Abstract
Mild cognitive impairment (MCI) is considered to be an early stage in the progression of Alzheimer’s disease (AD) providing an opportunity to investigate brain pathogenesis prior to the onset of dementia. Neuroimaging studies have identified the posterior cingulate gyrus (PostC) as a cortical region affected early in the onset of AD. This association cortex is involved in a variety of different cognitive tasks and is intimately connected with the hippocampal/entorhinal cortex region, a component of the medial temporal memory circuit that displays early AD pathology. We quantified the total number of synapses in lamina 3 of the PostC using unbiased stereology coupled with electron microscopy from short postmortem autopsy tissue harvested from cases at different stage of AD progression. Individuals in the early stages of AD showed a significant decline in synaptic numbers compared to individuals with no cognitive impairment (NCI). Subjects with MCI exhibited synaptic numbers that were between the AD and NCI cohorts. Adjacent tissue was evaluated for changes in both pre and postsynaptic proteins levels. Individuals with MCI demonstrated a significant loss in presynaptic markers synapsin-1 and synaptophysin and postsynaptic markers PSD-95 and SAP-97. Levels of [3H]PiB binding was significantly increased in MCI and AD and correlated strongly with levels of synaptic proteins. All synaptic markers showed a significant association with mini mental status examination scores. These results support the idea that the PostC synaptic function is affected during the prodromal stage of the disease and may underlie some of the early clinical sequela associated with AD.
Keywords: Alzheimer’s disease, dementia, synapses, memory, mild cognitive impairment
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurological degenerative disease that is commonly seen in older individuals, although aging may not necessarily be a risk factor [1]. Clinically it affects recent memory, verbal fluency, and executive functions. As the disease progresses there are changes in emotions, increased psychotic symptoms, depression, and personality changes. It afflicts over 5 million individuals in the United States and more than 25 million world wide and growing. Early brain structural damage occurs in the hippocampus and interconnected cortical areas, which play a role in memory function.
The major pathological hallmarks of sporadic Alzheimer’s disease (AD) are intraneuronal neurofibrillary tangles (NFT) composed of hyperphosphorylated and misfolded tau, and extracellular deposits of aggregated amyloid beta (Aβ) peptide surrounding a dense core termed a neuritic amyloid plaque (NP). It is both the accumulation and distribution of these histopathological hallmarks that confirms the clinical diagnosis of AD [2–4]. Although the precise cause of AD remains an enigma, dysfunctional amyloid beta (Aβ) metabolism [5–7] and hyperphosphorylated tau [8] have been proposed as the major factors driving the disease. Accumulation of amyloid plaques does not correlate strongly with changes in cognition in AD. On the other hand, change in cognition associates better with the accumulation of NFTs [4]. It is becoming more evident that the loss of synapses and synaptic function is a prominent feature of AD that synaptic loss in the cortex and hippocampus is an early event that associates strongly with cognitive dysfunction (see reviews [9, 10]), although synaptic loss is not unique to this type of dementia [11].
Several studies have reported neuronal and synaptic changes in individuals with mild cognitive impairment (MCI), a prodromal stage of AD [12, 13]. MCI is defined as a slow developing cognitive impairment that can be demonstrated on neuropsychological testing but not severe enough to interfere with activities of daily living and does not meet the criteria for dementia [14]. The most common form of MCI is amnestic in which individuals present with a memory deficit, although some cases also have impairments in other domains [15]. The criteria are strictly clinical and are often subjective although heavily influenced by neuropsychological test scores. These individuals have an increased risk for progression to AD [16].
Most neuropsychiatric diseases are the result of a multilevel neural dysfunction. The cingulate cortex, because of its varied structural architectonics and behavioral functions as well as its extensive connectivity with multiple different cortical areas involved in diverse behaviors, has been implicated in many brain diseases. Clinicians have recently begun to rely upon structural and functional imaging to help determine whether or not an individual has AD. Positron emission tomography (PET) using [11C]PiB (Pittsburgh compound B), which binds to amyloid, have identified several regions of the cortex that may be among the earliest involved in the disease process including the precuneus and posterior cingulate (PostC) regions of the medial parietal cortex [17–33]. A recent functional MRI (fMRI) study also supports the involvement of the PostC very early progression of the disease [34]. The PostC has been shown to have decreased perfusion in patients that convert to AD from MCI [35]. Yoshida et al reported [36] that regional cerebral blood flow (rCBF) and regional cerebral protein synthesis (rCPS) was significantly decreased in the PostC but not in the frontal, parietal or mid temporal regions of the cortex in the early stages of AD. This is consistent with earlier studies showing significantly reduced rCBF in the PostC [25]. Together these studies point to early involvement of the PostC in AD. Therefore, the present study was designed to test the hypothesis that there is a significant change in the synapses and/or synaptic protein markers in the PostC as a function of the progression of AD and that these changes associate with cognitive test scores.
MATERIALS AND METHODS
Postmortem human brains
Tissue was examined from 48 individuals (mean age 86.5 ± 5.9 years; range 67 to 97 years; table 1) who were participants in either the University of Kentucky Alzheimer’s Disease Center (UKADC) [37], or the Rush Religious Orders Study (RROS), a longitudinal clinical-pathologic study of aging and Alzheimer’s disease composed of older Catholic nuns, priests, and brothers [38–40]. The Human Investigations Committee of the University of Kentucky College of Medicine and the Rush University Medical Center approved the studies. Individuals included in these studies agreed to annual clinical evaluation and brain donation at the time of death. For all subjects, cognitive test scores were available within the last year of life; the average interval from last evaluation to time of death was 9.4 ± 5.2 months, with no differences among the three diagnostic groups (p > 0.1). Subjects were categorized as no cognitive impairment (NCI; n = 20) or MCI (n = 12), based on cognitive testing prior to death using previously reported criteria for MCI [38, 41], or AD (n = 16) based upon cognitive testing and postmortem neuropathological examination. The NCI subjects were without a history of dementia or other neurological disorders. Standard criteria for exclusion included the presence of 1) significant cerebral stroke regardless of ante mortem date, 2) large cortical infarcts identified in the postmortem neuropathologic evaluation, 3) significant trauma within 12 months before autopsy, 4) individuals on a respirator longer than 12 hours before death, 5) individuals in coma longer than 12 hours immediately before death, 6) individuals currently undergoing radiation therapy for CNS tumor, and 7) individuals with Lewy bodies in the area of interest.
Table 1.
