a time-dependent fashion following TBI [F (8, 45) = 8.705, p > 0.0001] (Fig. 3A & B). This elevation occurs as early as 1h post trauma and is maintained for at least 96h (p < 0.01). Maximum levels in the ipsilateral hippocampus were observed at 12-48 h (~225%).
Figure 3.
Time-dependent changes in hippocampal phosphorylated eNOS (p-eNOS) and level total nitric oxide (NO) after a moderate cortical contusion. (A) Western-blot analysis shows p-eNOS was significant increase in the ipsilateral hippocampus, as early as 1 h and maintained elevated levels at 96 h post trauma (B). Total NO levels in ipsilateral hippocampus were significantly increased at 24 h after TBI (C). Each bar represents the group mean ± SD of six animals/group. *p < 0.05 versus sham operated rats.
NO was evaluated in the same samples and displayed a delayed time-dependent increase [F (8, 45) = 3.313, p < 0.005] (Fig. 3C). Peak elevation of NO was observed at 24 h after TBI with levels approaching pre injury values at 72 h post trauma.
NOX up-regulation, p-eNOS, upsurge of O2− and NO
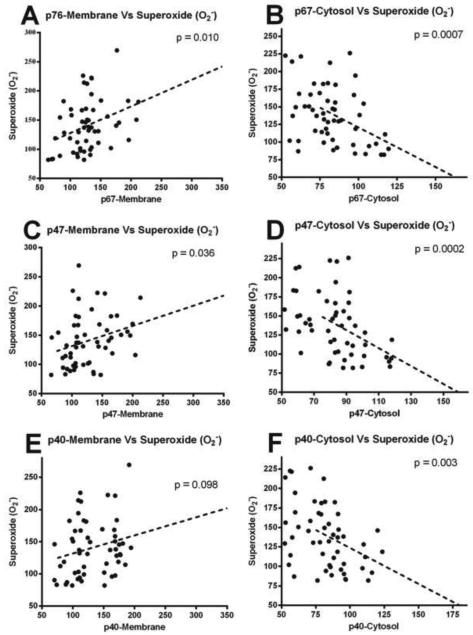
Association between the time dependent changes in individual NOX proteins (increase in membrane), NOX function and produced O2.− was assessed in the hippocampus following TBI. Time dependent increase of p-67Phox and p-47Phox proteins in the membrane fraction was strongly associated (p < 0.05) with both the increased NOX activity and surge of O2.− (Fig. 4A, B, C & D). However, increased p-40Phox in the membrane fraction was significantly associated with the increased NOX activity (p < 0.05), but it failed to show a strong association with increased O2.− levels (Fig. 4E & F).
Figure 4.
Scatterplot (A, C, E) showing the relationship between key NOX subunits (p-67Phox, p-47Phox, & p-40Phox) in the membrane fraction and changes in NOX activity Increases in the subunits significantly correlated with the elevation in total NOX activity. Scatterplot (B, D, F) shows the relationship between key NOX subunits (p-67Phox, p-47Phox, & p-40Phox) in the membrane fraction and changes in O2.− activity. Lines are shown to represent the direction of the correlation.
