Abstract
The rapid identification of bacteria and fungi directly from the blood of patients with suspected bloodstream infections aids in diagnosis and guides treatment decisions. The development of an automated, rapid, and sensitive molecular technology capable of detecting the diverse agents of such infections at low titers has been challenging, due in part to the high background of genomic DNA in blood. PCR followed by electrospray ionization mass spectrometry (PCR/ESI-MS) allows for the rapid and accurate identification of microorganisms but with a sensitivity of about 50% compared to that of culture when using 1-ml whole-blood specimens. Here, we describe a new integrated specimen preparation technology that substantially improves the sensitivity of PCR/ESI-MS analysis. An efficient lysis method and automated DNA purification system were designed for processing 5 ml of whole blood. In addition, PCR amplification formulations were optimized to tolerate high levels of human DNA. An analysis of 331 specimens collected from patients with suspected bloodstream infections resulted in 35 PCR/ESI-MS-positive specimens (10.6%) compared to 18 positive by culture (5.4%). PCR/ESI-MS was 83% sensitive and 94% specific compared to culture. Replicate PCR/ESI-MS testing from a second aliquot of the PCR/ESI-MS-positive/culture-negative specimens corroborated the initial findings in most cases, resulting in increased sensitivity (91%) and specificity (99%) when confirmed detections were considered true positives. The integrated solution described here has the potential to provide rapid detection and identification of organisms responsible for bloodstream infections.
INTRODUCTION
Although molecular tests have been available for decades, there is no widely used molecular method to rapidly identify the organisms causing bloodstream infections and bacteremia directly from the blood. In the most serious cases. those of sepsis and septic shock the risk of mortality increases by the hour if appropriate antimicrobial therapy is delayed (1). The long delays associated with culture methods for the detection and identification of organisms in blood force physicians to empirically treat patients with multiple broad-spectrum antimicrobial agents rather than to wait for more specific microbiological data. This is not ideal because of the toxicities of broad-spectrum agents, the fact that the empirical antimicrobial therapy might not be optimal for the infection being treated, the high costs associated with increased hospitalization time, and the impact on antimicrobial stewardship (1–5).
Direct molecular identification of infecting microbes in blood would mitigate these issues. PCR and mass spectrometry methods used on positive blood cultures to identify microbes have been reported to decrease the time to an answer, but these strategies are still far from ideal, as they depend on the cultures to grow. More importantly, blood cultures are negative in >50% of the cases for which true bacterial or candidal infections are believed to exist (3, 6, 7). Some of these apparent false negatives result from a lack of bacteria in any one sample; however, the bacteria present in such samples are often rendered nonviable (and hence unculturable) with concurrent antibiotic treatment (8). Molecular methods have an inherent advantage in detecting such cryptic infections, as they do not rely on viability.
A rapid and sensitive molecular method that detects a broad range of microbes directly in blood specimens would have a significant impact on the management of patients with suspected infections. However, the molecular detection and identification of microbes that may be present in low quantities in a large volume of blood are challenging. First, a relatively large volume of blood must be analyzed to provide a reasonable sampling of the blood compartment, where the distribution of the microbe may not be homogeneous (8–10). Second, diverse microbes must be efficiently lysed in a dense complex matrix of cellular material. Third, high quantities of human genomic DNA from white blood cells must be either copurified with microbial DNA or separated without losing the microbial DNA. Finally, amplification of target DNA and signal capture (by sequencing, probe capture, mass spectrometry, or another method) must be robust. This can be very challenging if genomic DNA is copurified with targeted microbial DNA (11–13). A number of solutions to this problem have been proposed (14–19) but, to date, none have achieved the desired sensitivity (20). It should be noted that many of these methods do appear to capture a significantly higher number of total positives than culture, and the “extra” detections are often correlated with clinical indications of bloodstream infection; however, an inability to capture all positives detected by culture (false negatives) has been an issue.
We previously described PCR followed by electrospray ionization mass spectrometry (ESI-MS) technology using a broad bacterial and candida detection research assay, which is able to detect and identify >800 clinically relevant bacteria and Candida spp. associated with human infections, including unculturable organisms, directly from blood specimens (21–27). The assay can also detect three classes of antibiotic resistance markers associated with resistance to methicillin (mecA), vancomycin (vanA and vanB), and carbapenems (blaKPC). Two previous PCR/ESI-MS studies using direct blood specimens reported on the speed, breadth of bacterial coverage, and accuracy of bacterial and Candida identification (28, 29) of this research assay. In these studies, PCR/ESI-MS consistently identified more positive specimens than culture due to the well-known phenomenon that only about half of the blood specimens are positive in cases for which true infections are believed to exist (3, 6, 7). When using blood culture as a direct comparator method, however, PCR/ESI-MS of 1-ml direct blood specimens identified only about 50% of the culture-positive specimens, leaving a significant number of culture-confirmed infections undetected. Thus, our goal was to improve the sensitivity of direct detection from human blood specimens compared to that of culture-positive specimens without introducing additional steps that would compromise laboratory workflow.