Characteristics of no cognitive impairment (NCI), mild cognitive impairment (MCI), and Alzheimer’s disease (AD) subjects
| Subjects | Age (y) | Gender | Brain wt (g) | Education (y) | MMSE | NIA/Reagan | Braak | PMI (h) |
|---|---|---|---|---|---|---|---|---|
| NCI | 77 | M | 1340 | 16 | 30 | I | IV | 2.6 |
| NCI | 78 | M | 1130 | 16 | 28 | No | II | 1.0 |
| NCI | 81 | M | 1390 | 16 | 29 | L | II | 4.0 |
| NCI | 83 | F | 1036 | 16 | 29 | I | IV | 4.0 |
| NCI | 85 | F | 1177 | 16 | 26 | L | III | 6.0 |
| NCI | 86 | F | 1200 | 18 | 28 | NO | 0 | 2.5 |
| NCI | 86 | F | 1130 | 14 | 26 | I | III | 3.3 |
| NCI | 87 | F | 1220 | 12 | 30 | H | V | 2.3 |
| NCI | 87 | M | 1473 | 14 | 30 | L | III | 6.0 |
| NCI | 87 | F | 1115 | 14 | 30 | I | III | 2.4 |
| NCi | 88 | M | 1290 | 20 | 30 | L | II | 2.1 |
| NCI | 88 | F | 1100 | 16 | 30 | NO | I | 2.5 |
| NCI | 88 | F | 1070 | 16 | 29 | L | III | 2.5 |
| NCI | 90 | F | 1045 | 17 | 30 | NO | II | 2.2 |
| NCI | 92 | F | 1220 | 16 | 30 | I | III | 3.3 |
| NCI | 93 | M | 1220 | 20 | 28 | L | 0 | 3.5 |
| NCI | 93 | F | 1210 | 20 | 30 | NO | II | 2.3 |
| NCI | 94 | F | 1090 | 16 | 30 | I | IV | 2.6 |
| NCI | 94 | F | 1000 | 13 | 29 | I | III | 4.5 |
| NCI | 96 | F | 1060 | 16 | 30 | NO | I | 2.0 |
|
| ||||||||
| Mean ± SD | 87.7 ± 5.2 | 1176 ± 125 | 16.1 ± 2.2 | 29.1 ± 1.3 | 3.0 ± 1.3 | |||
|
| ||||||||
| eMCI | 83 | F | 1110 | 16 | 28 | L | II | 7.0 |
| eMCI | 84 | F | 1050 | 16 | 28 | L | II | 4.0 |
| MCI | 84 | M | 1350 | 18 | 24 | I | IV | 3.0 |
| MCI | 85 | F | 1170 | 16 | 23 | L | I | 2.0 |
| MCI | 86 | M | 1210 | 16 | 28 | L | II | 3.8 |
| MCI | 87 | F | 1130 | 16 | 28 | I | III | 3.0 |
| MCI | 87 | M | 1290 | 16 | 24 | I | IV | 2.1 |
| MCI | 89 | M | 1330 | 20 | 26 | L | I | 3.0 |
| eMCI | 90 | F | 1010 | 18 | 30 | H | V | 6.3 |
| eMCI | 91 | M | 1308 | 25 | 23 | L | III | 5.0 |
| MCI | 93 | F | 1050 | 14 | 28 | L | II | 3.0 |
| MCI | 97 | F | 970 | 18 | 27 | NO | IV | 2.5 |
|
| ||||||||
| Mean ± SD | 88.0 ± 4.2 | 1164 ± 133 | 17.4 ± 2.8 | 26.4 ± 2.4* | 3.7 ± 1.6 | |||
|
| ||||||||
| AD | 67 | M | 1110 | 10 | 11 | H | VI | 2.0 |
| AD | 73 | M | 1130 | 16 | 16 | H | VI | 2.0 |
| AD | 79 | F | 1100 | 14 | 10 | H | VI | 2.9 |
| AD | 80 | F | 1130 | 12 | 14 | H | VI | 4.0 |
| AD | 80 | F | 1210 | 16 | 22 | H | VI | 3.3 |
| AD | 81 | M | 1280 | 12 | 22 | L | V | 6.0 |
| AD | 82 | F | 1200 | 14 | 26 | H | V | 5.0 |
| AD | 84 | F | 1050 | 16 | 13 | I | IV | 2.5 |
| AD | 85 | M | 1020 | 7 | 15 | H | VI | 2.8 |
| AD | 86 | M | 1150 | 16 | 10 | H | VI | 3.0 |
| AD | 89 | F | 1150 | 16 | 24 | H | V | 3.2 |
| AD | 90 | M | 1085 | 21 | 20 | H | V | 2.0 |
| AD | 90 | M | 1190 | 16 | 20 | H | VI | 4.0 |
| AD | 91 | M | 1210 | 13 | 22 | I | IV | 2.0 |
| AD | 92 | F | 1109 | 16 | 23 | I | III | 5.0 |
| AD | 92 | M | 1100 | 16 | 13 | L | III | 7.0 |
|
| ||||||||
| Mean ± SD | 83.8 ± 7.1 | 1139 ± 67 | 14.4 ± 3.2* | 17.6 ± 5.4* | 3.5 ± 1.5 | |||
NCI (no cognitive impairment); MCI (mild cognitive impairment); AD (Alzheimer’s disease); M (Male); F (Female); MMSE (Mini Mental State Examination); NIA/Reagan (National Institute of Aging/Reagan Institute); PMI (Post mortem interval); SD (standard deviation)
p < 0.05 vs NCI
Clinical Evaluations
Details of the RROS and UKADC have been published elsewhere [38, 42, 43]. All subjects have detailed mental status testing annually and have neurologic and physical examinations annually. Subjects were followed for 2 to 17 years (mean 8 ± 4 years). For the MCI cohort, 8 were amnestic without multi domain involvement and 4 were executive MCI. Tissue from questionable cases was not included in the study.
Morphological assessment
At autopsy, brain tissue was processed as previously described [40, 44]. Tissue from the PostC was evaluated for changes in both pre synaptic and postsynaptic proteins as well as soluble Aβ1-42 concentration and [3H]PiB binding. A subset of these cases (10/cognitive category) was also evaluated for ultrastructural assessment of total synaptic numbers in PostC cortical lamina 3.
Electron Microscopy
The procedure used for ultrastructural assessment of synapses was identical to that described previously [10, 45]. In brief, at the time of autopsy, the entire left PostC was removed in toto, defined as that portion of the medial aspect of the parietal lobe bordered by the cingulate sulcus superiorly, the posterior portion of the subparietal sulcus posteriorly, the inferiorly-directed side branch of the cingulate sulcus anteriorly, and the dorsal aspect of the corpus callosum inferiorly [46, 47]. Within the first 0.5 cm, a random starting point was chosen according to unbiased stereologic sampling methods [48] and the remaining entire gyrus was subsequently sectioned into 0.5 cm coronal slabs and immediately immersion fixed for 24 hours in 4% paraformaldehyde with 1% glutaraldehyde. Slabs were subsequently exhaustively sectioned at 100μm with a vibratome (Vibratome Co., St. Louis, MO), and a random number table used to identify sections for ultrastructural investigation. Designated sections were post fixed in 1% osmium tetroxide (OsO4), stained en bloc with 0.5% uranyl acetate, dehydrated in a graded series of ethanol, infiltrated with epoxy embedding resin, and flat embedded in circular molds (Ted Pella, Redding, CA).
A random number table was used to determine which portions of lamina 3 of the PostC to analyze resulting in a total of 15 to 24 different regions assessed depending on brain size. The physical disector method [49] was used to approximate the total number of synapses per unit volume (Nv). Electron micrographs were taken with a Zeiss EM-902 (Oberkochen, West Germany) at X4,400 and photographically enlarged to approximately X20,000. Every synaptic profile on each micrograph was identified by the presence of the postsynaptic density in association with the postsynaptic element and synaptic vesicles in a presynaptic terminal and marked by an investigator blind to case demographics (Figure 1). An unbiased counting frame was randomly superimposed over the micrographs. Only those synaptic profiles observed on the reference micrograph within the counting frame that did not violate the counting frame rules [48] and were not on the look-up micrograph were counted. To increase efficiency, the look-up and reference sections were reversed, and the counting frame was again applied in a random fashion. The thickness of the ultrathin sections was estimated with the Small method of minimal folds [50].
Figure 1.

Representative electron micrographs of lamina 3 of the posterior cingulate gyrus showing synaptic complexes in tissue from the three different cohorts studied: no cognitive impairment (NCI); mild cognitive impairment (MCI); Alzheimer’s disease (AD). In all tissues, the synaptic complexes appeared normal with synaptic vesicles observed in the presynaptic compartment and a synaptic density observed in the postsynaptic compartment. White arrows indicate a few of the synaptic complexes that were counted. Calibration bar = 0.5 μm.
The numerical density of synapses per unit volume, Nv, was calculated using the following formula: Nv = Q−/Vdis, where Q− is the mean number of synapses counted in each disector and Vdis is the mean disector volume. The total number of synapses, Nsyn, was calculated for each case using the following formula Nsyn = Nv • Vref.