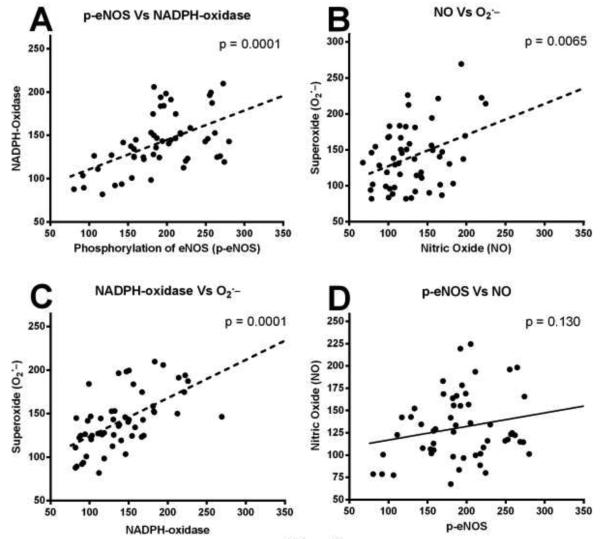
Across all the injured rats, it is observed that increased p-eNOS was almost in parallel with the NOX up-regulation after TBI. NOX up-regulation was strongly associated with the increased levels of O2.− (p < 0.001) and appeared to be a major source of O2.− increase in the ipsilateral hippocampus (Fig. 5A). Notably, there p-eNOS was failed to show strong association with total NO (p = 0.130) and appeared to be a non-significant contributor in NO increase after TBI (Fig. 5B). Though, these results are not to show cause and effect, but the correlation analysis among the changes in NOX and level of total NO shows there was a substantial associations between them (Fig. 5C). NOX up-regulation after injury mirrored total NO production. Increased levels of total NO was significantly associated with the upsurge of O2− (p < 0.01), as the levels of O2− increased so did the levels of NO (Fig. 5D).
Figure 5.
Scatterplot showing the significant relationship between levels of NOX activity and O2.− in the ipsilateral hippocampus following a moderate TBI (A). As the NOX levels increased they were paralleled by an increase in O2.−. NOX levels also significantly correlated with changes in the levels of NO (C). Levels of NO in the hippocampus following the moderate TBI significantly associated with increased O2.−. The analysis failed to demonstrate a significant correlation between p-eNOS levels and NO following the injury (B). Lines are shown to represent the direction of the correlation.
Discussion
This study evaluated very early changes in NOX in the hippocampus following TBI. As part of this evaluation, changes in the levels of several NOX subunits were quantified in both the cytosol and cell membrane fractions extracted from the hippocampus. Recent studies have shown significant NOX activation occurs between 48-72 h after a diffuse brain injury [49] and remains active even at 28 days after a focal brain injury [41]. In the present study, we focused on the first 96 hours after a defined cortical contusion. Previous studies from the laboratory have characterized the overall oxidative stress response in both the cortex and hippocampus [10-12,15]. Multiple different markers demonstrated the fact that the response following brain injury peaks between 12 and 24 h, but it is unclear what the source is of that oxidative stress. Here we demonstrate that NOX may be a major contributor to overall oxidative stress and is significantly elevated at 6 h post trauma similar to other markers [11]. NOX up-regulation remains for an extended period of time post injury, thus contributing to SIC and the spreading of neuronal injury. NOX is dedicated to the specific and deliberate production of O2.− [46,47,54,55,57]. Increased O2.− acts as a key component in oxidative stress and neuroinflammation [14,26,28,32] mediated SIC, that contributes to neuronal loss [11], neuronal circuitry failure [72,73] and eventually behavioral problems after TBI [23,49,74-76].
In our study, both NOX activity and its derived O2.− increased in a time dependent fashion, with the maximum values at 24 h. NOX up-regulation and O2.− production occurred early after injury and remains elevated for an extended period of time after head trauma. A recent study reported NOX activation and O2.− production maximal (peak value) at 1 h after injury. With the minimum values at 6h, both NOX activity and O2.− were significantly elevated at 96 h as compared to sham control [25]. Reasons for these differences may reflect the fact that a different animal (mice) model was used. It is also possible that the detection procedure may be lacking inhibitors for other sources of O2.− and performed by estimation of florescence of hydroethidine (redox-reaction) [64] administered intravenously before injury [25]. Such an early peak (at 1 h) of O2− is most probably associated with mitochondrial dysfunction that is known to occur within 30 min after TBI [77].