Under the assumption that the sensitivity of molecular methods is limited in part by small sample volumes paired with low titers and/or uneven distributions of pathogens in the bloodstream, we developed a nucleic acid extraction system capable of processing 5 ml of blood. Lysis of all the cellular components of the blood, including human, bacterial, and candidal cells, was achieved by high-impact percussive beating with zirconium-yttrium beads, followed by automated extraction and purification of the total nucleic acids. The extracts were tested using a small number of conserved-site PCRs targeting all bacterial and candidal species, with amplicon detection and organism identification achieved through ESI-MS. Interference by human DNA was limited by two strategies. First, PCR primers and formulations were optimized to be robust to large amounts of human DNA. Second, we developed a post-PCR analytical method that selectively enriches for PCR amplicons and debulks the specimen of human DNA to enable detection of the amplicon by ESI-MS.
Here, we evaluated the performance of the new extraction system with high-blood-volume specimen preparation technology coupled to a PCR/ESI-MS detection platform (available for research applications only; Ibis Biosciences, Abbott, Carlsbad, CA) through analytical limit of detection and breadth-of-coverage studies using culture-quantified microbes spiked in whole blood. Whole-blood specimens from patients suspected to have bloodstream infections were also evaluated using the new system. The PCR/ESI-MS results were compared to the culture results to evaluate sensitivity and specificity, and a subset of PCR/ESI-MS-positive/culture-negative samples were subjected to both direct sequencing and repeat PCR/ESI-MS analysis of duplicate blood specimens in an effort to confirm the presence of the initially identified microorganism. The system is able to rapidly detect a broad range of microorganisms with a workflow that is suitable for hospital laboratories. The integrated processes that collectively improve sensitivity and workflow are summarized in Fig. 1.
FIG 1.
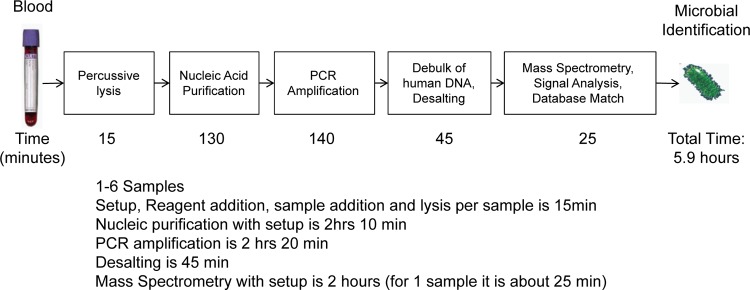
Workflow and timing of the steps in sample preparation and PCR/ESI-MS analysis of a single whole-blood specimen, with results reported in <6 h. A batch of up to 6 samples can be tested simultaneously and the results reported in 8 h.
MATERIALS AND METHODS
Extraction and analysis of DNA from 5 ml of whole blood.
In this research application, genomic DNA was isolated from 5 ml of EDTA-treated whole-blood clinical specimens or 5 ml healthy volunteer blood samples spiked with cultured microbes. Two tubes of blood, one from each arm, were drawn (usually within 30 min of each other) as part of the standard of care per hospital protocol. One additional sample was drawn from one arm (using the same venipuncture used for one of the culture samples) for PCR/ESI-MS testing, yielding between 5 and 15 ml of blood. This single blood sample was distributed into multiple aliquots of 5 ml and stored. In cases for which >10 ml was collected, the first 5 ml was used for the primary PCR/ESI-MS analysis and the second 5 ml was used for repeat testing as needed. Each patient was sampled only once during any hospital visit or admission for PCR/ESI-MS testing. The specimens were kept at 4°C refrigeration within 30 min of collection, shipped at 4°C, and kept frozen until analysis.
The blood samples were lysed in the presence of 665 μl lysis buffer (Abbott Molecular, Des Plaines, IL) 100 mM Tris solution containing guanidinium thiocyanate and detergent, 145 μl of 10% bovine serum albumin (BSA) containing a pumpkin DNA extraction control (24), and 3 g of 0.2-mm yttria-stabilized zirconium oxide beads using a large-volume bead mill homogenizer (under development at Ibis Biosciences, Abbott, Carlsbad, CA, and Omni International, Kennesaw, GA) (speed, 6.6 m/s; three 90-s cycles with 20-s dwell time). The sample tubes were centrifuged at 3,220 × g for 5 min. The supernatant fractions were processed by an automated DNA extraction and PCR setup instrument (under development at Ibis Biosciences, and Precision System Science, Co. Ltd., Matsudo, Japan). The extraction system uses prefilled disposable cartridges containing DNA-free reagents and silica-coated magnetic particles (Abbott Molecular).