Estimation of total volume of lamina 3
As part of the procedure for estimating the total number of synapses using the physical dissector, it is necessary to estimate the total reference volume (Vref) of the region of interest. This was accomplished as previously described [51]. Briefly, 100 μm thick sections immediately adjacent to those used for ultrastructural analysis were designated for determination of Vref and processed using procedures identical to that for ultrastructural evaluation. These sections were infused with embedding resin, flat embedded on glass microscope slides held in rubber molds (Ted Pella, Redding, CA) and coverslipped using embedding resin as the mounting medium. Using imaging software (Scion Image, PC version of Macintosh-compatible NIH Image), interfaced with a light microscope (VANOX-S AH-2, Olympus Optical, Tokyo, Japan) and a CCD KP-M1A camera (Hitachi, Tokyo), lamina 3 of the PostC was determined on each section using well described anatomical landmarks [52]. The total volume was determined using the Cavalieri method [48].
Coefficient of error and coefficient of variance for ultrastructural studies
The procedures for calculating the coefficient of error (CE), representing the sampling variance, and the coefficient of variance (CV), representing the biologic variance, for estimating the total synaptic numbers were according to standard stereologic procedures [53]. The mean CE for all subjects in the current study was 0.05, representing the intrasubject variance, indicating the precision of the counting scheme. The mean CV among the three diagnostic groups was 0.15. This observed CV is a combination of the inherent biologic and intersubject variation. The ratio CE2/CV2 is 0.11, indicating that the precision of the estimate observed with this sampling scheme meets the criterion for optimal sampling [53].
Biochemical analysis
All tissue samples were frozen on dry ice or in liquid nitrogen at time of autopsy and stored at −80°C until used for analysis. Tissues were homogenized using an ultrasonic cell disruptor (Microson, Farmingdale, NY) in a lysis buffer containing 10mM HEPES, 137mM NaCl, 0.6 mM MgSO4, 4.6mM KCL, 1.1 mM KH2PO4, pepstatin A, leuppeptin, aprotinin, and phenylmethylsulfonyl fluoride. Samples were centrifuged at 1000g for 10 min/4°C to remove cell debris, and the collected supernatant was centrifuged at 15,000g for 10 min/4°C. Supernatants were used for the analyses. Total protein concentration was determined by the BCA method (Sigma, St. Louis, MO).
Assessment of synaptic proteins
Synaptic proteins were evaluated by Western blot as previously described [54, 55]. Supernatants were probed for possible changes in synapsin I (Millipore, Temecula, CA AB1543), synaptophysin (Millipore, Temecula, CA AB9272), postsynaptic density – 95 (Santa Cruz Biotech, CA sc-28941), synapse associate protein 1 (Santa Cruz Biotech sc-25661), and drebrin (Sigma, St. Louis, MO D3816). For the analysis, 50 μg of protein was loaded with the appropriate marker (β-actin) (Santa Cruz Biotech, Santa Cruz, CA sc-47778) on a gradient gel (4–20% Tris-HC), followed by transfer to polyvinylidene fluoride membrane using a semidry transfer system (Bio-Rad, Hercules, CA) in transfer buffer (25mM Tris, 150 mM glycine) at 15V for 2h. The membrane was blocked with 5% milk or BSA in Tris/saline buffer –Tween 20 (TBST). Primary antibodies were added and incubated overnight at 4°C. Blots were washed three times in TBST and incubated for 1h with alkaline phosphatase conjugated secondary antibodies. The membrane was washed three times in TBST for 5 min and developed in Sigma Fast tablets (BCIP/NBT substrate). Blots were dried, scanned with Adobe Photoshop, and quantified with Scion Image. Membranes were also incubated with an antibody for beta-actin as a loading control and synaptic proteins levels were normalized to beta-actin levels prior to statistical analysis.
Measurement of soluble Aβ42 and [3H]PiB binding assay
Measurements for both soluble Aβ and [3H]PiB binding were identical to that used previously [56]. Briefly, soluble amyloid-β1-42 (Aβ42) peptide concentration was quantified in diethylamine (DEA)-soluble Aβ fractions, assayed using a fluorescent-based ELISA (Biosource, Camarillo, CA) with a capture antibody specific for the NH2 terminus of human Aβ (amino acids 1-16). Values for detection antibodies specific for the neoepitope at the 42-amino acid end of Aβ were determined from standard curves using synthetic Aβ42 peptide (Biosource) and expressed as picomoles per gram wet weight. For the [3H]PiB binding, unlabeled PiB was dissolved in dimethyl sulfoxide (DMSO) at 400 mM to yield < 1% DMSO in the final assay. [3H]PiB (American Radiolabled Chemicals, St. Louis, MO) was incubated with 100 mg tissue in 1 mL PBS. The binding mixture was filtered through a Whatman FG/B glass filter, rapidly washed with 3 mL PBS, vortexed overnight. Filters were counted in CytoScint ES, corrected for nonspecific, non-displaceable binding in the presence of 1 mM PiB and final values expressed as Pico moles of [3H]PiB bound per gram of wet tissue weight.
Statistical Analysis
The relationship between dependent variables and clinical diagnostic group was examined with an analysis of variance (ANOVA) using Statview 5.0 (SAS Institute, Cary, NC). If a significant ANOVA was found, a priori tests (Fischer’s PLSD) were used to identify pairs of diagnostic groups that differed significantly. When necessary due to a unpredicted significant ANOVA, post hoc testing employed the Newman-Keuls test. Because the diagnostic groups were heterogeneous in their clinical features, the relationship between dependent variables (e.g. total synaptic counts) and performance on neuropsychological tests at last clinical evaluation was examined using Spearman correlation. Level of significance was set at p < 0.05.
RESULTS
Demographics
Table 1 shows the subject characteristics grouped by consensus diagnosis. The three different groups did not differ significantly (p > 0.05) in gender, age, and postmortem interval (PMI), eliminating these variables as possible major contributors for observed differences. An ANOVA did reveal a difference in education [F(2,45) = 4.259, p < 0.05] with the AD group significantly lower than the MCI but not the NCI cohort. As expected there was a significant difference in the MMSE [F(2,45) = 52.848, p < 0.0001]. Post hoc analysis revealed that all three cohorts differed significantly from each other. Braak score distribution showed a greater percentage of individuals with scores in the IV–VI range in both the MCI (34%) and AD (88%) groups compared to the NCI (20%) cohort.
Total synaptic contacts: ultrastructure
The total synaptic counts for lamina 3 of the PostC are shown in figure 2. An ANOVA revealed a difference between the groups [F(2,27) = 3.856, p = 0.0337]. Comparisons showed that the AD group was significantly lower (24%) than the NCI cohort (p < 0.01) but not from the MCI group. The MCI cohort was a non- significant 17% lower than the NCI group (p > 0.05). An ANOVA showed a difference in group means [F(2,27) = 4.301; p = 0.0239] in the volume of lamina 3 of the PostC. Analysis revealed a significantly lower volume (21%) in the AD group (p < 0.005). There was an 8% decline in volume in the MCI group that was not significantly different from the NCI cohort (Figure 2). There was no significant association between total number of synapses in lamina 3 and the PMI (p > 0.1) or age at the time of death (p > 0.1). There was no significant association between the individual’s level of education and the total number of synapses in lamina 3.
Figure 2.
(A) Estimate of the total number of synapses in lamina 3 of the posterior cingulate cortex. Subjects were categorized clinically as no cognitive impairment (NCI), mild cognitive impairment (MCI), or Alzheimer’s disease (AD). Estimates were obtained using unbiased stereology coupled with electron microscopic imaging of synapses. (B) The total volume of lamina 3 of the posterior cingulate gyrus was estimated with the Cavalieri method directly from tissue sections immediately adjacent to regions used for synaptic counts. Single points represent individual subjects. Horizontal lines indicate group median. *p<0.05 compared to NCI.