The present study showed a strong correlation in cytosolic NOX subunit translocation and O2− production. Activation of NOX results from the translocation and assembly of the cytosolic proteins (p67Phox, p47Phox, and p-40Phox) into membrane subunits. Increases in the membrane fraction did not exactly mirror the declines in the cytosolic fraction. For example, at 48 h, while cytosolic levels of subunits remained significantly low, levels in the membrane fraction began to decline. Such a difference may illuminate possible degradation of cytosolic proteins. It is well known that the activation of NOX catalyzes O2− production [51-53], and consumes the secondary energy molecules NADPH. Increased NOX derived O2− in endothelial and smooth muscle cells contribute to a compromised blood brain barrier resulting in impaired cerebral blood flow [37,78-80]. Mechanistically, NOX activation is associated with increased intracellular Ca2+ [46,47], protein kinase C (PKC) functions [81-83] and participates in the cell signaling processes. NOX derived O2.− contributes to the expression of matrix metalloproteinase-9 (MMP-9) and the activation of extracellular signal-regulated kinase (ERK) [81,82], c-Jun-N-terminal kinase (JNK) and nuclear factor kappa-B (NF-kB) [82-84]. Consequently, NOX up-regulation has been implicated in microglia activation/proliferation [21,63,85], inflammation [39,40,86,87] and mediated apoptotic neuronal death [44,45,88]. Activated microglia produces both O2.− and NO, as a mechanistic tool in neurodegenerative processes [26,32,34,39]. Up-regulation of NOX showed a positive significant correlation with increased levels of NO. Our study is the first to show a significant association between the increase in NO and O2.− in the hippocampus following TBI.
In the present set of experiments we found a time-dependent activation, phosphorylation of eNOS (p-eNOS). This activation occurred very early (1 h) and remained significantly higher than uninjured animals up to 96 h while significant elevation in the total NO was observed at 24 h after TBI. It was surprising that p-eNOS did not strongly associated with the total NO levels supporting the idea that p-eNOS is not driving the increase in NO. These result suggest that perhaps either 1) early after injury eNOS derived NO have been consumed in the various biological processes [67-69] or 2) a significant elevation in total NO occurred after the expression/activation of iNOS and nNOS [66]. Oxygen radicals can also become a controlling factor for NO, such as, O2.− can favor NO production [89] and also control their bioavailability [90]. Several studies have shown that NO and its metabolites contribute to pathology [34-36,91,92] and can be considered as a biomarker in severe-TBI [91]. Endothelial NOS derived NO participates in various physiological functions [67-69] including regulation of blood flow [67,90], arterial pressure [93], and signal transduction [94]. Similar to NOX, eNOS is also activated with the increased intracellular Ca2+ and NO function involves the activation of ERK [64,68], NF-kB [40,84] and protein kinase B (Akt) [68,70,84]. In the reaction of O2.−, NO generates various other ROS/RNS that may cause protein damages [2,11], i.e., protein’s nitration [2,3], hence, play in SIC after TBI.
Conclusions
This study revealed an early, time dependent, up-regulation of NOX and p-eNOS after TBI. These enzymes increase O2.− and NO production, and play an important role in SIC. The weak association between p-eNOS and total NO suggests that targeting eNOS activation after TBI may not be an effective therapy. A strong association between the up-regulation of NOX and level of O2− suggests that the NOX is a major source of free radicals in the ipsilateral hippocampus. Targeting NOX function is recognized for therapeutic intervention following TBI. Our result suggests that treatment should begin early (within the first 12 hours) that may target translocation of cytosolic subunit into membrane for the modulation of NOX.
Figure 6.
Figure 7.
Acknowledgments
This research was supported by the NIH 5R21NS66117 and the Kentucky Spinal Cord Head Injury Research Trust # 12-16A.
