Abstract
The synthesis of full-length complementary DNA copies of avian myeloblastosis virus RNA is described. The cDNA is estimated by electrophoresis in agarose-methylmercury gels to have a molecular weight of 2.6 X 10(6), equivalent in size to that of the RNA template. Most (60-70%) of the reaction product is the full-length material and yields correspond to 60-70% of the input RNA.
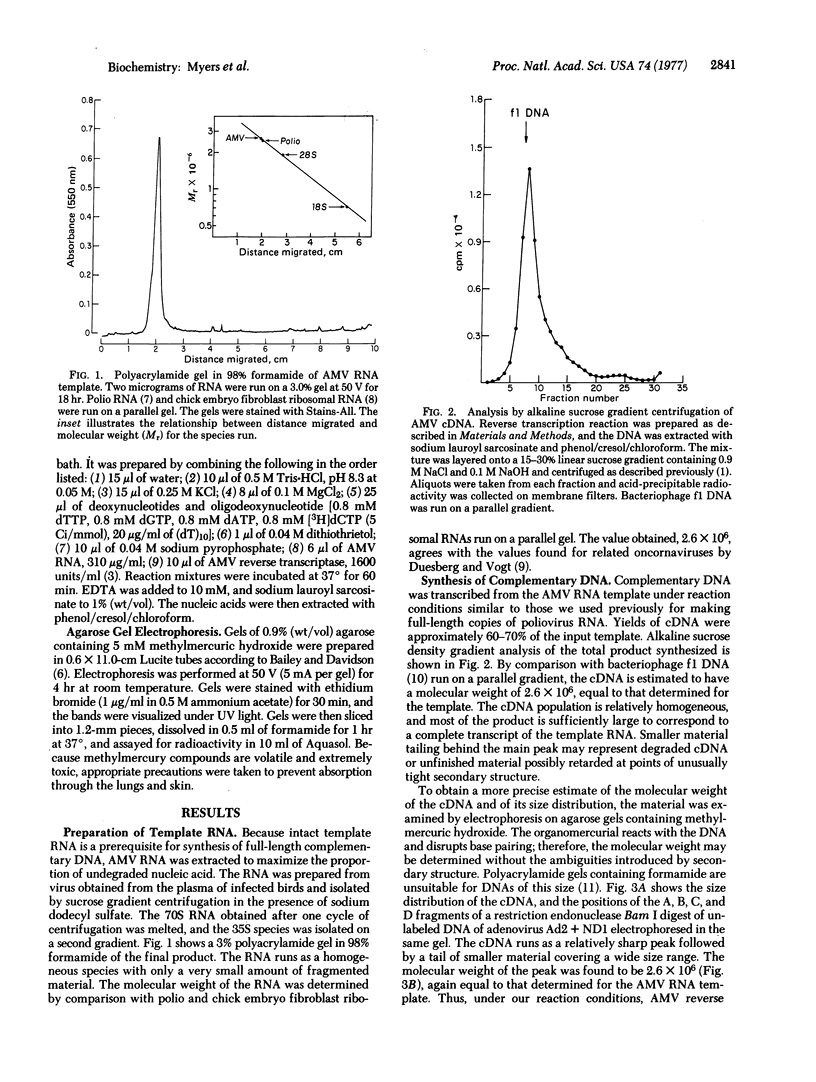
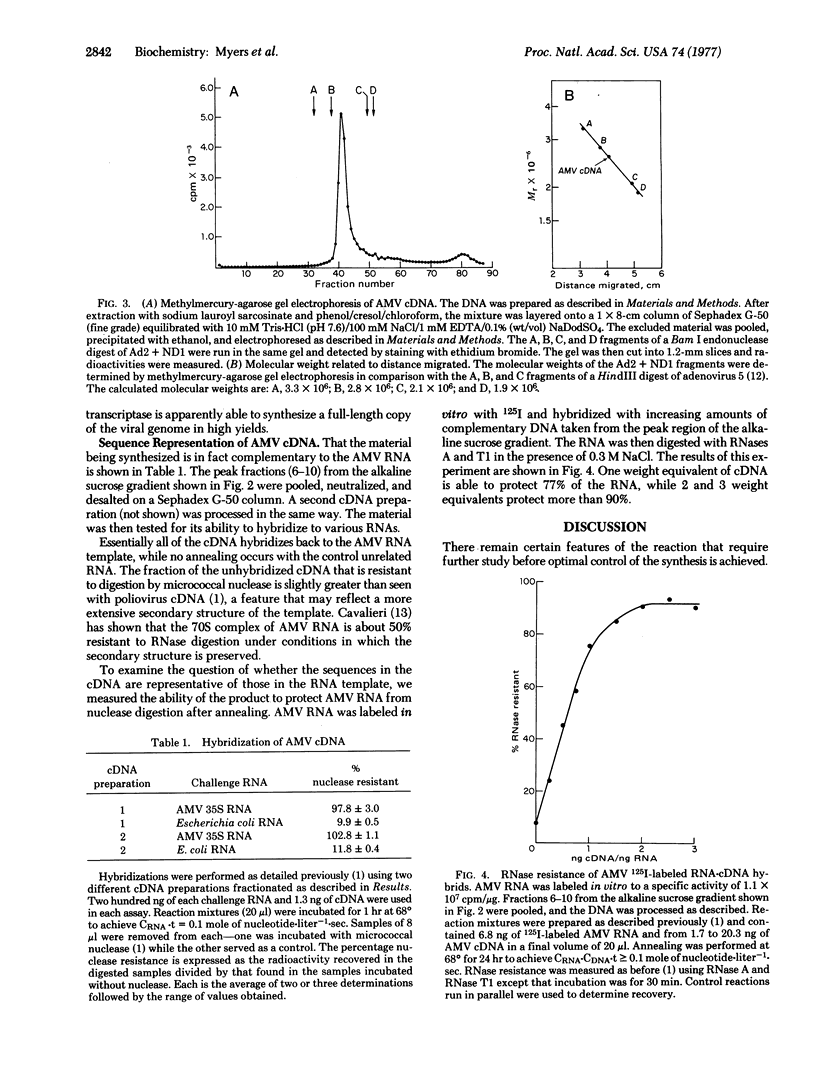
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Canaani E., Duesberg P., Dina D. Cleavage map of linear mouse sarcoma virus DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):29–33. doi: 10.1073/pnas.74.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri L. F. Extent of double strandedness in avian myeloblastosis virus RNA. J Virol. 1974 Dec;14(6):1458–1462. doi: 10.1128/jvi.14.6.1458-1462.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill P., Kabat D. Unexpectedly large size of globin messenger ribonucleic acid. Proc Natl Acad Sci U S A. 1971 Jan;68(1):72–75. doi: 10.1073/pnas.68.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granboulan N., Girard M. Molecular weight of poliovirus ribonucleic acid. J Virol. 1969 Oct;4(4):475–479. doi: 10.1128/jvi.4.4.475-479.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Vovis G. F., Zinder N. D. Effect of deoxyribonucleic acid length on the adenosine triphosphatase activity of Escherichia coli restriction endonuclease B. J Biol Chem. 1974 Jan 25;249(2):543–552. [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Site-specific cleavage of single-stranded DNA by a Hemophilus restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2555–2558. doi: 10.1073/pnas.72.7.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Anticomplementary nature of smaller DNA produced during synthesis of extensive DNA copies of poliovirus RNA. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3408–3412. doi: 10.1073/pnas.73.10.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. J., Ginsberg H. S. Persistence of type 5 adenovirus DNA in cells transformed by temperature-sensitive mutant, H5ts125. Proc Natl Acad Sci U S A. 1977 Feb;74(2):785–788. doi: 10.1073/pnas.74.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Pennings L., Schoenmakers J. G. Restriction-enzyme-cleavage maps of bacteriophage M13. Existence of an intergenic region on the M13 genome. Eur J Biochem. 1976 Sep;68(1):55–70. doi: 10.1111/j.1432-1033.1976.tb10764.x. [DOI] [PubMed] [Google Scholar]