Synaptic proteins
Five different synaptic proteins were evaluated for each of the subjects representing three different stages of the disease progression. Disease affected all five proteins examined (see Figure 3).
Figure 3.
Changes in synaptic protein levels in the posterior cingulate gyrus. Five different synaptic proteins (2 presynaptic and 3 postsynaptic) were analyzed by Western blot and beta actin was used as a loading control on the gels (A). Dot blots showing changes in different synaptic proteins within the posterior cingulate gyrus for each subject from the three different cohorts representing different stages in the progression of the disease: No cognitive impairment (NCI), mild cognitive impairment (MCI), Alzheimer’s disease (AD). Antibodies directed against both post-synaptic (B) Drebrin, (C) SAP-97, (D) PSD-95, and presynaptic (E) Synapsin I, (F) Synaptophysin showed a significant change as a function of the disease progression. Single points represent individual subjects. Horizontal lines indicate group median. **p<0.005 ***p<0.0001 compared to NCI. #p<0.05 compared to MCI.
Presynaptic
Synapsin-1 is a major protein in the presynaptic portion of the synapse. Analysis showed a significant difference between clinical groups [F(2,45) = 9.246, p < 0.0005]. Levels were significantly lower in both the MCI (p < 0.0005) and AD (p < 0.0001) compared to the NCI cases (Figure 3E). The MCI and AD groups were not significantly different. Very similar results were found when assessing levels of the synaptic vesicular protein, synaptophysin, [F(2,45) = 12.332, p < 0.0001] in which both MCI (p < 0.005) and AD (p < 0.0001) were significantly lower than the NCI group (Figure 3F).
Postsynaptic
SAP-97 is a membrane-associated phosphoprotein which participates in AMPA-type glutamate receptors. There was a significant change [F(2,45) = 4.872, p < 0.02] with the AD cohort demonstrating a significant decline (p < 0.005) compared to NCI (Figure 3). There was no significant difference between MCI and either AD or NCI. PSD-95 is a core scaffolding component of the postsynaptic element. Levels were significantly decreased [F(2,45) = 14.998, p < 0.0001] for both the MCI (p < 0.005) and AD (p < 0.0001) cohorts compared to the NCI group. The MCI and AD groups did not differ statistically. Drebrin, an actin binding synaptic scaffolding protein is found in dendritic spines. Quantitative analysis revealed a significant change [F(2,45) = 9.246, p < 0.0005] in the PostC with the AD cohort showing a significant decline compared to NCI (p < 0.0001) and MCI (p < 0.05). MCI and NCI did not differ statistically. There was no significant relationship between PMI and levels of various synaptic proteins (p > 0.05).
Pittsburgh compound B ([3H]PiB) binding and soluble amyloid-β1–42 concentration
An ANOVA revealed a significant group effect for [3H]PiB binding [F(2,45) = 11.588, p < 0.0001] and post hoc testing revealed a significant increase in AD compared to both MCI (p < 0.005) and NCI (p < 0.0001). The two latter groups were not significantly different from each other. There was a significant association between [3H]PiB binding and total synaptic numbers (r = .419; p < 0.05) and also between [3H]PiB binding and various synaptic proteins (Figure 4). The strongest association was with the presynaptic protein synaptophysin (r = .520; p < 0.0002). Analysis also revealed a significant correlation between [3H]PiB binding in the PostC and the individuals MMSE score (r = - .470; p < 0.005). The analysis of the sAβ failed to demonstrate a significant group effect [F(2,45) = 0.234, p > 0.1]. There were no significant associations between synaptic protein levels and levels of sAβ (p > 0.05). Analysis failed to reveal a significant correlation between sAβ and MMSE scores (r = −.165; p > 0.1).
Figure 4.
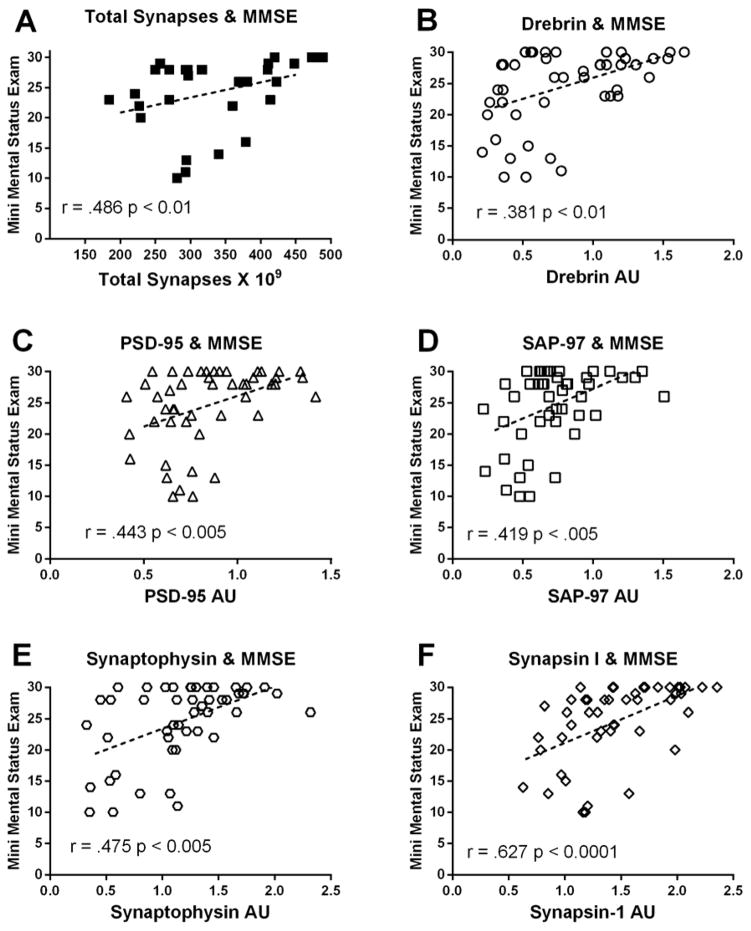
(A) Scatterplots showing the relationship between estimates of total number of synapses in lamina 3 (of the posterior cingulate gyrus and the subject’s Mini Mental Status Examination (MMSE) score. The same type of relationship is shown for both post- (B–D) and pre-synaptic (E, F) proteins. Single points represent individual subjects. Lines are shown to represent direction of the correlation. AU = arbitrary units
Neuropsychological cognitive testing and synaptic changes
We evaluated the possible relationship between total number of synapses in lamina 3 of the PostC and also the individual’s most recent MMSE score. As the total number of synapses decreased in lamina 3 there was a significant decline in the MMSE score (r = .486; p < 0.01) (Figure 5). There was also a significant association between the MMSE scores and the various pre and postsynaptic proteins (Figure 5). Statistical analysis failed to find any relationship between the individual’s level of education attained and the last MMSE (p > 0.1).
Figure 5.
(A) Scatterplots showing the relationship between estimates of the total number of synapses in lamina 3 of the posterior cingulate gyrus and the subject’s level of [3H]PiB binding in tissue immediately adjacent to that used for ultrastructural examination. Similar relationship is shown for both post- (B–D) and presynaptic (E, F) proteins. Single points represent individual subjects. Lines are shown to represent direction of the correlation. AU = arbitrary units
ApoE genotype and synaptic changes
Apolipoprotein E (APOE) genotype (ε2/2, ε2/3, ε3/3, ε3/4, ε4/4) was determined for each of the subjects and the changes in synaptic proteins in each group analyzed (Table 2). ANOVAs failed to reveal any statistically significant effect of differences in genotype and levels of synaptic proteins (p > 0.1). Changes in synaptic proteins were further grouped by whether or not the individual had any APOE ε4 allele. Unpaired t-tests failed to identify a significant difference between these two groups for any of the synaptic proteins analyzed. These results indicate that in the present study APOE genotype did not influence changes in synaptic proteins in the PostC.