Abbreviations
- Akt
protein kinase B
- Akt
protein kinase B
- BCIP/NBT
5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium
- CCI
controlled cortical impact
- EDTA
ethylenediaminetetraacetic acid
- ERK
extracellular signal-regulated kinase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GTPase
guanosine triphosphatase
- JNK
c-Jun-N-terminal kinase
- MMP-9
matrix metalloproteinase
- Na+/K+-APTase
sodium-potassium adenosine triphosphatase
- NF-kB
nuclear factor kappa-B
- PKC
protein kinase-C
- PMS
post mitochondrial supernatant
- SIC
secondary injury cascades
References
- [1].Langlois JA, Marr A, Mitchko J, Johnson RL. Tracking the silent epidemic and educating the public: CDC's traumatic brain injury-associated activities under the TBI Act of 1996 and the Children's Health Act of 2000. J Head Trauma Rehabil. 2005;20:196–204. doi: 10.1097/00001199-200505000-00003. [DOI] [PubMed] [Google Scholar]
- [2].Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- [3].Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- [4].Siesjo BK. Calcium-mediated processes in neuronal degeneration. Ann N Y Acad Sci. 1994;747:140–161. doi: 10.1111/j.1749-6632.1994.tb44406.x. [DOI] [PubMed] [Google Scholar]
- [5].Gilmer LK, Ansari MA, Roberts KN, Scheff SW. Age-related mitochondrial changes after traumatic brain injury. J Neurotrauma. 2010;27:939–950. doi: 10.1089/neu.2009.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gilmer LK, Ansari MA, Roberts KN, Scheff SW. Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mech Ageing Dev. 2010;131:133–143. doi: 10.1016/j.mad.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singh IN, Sullivan PG, Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. J Neurosci Res. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- [8].Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- [9].Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci U S A. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ansari MA, Roberts KN, Scheff S. Dose and time dependent neuroprotective effects of Pycnogenol following traumatic brain injury. J Neurotrauma. 2013;30:1542–1549. doi: 10.1089/neu.2013.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008;25:513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- [13].Holmin S, Schalling M, Hojeberg B, Nordqvist AC, Skeftruna AK, Mathiesen T. Delayed cytokine expression in rat brain following experimental contusion. J Neurosurg. 1997;86:493–504. doi: 10.3171/jns.1997.86.3.0493. [DOI] [PubMed] [Google Scholar]
- [14].Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- [15].Scheff SW, Ansari MA, Roberts KN. Neuroprotective effect of Pycnogenol(R) following traumatic brain injury. Exp Neurol. 2013;239:183–191. doi: 10.1016/j.expneurol.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shohami E, Bass R, Wallach D, Yamin A, Gallily R. Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab. 1996;16:378–384. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- [17].Shao C, Roberts KN, Markesbery WR, Scheff SW, Lovell MA. Oxidative stress in head trauma in aging. Free Radic Biol Med. 2006;41:77–85. doi: 10.1016/j.freeradbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- [18].Ozdemir D, Uysal N, Gonenc S, Acikgoz O, Sonmez A, Topcu A, Ozdemir N, Duman M, Semin I, Ozkan H. Effect of melatonin on brain oxidative damage induced by traumatic brain injury in immature rats. Physiol Res. 2005;54:631–637. [PubMed] [Google Scholar]
- [19].Ansari MA, Ahmad AS, Ahmad M, Salim S, Yousuf S, Ishrat T, Islam F. Selenium protects cerebral ischemia in rat brain mitochondria. Biol Trace Elem Res. 2004;101:73–86. doi: 10.1385/BTER:101:1:73. [DOI] [PubMed] [Google Scholar]
- [20].Gahm C, Holmin S, Mathiesen T. Temporal profiles and cellular sources of three nitric oxide synthase isoforms in the brain after experimental contusion. Neurosurgery. 2000;46:169–177. [PubMed] [Google Scholar]