Table 2.
Distribution of ApoE categories by diagnosis
| ApoE | NCI | MCI | AD |
|---|---|---|---|
| E2/2 | 0 | 1 (8%) | 0 |
| E2/3 | 3 (15%) | 0 | 1 (6%) |
| E2/4 | 0 | 0 | 1 (6%) |
| E3/3 | 14 (70%) | 6 (50%) | 6 (38%) |
| E3/4 | 2 (10%) | 5 (42%) | 6 (38%) |
| E4/4 | 1 (5%) | 0 | 2 (12%) |
NCI (no cognitive impairment); MCI (mild cognitive impairment); AD(Alzheimer’s disease)
Synaptic number and Braak stage
Total synapse number was analyzed by classifying subjects into three different Braak staging groups (0–II; III–IV; V–VI). ANOVA analysis failed to show a main effect for Braak staging [F (2,27) = 0.676; p > 0.1]. When this analysis was applied to the levels of the different synaptic proteins examined there were multiple statistically significant difference observed. Both drebrin and PSD-95 showed main effects for Braak grouping ([F(2,45) = 3.834; p < 0.03]; [F(2,45) = 5.329; p < 0.01] respectively). Post hoc testing showed that subjects in the V–VI group displayed significantly lower protein levels than the other two groups. A similar effect was observed for the presynaptic protein synapsin [F(2,45) = 4.184; p < 0.03] with the V–VI group significantly lower than the other two Braak categories. Synaptophysin and SAP-97 failed to show a main effect when grouped according the Braak staging (p > 0.05).
DISCUSSION
We previously reported significant synapse loss in both the anterior and posterior cingulate gyrus in advanced stages of AD [57]. The present set of studies is the first to investigate whether or not synaptic decline can be detected in the posterior cingulate region during the early stages of the disease. The present results support and extend our previous findings and shows synaptic change in the prodromal stage of the disease. A subset of the individuals in the three different stages of the disease was evaluated with quantitative ultrastructural techniques coupled with unbiased stereological sampling. Individuals in a relatively early stage of dementia demonstrated a 24% decline in synapses while the MCI cohort showed a statistically non-significant 17% decline compared to the group with no cognitive impairment. This is very similar to a prior study in the hippocampus that also showed a trend for synapse loss in the MCI group which did not reach statistical significance [51]. Individuals that are classified as amnestic MCI have noticeable recent memory complaints but have no obvious deficits associated with activities of daily living. Many of these individuals transition to AD, although the time course is variable and may occur over a number of years [58, 59]. There was some overlap in the estimation of total synaptic numbers between the three diagnostic groups with a subset of AD and MCI subjects in the range observed for the NCI group. This is similar to other ultrastructural studies evaluating synaptic numbers in the progression of the disease [45, 51, 60]. Accompanying the synaptic loss was a decline in cortical thickness, which supports previous studies demonstrating increased atrophy in this region in MCI and AD [17, 21, 24, 61–64].
Multiple studies have previously reported a decline in a wide variety of different synaptic proteins in mid to late stage AD in multiple regions of the cortex and hippocampus (see review by Honer [65]). Relatively few studies have evaluated possible synaptic protein changes in MCI. Counts and coworkers [66] evaluated both synaptophysin and drebrin levels in five different cortical regions in individuals who died with a clinical diagnosis of NCI, MCI and AD. Levels of the presynaptic protein synaptophysin did not decline in any of the regions in MCI subjects, although it did in the AD cases. Only the superior temporal cortex demonstrated a significant decline in the postsynaptic protein, drebrin, in the MCI cohort. In those same subjects, the superior frontal region of the MCI cohort demonstrated a significant increase in drebrin compared to the NCI group. Pham et al [67] evaluated the level of four different proteins in the frontal cortex in a small group of MCI subjects. Neither of the presynaptic markers (syntaxin or SNAP-25) demonstrated a significant decline when the MCI cohort was compared to the NCI group. However, both postsynaptic markers (VAMP2, PSD-95) showed significant declines in MCI. Two studies have evaluated possible MCI-related declines in synaptic proteins in the hippocampus. Sultana et al [68] reported a significant decline in PSD-95 in a very small cohort of MCI subjects and Counts et al [69] reported a significant loss of the postsynaptic protein drebrin but no change in either synaptophysin or synaptotagmin. An interesting study by Reddy et al [70] examined seven different synaptic proteins in both the frontal and parietal cortex in individuals with very low Braak scores and compared them to individual with no tangles. Since cognitive testing was not available for these subjects it is unclear if they were aMCI or very early AD. The cases with low Braak scores were classified as early AD and failed to show significant loss for many of the synaptic proteins when compared to the no tangle control group. Significant changes were observed for synaptotagmin and synaptopodin in the frontal cortex, and synaptophysin, neurogranin, and synaptopodin in the parietal region. Surprisingly they also found a significant increase in some of the synaptic proteins in the early AD cohort. The present results for the PostC provides further characterization of possible synaptic changes in MCI.
There clearly are regional differences in synaptic changes which may indicate areas of the cortex affected early in the disease process. The earlier reported loss in synaptic number in the cingulate in advanced AD was limited to upper lamina 3 in both anterior and posterior cingulate regions, with the lower lamina 5 only showing a loss in the posterior not the anterior cingulate [57]. The anterior and posterior cingulate have very different connections not only with the thalamus but also other cortical regions [71, 72] which probably accounted for this regional difference. The greatest changes in the PostC in the present investigation were observed in the presynaptic markers, synaptophysin and synapsin-1, in contrast to earlier MCI studies. For example, a previous study reported a significant decline in concentrations of several presynaptic proteins in multiple regions of the cortex in AD compared to NCI [73]. There was a significant decline in both the MCI and AD cohorts compared to the age-matched NCI cohort. Synaptophysin is an integral membrane protein of synaptic vesicles and has been used by many laboratories around the world as a marker to study the distribution of synapses in AD [65, 74], while synapsin-1 is a neuron specific phosphoprotein localized to the cytoplasmic side of small synaptic vesicles that plays an important role in the release of neurotransmitter [75, 76]. PSD-95 was the only postsynaptic marker in the PostC to show a significant decline in MCI, similar to the studies described above. PSD-95 provides essential scaffolding in the postsynaptic element that interacts with postsynaptic receptors and various ion channels [77]. The moderate AD group in the present study demonstrated a significant decline in both drebrin and SAP-97. Drebrin is a major actin-binding protein located in dendritic spines that plays a critical role in the morphology of spines and implicated in synaptic plasticity [78–81]. Dendritic spine pathology can have severe consequences in terms of retardation and dementia, especially in progressive neurodegenerative diseases such as AD [82]. SAP-97 is a postsynaptic protein found at postsynaptic densities (PSD). Unlike PSD-95, which is highly concentrated at the PSD, SAP-97 is more closely associated with the cytoplasm and plays a major role in regulating AMPA receptors [83–85].
Unlike other studies evaluating synaptic change in the progression of AD, values for each of the synaptic proteins and actual ultrastructural synaptic counts were evaluated as a function of the subject’s recent MMSE score. As shown in figure 4, each of the different synaptic proteins displayed a significant association with the cognitive testing. As the levels of synaptic proteins declined so did the MMSE scores. The greatest correlations were with both of the presynaptic proteins and the postsynaptic protein PSD-95. These results coincide with the group differences for the different proteins described above. Actual synaptic counts using the unbiased ultrastructural protocol also demonstrated a strong relationship with the cognitive testing results. Previous studies from our laboratory and others have documented a similar relationship between synaptic numbers/markers in several regions of the cortex and hippocampus and cognitive testing [10, 45, 60, 86–89].
Imaging studies have reported increased [C11]PiB PET retention binding patterns in several cortical regions, including the PostC [28, 29, 90], that display hypometabolism in AD. In the present study, [3H]PiB binding was elevated as a function of the disease progression. Hypometabolism in the PostC has been directly related to the severity of AD dementia [91, 92]. [3H]PiB binding demonstrated a statistically significant association with all of the synaptic marker proteins including the ultrastructural assessment of total synapses in lamina 3. As the [3H]PiB levels increased the level of various synaptic markers decreased. Our analysis also revealed a significant correlation between [3H]PiB binding and the individual’s cognitive test score. This coincides with previous findings in the precuneus [93, 94]. Recent studies have questioned the relationship of [C11]PiB PET retention in imaging studies with actual cognitive performance and as a marker for amyloid toxicity [95], although some regional localization may be indicative of very early or preclinical stages of the disease [96–102].
Numerous studies have focused on levels of both soluble and insoluble Aβ as possible mechanisms underlying both synapse loss and dementia in AD [7, 103]. While it is controversial as to whether or not increased oligomeric Aβ plays a major role in the onset of AD, studies indicate that its accumulation strongly associates with cognitive deficits associated with AD [104]. Still unanswered is the possibility that soluble Aβ in conjunction with other significant AD-related factors alter the activity of specific types of synapses thus interfering with higher level cognitive functioning such as memory [104–107]. Previous studies have shown that the cognitive deficits correlate strongly with the levels of oligomeric Aβ while levels of insoluble Aβ did not associate strongly with synaptic change [108]. Takahashi et al [109] demonstrated that Aβ42 localizes to multivesicular bodies in neurons, and there is strong evidence that Aβ oligomers bind to postsynaptic densities, which may explain in part some of the synapse loss in AD [110, 111]. The association between the levels of sAβ and synaptic markers, in the present set of studies, was not significant although it did significantly correlate with the [3H]PiB binding. This is somewhat puzzling and may relate to the fact that the [3H]PiB binding essentially marks only fibrillar amyloid and not soluble oligomeric Aβ. There are both soluble and insoluble forms of Aβ in the brain of individuals with AD [112].
Several questions that require further investigation is whether MCI is truly a transition point for synaptic change, has synaptic loss occurred prior to the first clinical expression of cognitive dysfunction (i.e. memory) and is MCI significantly different from other early stages of AD? Perhaps individuals with normal cognition and high AD-like pathology represent the earliest stage in the transition to MCI. In the current study, several NCI subjects could be considered preclinical AD based on Braak score and amyloid load (Table 1). Although there didn’t appear to be any significant differences between the low pathology and high pathology NCI groups in terms of synaptic markers, the number of cases in the high pathology group was low. Most likely the observed changes in synaptic protein levels seen in the MCI cohort is just one aspect of the multifactorial cascade that leads to early synaptic decline and cognitive dysfunction. Other important early factors that could impact synapse dysfunction include neuroinflammation [113–118], mitochondrial dysfunction [119–125], oxidative stress [126–130], cerebrovascular dysfunction [131–133], tau modification [8, 134–136], and epigenetics.
It is important to note that the ultrastructural assessment, while carried out with unbiased sampling, used a relatively small number of cases in each clinical cohort, which should be taken into consideration when generalizing the relationship with total synaptic counts and cognitive test results. The synaptic protein assessments were carried out on the full thickness of the cortex and were not limited to lamina 3. Further studies are needed to using larger cohorts to fully substantiate the current observations. Despite these caveats, evidence is accumulating that synaptic dysfunction plays a pivotal role in the onset of cognitive decline early in the progression of AD.
Acknowledgments
This work was supported by the National Institute of Health grants: AG028383, PO1AG014449, AG027219, AG042475, AG025204, AG043375 and the Mansbach Chair in Alzheimer’s Disease. We are indebted to the altruism of the participants in the Sanders-Brown Center on Aging and the Religious Orders Study (P30AG10161).
References
- 1.Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, Abner EL, Smith CD, Van Eldik LJ, Kryscio RJ, Scheff SW. Alzheimer’s disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta neuropathologica. 2011;121:571–587. doi: 10.1007/s00401-011-0826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, Alzheimer’s A National Institute on A. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta neuropathologica. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. Journal of neuropathology and experimental neurology. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 7.Krafft GA, Klein WL. ADDLs and the signaling web that leads to Alzheimer’s disease. Neuropharmacology. 2010;59:230–242. doi: 10.1016/j.neuropharm.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Maccioni RB, Farias G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Archives of medical research. 2010;41:226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Scheff SW, Price DA. Synaptic pathology in Alzheimer’s disease: a review of ultrastructural studies. Neurobiology of aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Scheff SW, Price DA. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. Journal of Alzheimer’s disease: JAD. 2006;9:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 11.Scheff SW, Neltner JH, Nelson PT. Is synaptic loss a unique hallmark of Alzheimer’s disease? Biochemical pharmacology. 2014;88:517–528. doi: 10.1016/j.bcp.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo-Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW. Mild cognitive impairment: pathology and mechanisms. Acta neuropathologica. 2012;123:13–30. doi: 10.1007/s00401-011-0884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheff SW, Ginsberg SD, Counts SE, Mufson EJ. Synaptic integrity in mild cognitive impairment and Alzheimer’s disesae. In: Sun M-K, editor. Research progress in Alzheimer’s disease and dementia. Nova Science Publishers; Hauppauge, NY: 2012. pp. 23–49. [Google Scholar]
- 14.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC. Mild cognitive impairment clinical trials. Nature reviews Drug discovery. 2003;2:646–653. doi: 10.1038/nrd1155. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 17.Barnes J, Godbolt AK, Frost C, Boyes RG, Jones BF, Scahill RI, Rossor MN, Fox NC. Atrophy rates of the cingulate gyrus and hippocampus in AD and FTLD. Neurobiology of aging. 2007;28:20–28. doi: 10.1016/j.neurobiolaging.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. NeuroImage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 19.Boxer AL, Rankin KP, Miller BL, Schuff N, Weiner M, Gorno-Tempini ML, Rosen HJ. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Archives of neurology. 2003;60:949–956. doi: 10.1001/archneur.60.7.949. [DOI] [PubMed] [Google Scholar]
- 20.Jones BF, Barnes J, Uylings HB, Fox NC, Frost C, Witter MP, Scheltens P. Differential regional atrophy of the cingulate gyrus in Alzheimer disease: a volumetric MRI study. Cerebral cortex. 2006;16:1701–1708. doi: 10.1093/cercor/bhj105. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann M, Rohrer JD, Clarkson MJ, Ridgway GR, Scahill RI, Modat M, Warren JD, Ourselin S, Barnes J, Rossor MN, Fox NC. Reduced cortical thickness in the posterior cingulate gyrus is characteristic of both typical and atypical Alzheimer’s disease. Journal of Alzheimer’s disease. 2010;20:587–598. doi: 10.3233/JAD-2010-1401. [DOI] [PubMed] [Google Scholar]
- 22.Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Annals of neurology. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 24.Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Annals of neurology. 2003;54:343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Annals of nuclear medicine. 2001;15:85–92. doi: 10.1007/BF02988596. [DOI] [PubMed] [Google Scholar]
- 26.Chetelat G, Villain N, Desgranges B, Eustache F, Baron JC. Posterior cingulate hypometabolism in early Alzheimer’s disease: what is the contribution of local atrophy versus disconnection? Brain: a journal of neurology. 2009;132:e133. doi: 10.1093/brain/awp253. author reply e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii K, Mori T, Hirono N, Mori E. Glucose metabolic dysfunction in subjects with a clinical dementia rating of 0.5. Journal of the neurological sciences. 2003;215:71–74. doi: 10.1016/s0022-510x(03)00206-5. [DOI] [PubMed] [Google Scholar]