- [21].Jafarian-Tehrani M, Louin G, Royo NC, Besson VC, Bohme GA, Plotkine M, Marchand-Verrecchia C. 1400W, a potent selective inducible NOS inhibitor, improves histopathological outcome following traumatic brain injury in rats. Nitric Oxide. 2005;12:61–69. doi: 10.1016/j.niox.2004.12.001. [DOI] [PubMed] [Google Scholar]
- [22].Choi BY, Jang BG, Kim JH, Lee BE, Sohn M, Song HK, Suh SW. Prevention of traumatic brain injury-induced neuronal death by inhibition of NADPH oxidase activation. Brain Res. 2012;1481:49–58. doi: 10.1016/j.brainres.2012.08.032. [DOI] [PubMed] [Google Scholar]
- [23].Ferreira AP, Rodrigues FS, Della-Pace ID, Mota BC, Oliveira SM, Velho Gewehr CD, Bobinski F, de Oliveira CV, Brum JS, Oliveira MS, Furian AF, de Barros CS, Ferreira J, Santos AR, Fighera MR, Royes LF. The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: Role of inflammatory and oxidative brain damage. Neurochem Int. 2013;63:583–593. doi: 10.1016/j.neuint.2013.09.012. [DOI] [PubMed] [Google Scholar]
- [24].Loane DJ, Stoica BA, Byrnes KR, Jeong W, Faden AI. Activation of mGluR5 and inhibition of NADPH oxidase improves functional recovery after traumatic brain injury. J Neurotrauma. 2013;30:403–412. doi: 10.1089/neu.2012.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang QG, Laird MD, Han D, Nguyen K, Scott E, Dong Y, Dhandapani KM, Brann DW. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One. 2012;7:e34504. doi: 10.1371/journal.pone.0034504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- [27].Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7:354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- [29].Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu XX, Zhou HJ, Cai L, Zhang W, Ma JL, Tao XJ, Yu JN. NADPH oxidase-dependent formation of reactive oxygen species contributes to transforming growth factor beta1-induced epithelial-mesenchymal transition in rat peritoneal mesothelial cells, and the role of astragalus intervention. Chin J Integr Med. 2012 doi: 10.1007/s11655-012-1176-x. [DOI] [PubMed] [Google Scholar]
- [31].Zhou H, Zhang F, Chen SH, Zhang D, Wilson B, Hong JS, Gao HM. Rotenone activates phagocyte NADPH oxidase by binding to its membrane subunit gp91phox. Free Radic Biol Med. 2012;52:303–313. doi: 10.1016/j.freeradbiomed.2011.10.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [33].Rooker S, Jander S, Van Reempts J, Stoll G, Jorens PG, Borgers M, Verlooy J. Spatiotemporal pattern of neuroinflammation after impact-acceleration closed head injury in the rat. Mediators Inflamm. 2006;2006;90123 doi: 10.1155/MI/2006/90123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- [35].Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl 1):S96–102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- [36].Hortobagyi T, Gorlach C, Benyo Z, Lacza Z, Hortobagyi S, Wahl M, Harkany T. Inhibition of neuronal nitric oxide synthase-mediated activation of poly(ADP-ribose) polymerase in traumatic brain injury: neuroprotection by 3-aminobenzamide. Neuroscience. 2003;121:983–990. doi: 10.1016/s0306-4522(03)00482-2. [DOI] [PubMed] [Google Scholar]
- [37].Fukui T, Lassegue B, Kai H, Alexander RW, Griendling KK. Cytochrome b-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochim Biophys Acta. 1995;1231:215–219. doi: 10.1016/0005-2728(95)00098-4. [DOI] [PubMed] [Google Scholar]
- [38].Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 2711996:H1626–1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- [39].Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- [40].Chang CC, Wang YH, Chern CM, Liou KT, Hou YC, Peng YT, Shen YC. Prodigiosin inhibits gp91(phox) and iNOS expression to protect mice against the oxidative/nitrosative brain injury induced by hypoxia-ischemia. Toxicol Appl Pharmacol. 2011;257:137–147. doi: 10.1016/j.taap.2011.08.027. [DOI] [PubMed] [Google Scholar]
- [41].Cooney SJ, Bermudez-Sabogal SL, Byrnes KR. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J Neuroinflammation. 102013:155. doi: 10.1186/1742-2094-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hur J, Lee P, Kim MJ, Kim Y, Cho YW. Ischemia-activated microglia induces neuronal injury via activation of gp91phox NADPH oxidase. Biochem Biophys Res Commun. 2010;391:1526–1530. doi: 10.1016/j.bbrc.2009.12.114. [DOI] [PubMed] [Google Scholar]
- [43].Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J Neuropathol Exp Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim YH, Koh JY. The role of NADPH oxidase and neuronal nitric oxide synthase in zinc-induced poly(ADP-ribose) polymerase activation and cell death in cortical culture. Exp Neurol. 2002;177:407–418. doi: 10.1006/exnr.2002.7990. [DOI] [PubMed] [Google Scholar]
- [45].Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:RC53. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Choi SH, Lee DY, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005;25:4082–4090. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Song SX, Gao JL, Wang KJ, Li R, Tian YX, Wei JQ, Cui JZ. Attenuation of brain edema and spatial learning de fi cits by the inhibition of NADPH oxidase activity using apocynin following diffuse traumatic brain injury in rats. Mol Med Rep. 2013;7:327–331. doi: 10.3892/mmr.2012.1147. [DOI] [PubMed] [Google Scholar]
- [50].Ansari MA, Keller JN, Scheff SW. Protective effect of Pycnogenol in human neuroblastoma SH-SY5Y cells following acrolein-induced cytotoxicity. Free Radic Biol Med. 2008;45:1510–1519. doi: 10.1016/j.freeradbiomed.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- [52].Ozaki M, Deshpande SS, Angkeow P, Bellan J, Lowenstein CJ, Dinauer MC, Goldschmidt-Clermont PJ, Irani K. Inhibition of the Rac1 GTPase protects against nonlethal ischemia/reperfusion-induced necrosis and apoptosis in vivo. Faseb J. 2000;14:418–429. doi: 10.1096/fasebj.14.2.418. [DOI] [PubMed] [Google Scholar]
- [53].Reeves EP, Dekker LV, Forbes LV, Wientjes FB, Grogan A, Pappin DJ, Segal AW. Direct interaction between p47phox and protein kinase C: evidence for targeting of protein kinase C by p47phox in neutrophils. Biochem J. 1999;344:859–866. Pt 3. [PMC free article] [PubMed] [Google Scholar]
- [54].Cross AR, Segal AW. The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Anantharam V, Kaul S, Song C, Kanthasamy A, Kanthasamy AG. Pharmacological inhibition of neuronal NADPH oxidase protects against 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress and apoptosis in mesencephalic dopaminergic neuronal cells. Neurotoxicology. 2007;28:988–997. doi: 10.1016/j.neuro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ansari MA, Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med. 2011;51:171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].McCann SK, Dusting GJ, Roulston CL. Early increase of Nox4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. J Neurosci Res. 2008;86:2524–2534. doi: 10.1002/jnr.21700. [DOI] [PubMed] [Google Scholar]
- [58].Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- [59].Gao HM, Zhou H, Hong JS. NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol Sci. 2012;33:295–303. doi: 10.1016/j.tips.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim JH, Jang BG, Choi BY, Kim HS, Sohn M, Chung TN, Choi HC, Song HK, Suh SW. Post-treatment of an NADPH oxidase inhibitor prevents seizure-induced neuronal death. Brain Res. 2013;1499:163–172. doi: 10.1016/j.brainres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- [61].Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hall ED, Wang JA, Miller DM. Relationship of nitric oxide synthase induction to peroxynitrite-mediated oxidative damage during the first week after experimental traumatic brain injury. Exp Neurol. 2012;238:176–182. doi: 10.1016/j.expneurol.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wada K, Chatzipanteli K, Busto R, Dietrich WD. Role of nitric oxide in traumatic brain injury in the rat. J Neurosurg. 1998;89:807–818. doi: 10.3171/jns.1998.89.5.0807. [DOI] [PubMed] [Google Scholar]