- 28.Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, Rossor M, Brooks DJ. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 29.Herholz K, Carter SF, Jones M. Positron emission tomography imaging in dementia. The British journal of radiology. 2007;80(Spec No 2):S160–167. doi: 10.1259/bjr/97295129. [DOI] [PubMed] [Google Scholar]
- 30.Koivunen J, Scheinin N, Virta JR, Aalto S, Vahlberg T, Nagren K, Helin S, Parkkola R, Viitanen M, Rinne JO. Amyloid PET imaging in patients with mild cognitive impairment: a 2-year follow-up study. Neurology. 2011;76:1085–1090. doi: 10.1212/WNL.0b013e318212015e. [DOI] [PubMed] [Google Scholar]
- 31.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 32.Small GW, Siddarth P, Kepe V, Ercoli LM, Burggren AC, Bookheimer SY, Miller KJ, Kim J, Lavretsky H, Huang SC, Barrio JR. Prediction of cognitive decline by positron emission tomography of brain amyloid and tau. Archives of neurology. 2012;69:215–222. doi: 10.1001/archneurol.2011.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Targosz-Gajniak MG, Siuda JS, Wicher MM, Banasik TJ, Bujak MA, Augusciak-Duma AM, Opala G. Magnetic resonance spectroscopy as a predictor of conversion of mild cognitive impairment to dementia. Journal of the neurological sciences. 2013;335:58–63. doi: 10.1016/j.jns.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Rami L, Sala-Llonch R, Sole-Padulles C, Fortea J, Olives J, Llado A, Pena-Gomez C, Balasa M, Bosch B, Antonell A, Sanchez-Valle R, Bartres-Faz D, Molinuevo JL. Distinct functional activity of the precuneus and posterior cingulate cortex during encoding in the preclinical stage of Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2012;31:517–526. doi: 10.3233/JAD-2012-120223. [DOI] [PubMed] [Google Scholar]
- 35.Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida T, Kazui H, Tokunaga H, Kito Y, Kubo Y, Kimura N, Morihara T, Shimosegawa E, Hatazawa J, Takeda M. Protein synthesis in the posterior cingulate cortex in Alzheimer’s disease. Psychogeriatrics: the official journal of the Japanese Psychogeriatric Society. 2011;11:40–45. doi: 10.1111/j.1479-8301.2010.00350.x. [DOI] [PubMed] [Google Scholar]
- 37.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. Journal of neuropathology and experimental neurology. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 39.DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Annals of neurology. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 40.Mufson EJ, Ma SY, Cochran EJ, Bennett DA, Beckett LA, Jaffar S, Saragovi HU, Kordower JH. Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer’s disease. The Journal of comparative neurology. 2000;427:19–30. doi: 10.1002/1096-9861(20001106)427:1<19::aid-cne2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 41.Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Experimental neurology. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt FA, Nelson PT, Abner E, Scheff S, Jicha GA, Smith C, Cooper G, Mendiondo M, Danner DD, Van Eldik LJ, Caban-Holt A, Lovell MA, Kryscio RJ. University of Kentucky Sanders-Brown healthy brain aging volunteers: donor characteristics, procedures and neuropathology. Current Alzheimer research. 2012;9:724–733. doi: 10.2174/156720512801322591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Archives of neurology. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 45.Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 46.Ono M, Kubik S, Abernathey CD. Atlas of the cerebral sulci. Georg Thieme Verlag; New York: 1990. [Google Scholar]
- 47.Vogt BA, Hof PR, Vogt LJ. Cingulate gyrus. In: Paxionos G, Mai JK, editors. The Human Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 915–949. [Google Scholar]
- 48.Mouton PR. Principles and practices of unbiased stereology. The Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- 49.Gundersen HJG. Stereology of arbitrary particles. Journal of Microscopy. 1986;143:3–45. [PubMed] [Google Scholar]
- 50.Weibel E. Point counting method. In: Weibel ER, editor. Stereological Method. Academic Press; London: 1979. pp. 101–161. [Google Scholar]
- 51.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiology of aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Zilles K. Architecture of the human cerebral cortex. In: Paxionos G, Mai JK, editors. The human nervous system. Elsevier Academic Press; London: 2004. pp. 997–1055. [Google Scholar]
- 53.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. The Anatomical record. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 54.Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. Journal of neurotrauma. 2008;25:513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- 55.Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free radical biology & medicine. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain: a journal of neurology. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheff SW, Price DA. Alzheimer’s disease-related synapse loss in the cingulate cortex. Journal of Alzheimer’s disease: JAD. 2001;3:495–505. doi: 10.3233/jad-2001-3509. [DOI] [PubMed] [Google Scholar]
- 58.Amieva H, Le Goff M, Millet X, Orgogozo JM, Peres K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Annals of neurology. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 59.Johnson JK, Pa J, Boxer AL, Kramer JH, Freeman K, Yaffe K. Baseline predictors of clinical progression among patients with dysexecutive mild cognitive impairment. Dementia and geriatric cognitive disorders. 2010;30:344–351. doi: 10.1159/000318836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2011;24:547–557. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Callen DJ, Black SE, Gao F, Caldwell CB, Szalai JP. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57:1669–1674. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- 62.Choo IH, Lee DY, Oh JS, Lee JS, Lee DS, Song IC, Youn JC, Kim SG, Kim KW, Jhoo JH, Woo JI. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer’s disease. Neurobiology of aging. 2010;31:772–779. doi: 10.1016/j.neurobiolaging.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 63.Shima K, Matsunari I, Samuraki M, Chen WP, Yanase D, Noguchi-Shinohara M, Takeda N, Ono K, Yoshita M, Miyazaki Y, Matsuda H, Yamada M. Posterior cingulate atrophy and metabolic decline in early stage Alzheimer’s disease. Neurobiology of aging. 2012;33:2006–2017. doi: 10.1016/j.neurobiolaging.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Brun A, Englund E. Regional pattern of degeneration in Alzheimer’s disease: neuronal loss and histopatholgical grading. Histopathaology. 1981;5:549–564. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 65.Honer WG. Pathology of presynaptic proteins in Alzheimer’s disease: more than simple loss of terminals. Neurobiology of aging. 2003;24:1047–1062. doi: 10.1016/j.neurobiolaging.2003.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Pham E, Crews L, Ubhi K, Hansen L, Adame A, Cartier A, Salmon D, Galasko D, Michael S, Savas JN, Yates JR, Glabe C, Masliah E. Progressive accumulation of amyloid-beta oligomers in Alzheimer’s disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. The FEBS journal. 2010;277:3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sultana R, Banks WA, Butterfield DA. Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: Insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer’s disease. Journal of neuroscience research. 2010;88:469–477. doi: 10.1002/jnr.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Counts SE, He B, Nadeem M, Wuu J, Scheff SW, Mufson EJ. Hippocampal drebrin loss in mild cognitive impairment. Neuro-degenerative diseases. 2012;10:216–219. doi: 10.1159/000333122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. Journal of Alzheimer’s disease: JAD. 2005;7:103–117. doi: 10.3233/jad-2005-7203. discussion 173–180. [DOI] [PubMed] [Google Scholar]
- 71.Shibata H, Yukie M. Thalamocingulate connections in the monkey. In: Vogt BA, editor. Cingulate neurogiology and disease. Oxford Univeristy Press; New York: 2009. pp. 95–111. [Google Scholar]
- 72.Yukie M, Shibata H. Temporocingulate interactions in the monkey. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; New York: 2009. pp. 145–162. [Google Scholar]
- 73.Gabriel SM, Haroutunian V, Powchik P, Honer WG, Davidson M, Davies P, Davis KL. Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with schizophrenia. Archives of general psychiatry. 1997;54:559–566. doi: 10.1001/archpsyc.1997.01830180077010. [DOI] [PubMed] [Google Scholar]
- 74.Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- 75.Bahler M, Benfenati F, Valtorta F, Greengard P. The synapsins and the regulation of synaptic function. BioEssays: news and reviews in molecular, cellular and developmental biology. 1990;12:259–263. doi: 10.1002/bies.950120603. [DOI] [PubMed] [Google Scholar]
- 76.De Camilli P, Benfenati F, Valtorta F, Greengard P. The synapsins. Annual review of cell biology. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- 77.Kim E, Sheng M. PDZ domain proteins of synapses. Nature reviews Neuroscience. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 78.Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:7161–7170. doi: 10.1523/JNEUROSCI.16-22-07161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shirao T. The roles of microfilament-associated proteins, drebrins, in brain morphogenesis: a review. Journal of biochemistry. 1995;117:231–236. doi: 10.1093/jb/117.2.231. [DOI] [PubMed] [Google Scholar]