- [64].Zhou YF, Li WT, Han HC, Gao DK, He XS, Li L, Song JN, Fei Z. Allicin protects rat cortical neurons against mechanical trauma injury by regulating nitric oxide synthase pathways. Brain Res Bull. 2014;100:14–21. doi: 10.1016/j.brainresbull.2013.10.013. [DOI] [PubMed] [Google Scholar]
- [65].Oddi S, Latini L, Viscomi MT, Bisicchia E, Molinari M, Maccarrone M. Distinct regulation of nNOS and iNOS by CB2 receptor in remote delayed neurodegeneration. J Mol Med (Berl) 2012;90:371–387. doi: 10.1007/s00109-011-0846-z. [DOI] [PubMed] [Google Scholar]
- [66].Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- [67].DeWitt DS, Smith TG, Deyo DJ, Miller KR, Uchida T, Prough DS. L-arginine and superoxide dismutase prevent or reverse cerebral hypoperfusion after fluid-percussion traumatic brain injury. J Neurotrauma. 1997;14:223–233. doi: 10.1089/neu.1997.14.223. [DOI] [PubMed] [Google Scholar]
- [68].Lee ST, Chu K, Sinn DI, Jung KH, Kim EH, Kim SJ, Kim JM, Ko SY, Kim M, Roh JK. Erythropoietin reduces perihematomal inflammation and cell death with eNOS and STAT3 activations in experimental intracerebral hemorrhage. J Neurochem. 2006;96:1728–1739. doi: 10.1111/j.1471-4159.2006.03697.x. [DOI] [PubMed] [Google Scholar]
- [69].Zhan X, Li D, Johns RA. Expression of endothelial nitric oxide synthase in ciliated epithelia of rats. J Histochem Cytochem. 2003;51:81–87. doi: 10.1177/002215540305100110. [DOI] [PubMed] [Google Scholar]
- [70].Yoshitomi H, Xu Q, Gao M, Yamori Y. Phosphorylated endothelial NOS Ser1177 via the PI3K/Akt pathway is depressed in the brain of stroke-prone spontaneously hypertensive rat. J Stroke Cerebrovasc Dis. 2011;20:406–412. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.014. [DOI] [PubMed] [Google Scholar]
- [71].Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002;306:79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- [72].Mathis DM, Furman JL, Norris CM. Preparation of acute hippocampal slices from rats and transgenic mice for the study of synaptic alterations during aging and amyloid pathology. J Vis Exp. 2011 doi: 10.3791/2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Norris CM, Scheff SW. Recovery of afferent function and synaptic strength in hippocampal CA1 following traumatic brain injury. J Neurotrauma. 2009;26:2269–2278. doi: 10.1089/neu.2009.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87:359–369. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- [75].Shapira M, Licht A, Milman A, Pick CG, Shohami E, Eldar-Finkelman H. Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci. 2007;34:571–577. doi: 10.1016/j.mcn.2006.12.006. [DOI] [PubMed] [Google Scholar]
- [76].Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- [78].Kuhlmann CR, Tamaki R, Gamerdinger M, Lessmann V, Behl C, Kempski OS, Luhmann HJ. Inhibition of the myosin light chain kinase prevents hypoxia-induced blood-brain barrier disruption. J Neurochem. 2007;102:501–507. doi: 10.1111/j.1471-4159.2007.04506.x. [DOI] [PubMed] [Google Scholar]
- [79].Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 2661994:H2568–2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- [80].Shin HK, Hong KW. Importance of calcitonin gene-related peptide, adenosine and reactive oxygen species in cerebral autoregulation under normal and diseased conditions. Clin Exp Pharmacol Physiol. 2004;31:1–7. doi: 10.1111/j.1440-1681.2004.03943.x. [DOI] [PubMed] [Google Scholar]
- [81].Hsieh HL, Lin CC, Shih RH, Hsiao LD, Yang CM. NADPH oxidase-mediated redox signal contributes to lipoteichoic acid-induced MMP-9 upregulation in brain astrocytes. J Neuroinflammation. 2012;9:110. doi: 10.1186/1742-2094-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hsieh HL, Wang HH, Wu CY, Yang CM. Reactive Oxygen Species-Dependent c-Fos/Activator Protein 1 Induction Upregulates Heme Oxygenase-1 Expression by Bradykinin in Brain Astrocytes. Antioxid Redox Signal. 2010;13:1829–1844. doi: 10.1089/ars.2009.2957. [DOI] [PubMed] [Google Scholar]
- [83].Wu DM, Lu J, Zheng YL, Zhang YQ, Hu B, Cheng W, Zhang ZF, Li MQ. Small interfering RNA-mediated knockdown of protein kinase C zeta attenuates domoic acid-induced cognitive deficits in mice. Toxicol Sci. 2012;128:209–222. doi: 10.1093/toxsci/kfs124. [DOI] [PubMed] [Google Scholar]
- [84].Chern CM, Liou KT, Wang YH, Liao JF, Yen JC, Shen YC. Andrographolide inhibits PI3K/AKT-dependent NOX2 and iNOS expression protecting mice against hypoxia/ischemia-induced oxidative brain injury. Planta Med. 2011;77:1669–1679. doi: 10.1055/s-0030-1271019. [DOI] [PubMed] [Google Scholar]
- [85].Grzybicki D, Moore SA, Schelper R, Glabinski AR, Ransohoff RM, Murphy S. Expression of monocyte chemoattractant protein (MCP-1) and nitric oxide synthase-2 following cerebral trauma. Acta Neuropathol. 1998;95:98–103. doi: 10.1007/s004010050770. [DOI] [PubMed] [Google Scholar]
- [86].Egea J, Martin-de-Saavedra MD, Parada E, Romero A, Del Barrio L, Rosa AO, Garcia AG, Lopez MG. Galantamine elicits neuroprotection by inhibiting iNOS, NADPH oxidase and ROS in hippocampal slices stressed with anoxia/reoxygenation. Neuropharmacology. 2012;62:1082–1090. doi: 10.1016/j.neuropharm.2011.10.022. [DOI] [PubMed] [Google Scholar]
- [87].Jin R, Song Z, Yu S, Piazza A, Nanda A, Penninger JM, Granger DN, Li G. Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke. 2011;42:2033–2044. doi: 10.1161/STROKEAHA.110.601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hwang JJ, Choi SY, Koh JY. The role of NADPH oxidase, neuronal nitric oxide synthase and poly(ADP ribose) polymerase in oxidative neuronal death induced in cortical cultures by brain-derived neurotrophic factor and neurotrophin-4/5. J Neurochem. 2002;82:894–902. doi: 10.1046/j.1471-4159.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- [89].Kim SM, Byun JS, Jung YD, Kang IC, Choi SY, Lee KY. The effects of oxygen radicals on the activity of nitric oxide synthase and guanylate cyclase. Exp Mol Med. 1998;30:221–226. doi: 10.1038/emm.1998.32. [DOI] [PubMed] [Google Scholar]
- [90].Fabian RH, Perez-Polo JR, Kent TA. Perivascular nitric oxide and superoxide in neonatal cerebral hypoxia-ischemia. Am J Physiol Heart Circ Physiol. 2008;295:H1809–1814. doi: 10.1152/ajpheart.00301.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kandasamy R, Kanti Pal H, Swamy M, Abdullah J. Cerebrospinal fluid nitric oxide metabolite levels as a biomarker in severe traumatic brain injury. Int J Neurosci. 2013;123:385–391. doi: 10.3109/00207454.2012.761983. [DOI] [PubMed] [Google Scholar]
- [92].Stoffel M, Rinecker M, Plesnila N, Eriskat J, Baethmann A. Role of nitric oxide in the secondary expansion of a cortical brain lesion from cold injury. J Neurotrauma. 2001;18:425–434. doi: 10.1089/089771501750171010. [DOI] [PubMed] [Google Scholar]
- [93].Campese VM, Sindhu RK, Ye S, Bai Y, Vaziri ND, Jabbari B. Regional expression of NO synthase, NAD(P)H oxidase and superoxide dismutase in the rat brain. Brain Res. 2007;1134:27–32. doi: 10.1016/j.brainres.2006.11.067. [DOI] [PubMed] [Google Scholar]
- [94].Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]