- 80.Sekino Y, Tanaka S, Hanamura K, Yamazaki H, Sasagawa Y, Xue Y, Hayashi K, Shirao T. Activation of N-methyl-D-aspartate receptor induces a shift of drebrin distribution: disappearance from dendritic spines and appearance in dendritic shafts. Molecular and cellular neurosciences. 2006;31:493–504. doi: 10.1016/j.mcn.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi H, Mizui T, Shirao T. Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurones. Journal of neurochemistry. 2006;97(Suppl 1):110–115. doi: 10.1111/j.1471-4159.2005.03536.x. [DOI] [PubMed] [Google Scholar]
- 82.Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- 83.Cai C, Coleman SK, Niemi K, Keinanen K. Selective binding of synapse-associated protein 97 to GluR-A alpha-amino-5-hydroxy-3-methyl-4-isoxazole propionate receptor subunit is determined by a novel sequence motif. The Journal of biological chemistry. 2002;277:31484–31490. doi: 10.1074/jbc.M204354200. [DOI] [PubMed] [Google Scholar]
- 84.Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 87.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Annals of neurology. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 88.Head E, Corrada MM, Kahle-Wrobleski K, Kim RC, Sarsoza F, Goodus M, Kawas CH. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiology of aging. 2009;30:1125–1134. doi: 10.1016/j.neurobiolaging.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sze CI, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. Selective regional loss of exocytotic presynaptic vesicle proteins in Alzheimer’s disease brains. Journal of the neurological sciences. 2000;175:81–90. doi: 10.1016/s0022-510x(00)00285-9. [DOI] [PubMed] [Google Scholar]
- 90.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, Schonknecht P, Ito K, Mielke R, Kalbe E, Zundorf G, Delbeuck X, Pelati O, Anchisi D, Fazio F, Kerrouche N, Desgranges B, Eustache F, Beuthien-Baumann B, Menzel C, Schroder J, Kato T, Arahata Y, Henze M, Heiss WD. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. NeuroImage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 92.Salmon E, Collette F, Degueldre C, Lemaire C, Franck G. Voxel-based analysis of confounding effects of age and dementia severity on cerebral metabolism in Alzheimer’s disease. Human brain mapping. 2000;10:39–48. doi: 10.1002/(SICI)1097-0193(200005)10:1<39::AID-HBM50>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ikonomovic MD, Klunk WE, Abrahamson EE, Wuu J, Mathis CA, Scheff SW, Mufson EJ, DeKosky ST. Precuneus amyloid burden is associated with reduced cholinergic activity in Alzheimer disease. Neurology. 2011;77:39–47. doi: 10.1212/WNL.0b013e3182231419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scheff SW, Price DA, Schmitt FA, Roberts KN, Ikonomovic MD, Mufson EJ. Synapse stability in the precuneus early in the progression of Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2013;35:599–609. doi: 10.3233/JAD-122353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perez SE, He B, Nadeem M, Wuu J, Scheff SW, Abrahamson EE, Ikonomovic MD, Mufson EJ. Resilience of Precuneus Neurotrophic Signaling Pathways Despite Amyloid Pathology in Prodromal Alzheimer’s Disease. Biological psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-beta associated cortical thinning in clinically normal elderly. Annals of neurology. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chetelat G, Desgranges B, Landeau B, Mezenge F, Poline JB, de la Sayette V, Viader F, Eustache F, Baron JC. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer’s disease. Brain: a journal of neurology. 2008;131:60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- 98.Chetelat G, Villemagne VL, Pike KE, Ellis KA, Ames D, Masters CL, Rowe CC Australian Imaging B, Lifestyle Study of Ageing Research G. Relationship between memory performance and beta-amyloid deposition at different stages of Alzheimer’s disease. Neuro-degenerative diseases. 2012;10:141–144. doi: 10.1159/000334295. [DOI] [PubMed] [Google Scholar]
- 99.Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain: a journal of neurology. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC Alzheimer’s Disease Neuroimaging I. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain: a journal of neurology. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ Alzheimer’s Disease Neuroimaging I. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain: a journal of neurology. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O’Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Annals of neurology. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nature reviews Molecular cell biology. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 104.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harbor perspectives in medicine. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer’s disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 106.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behavioural brain research. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer’s disease. Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. The American journal of pathology. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. The American journal of pathology. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Takahashi RH, Capetillo-Zarate E, Lin MT, Milner TA, Gouras GK. Co-occurrence of Alzheimer’s disease ss-amyloid and tau pathologies at synapses. Neurobiology of aging. 2010;31:1145–1152. doi: 10.1016/j.neurobiolaging.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiology of disease. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 113.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiology of aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain pathology. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nature medicine. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 116.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor perspectives in medicine. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2006;24:157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 118.McGeer EG, McGeer PL. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. Journal of Alzheimer’s disease: JAD. 2010;19:355–361. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- 119.Atamna H, Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. 2007;7:297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 120.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2006;9:167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 121.Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiology of aging. 2009;30:1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 122.Ly CV, Verstreken P. Mitochondria at the synapse. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2006;12:291–299. doi: 10.1177/1073858406287661. [DOI] [PubMed] [Google Scholar]
- 123.Mancuso M, Coppede F, Murri L, Siciliano G. Mitochondrial cascade hypothesis of Alzheimer’s disease: myth or reality? Antioxidants & redox signaling. 2007;9:1631–1646. doi: 10.1089/ars.2007.1761. [DOI] [PubMed] [Google Scholar]
- 124.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. Journal of Alzheimer’s disease: JAD. 2010;20(Suppl 2):S265–279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochimica et biophysica acta. 2013 doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ansari MA, Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free radical biology & medicine. 2011;51:171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR. Oxidative damage and progression to Alzheimer’s disease in patients with mild cognitive impairment. Journal of Alzheimer’s disease: JAD. 2010;21:1165–1177. doi: 10.3233/jad-2010-091723. [DOI] [PubMed] [Google Scholar]
- 129.Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW, Jr, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, Nakamura M, Nunomura A, Perry G. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. Journal of Alzheimer’s disease: JAD. 2010;19:363–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet neurology. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 131.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta neuropathologica. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutrition reviews. 2010;68(Suppl 2):S74–87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ostergaard L, Aamand R, Gutierrez-Jimenez E, Ho YC, Blicher JU, Madsen SM, Nagenthiraja K, Dalby RB, Drasbek KR, Moller A, Braendgaard H, Mouridsen K, Jespersen SN, Jensen MS, West MJ. The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiology of aging. 2013;34:1018–1031. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 134.Crimins JL, Pooler A, Polydoro M, Luebke JI, Spires-Jones TL. The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer’s disease. Ageing research reviews. 2013;12:757–763. doi: 10.1016/j.arr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pooler AM, Noble W, Hanger DP. A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology. 2014;76(Pt A):1–8. doi: 10.1016/j.neuropharm.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 136.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]