The eluates were transferred into 16 wells (30 μl per well) of a custom PCR assay strip prefilled (25 μl per well) with 18 unique primer pairs and concentrated PCR master mix. The primers of the bacteria and Candida assay for bloodstream infections (BAC BSI assay; available for research applications only) were designed to hybridize to conserved genomic sequences and amplify species-specific genetic signatures from a broad spectrum of bacteria and Candida spp.; target-specific primers yield signatures indicative of antibiotic resistance elements. The gene targets, primer sequences, and configuration were previously described in detail (28). The general PCR formulations and thermocycling conditions have also been described elsewhere (25). Due to the high loads of white blood cells in 5-ml whole-blood specimens, the primer and polymerase concentrations (detailed in Results) were optimized to enable the BAC BSI assay to withstand potentially extensive interference from high levels of human DNA (up to ∼12 μg per reaction). The PCR/ESI-MS data analysis and results include a report of the organism names, level, and Q score. The Q score is the output of a principal component analysis and represents a relative measure of the strength of the data supporting identification. It is a single figure of merit that describes the overall quality of the result derived from primer-dependent parameters, such as the number of primer pairs producing amplification products compared to the maximum number expected for the identified organism, the closeness of the match of those products to reference signatures in the database for that organism, and the consistency of signal amplitudes across multiple primer pairs. For the assay described here, organisms are reported above a threshold score of ≥0.85. The level is a reflection of signal abundance relative to a set of competitive PCR standards of known input quantity and thus serves as an indirect estimate of how much specific template was amplified. This is calculated with reference to an internal calibrant construct (the amplification control), as described previously (30), and provides a relative measure of the genome (or copy number) concentration of any detected target.
Sequencing and data analysis.
For identification by sequencing, portions of the 16S rRNA gene (for bacteria) or the 28S rRNA gene (for Candida spp.) were amplified with the primers specified in the CLSI guidelines for the identification of bacteria and fungi (31), including the primer pairs MM18-A (4F-TTGGAGAGTTTGATCCTGGCTC) and 108R (GGCGTGGACTACCAGGGTATCT), 28SF (GGACTACCCGCTGAACTTAAGCATATCAATA) and 28SR (GGTTTTACACCCAAACACTCGCATAGAC), and M13F (CCCAGTCACGACGTTGTAAAACG) and M13R (AGCGGATAACAATTTCACACAGG), using Platinum Taq high fidelity (Invitrogen). Platinum Taq buffer was used with 200 μM each deoxynucleoside triphosphate (dNTP), 2 mM MgSO4, and 250 nM each primer. The reactions were cycled with the following conditions: 95°C for 2 min, 8 cycles of 95°C for 15 s, 52°C for 45 s (increasing 0.6°C per cycle), and 68°C for 90 s, 27 cycles of 95°C for 15 s, 64°C for 15 s, and 68°C for 60 s, followed by 4 min at 68°C. SeqWright, Inc., (Houston, TX) performed all sequencing.
The sequences were analyzed with Phred and Phrap and aligned with BioLign (http://en.bio-soft.net/dna/BioLign.html). The primers were trimmed from the alignments and the sequences searched against GenBank using NCBI BLAST. Using the CLSI MM18-A guidelines (31), the best-matched organisms were reported at the genus and species levels with >99% identity. In some cases for which there were no perfect matches, >95% identity was used to report the most closely related genus, per the MM18-A guidelines.
Clinical specimens.
Blood samples were collected from prospectively consenting adults from January to April 2012 at The Johns Hopkins Hospital. The samples were obtained from subjects whose physicians ordered blood cultures due to clinical suspicion of a bloodstream infection. The subjects were considered eligible if they were ≥18 years old, were having blood cultures drawn as part of clinical care, and were able to provide informed consent. Two tubes of blood, one from each arm, were drawn (usually within 30 min of each other) as part of the standard of care per hospital protocol. One additional sample was drawn from one arm (using the same venipuncture used for one of the culture samples) for PCR/ESI-MS testing, yielding between 5 and 15 ml of blood. This single blood sample was distributed into multiple aliquots of 5 ml and stored. In cases for which >10 ml was collected, the first 5 ml was used for the primary PCR/ESI-MS analysis, and the second 5 ml was used for repeat testing as needed. Each patient was sampled only once during any hospital visit or admission for PCR/ESI-MS testing.
Microbiological methods.
The subjects suspected of sepsis had two sets of blood cultures obtained after appropriate skin decontamination. A set consisted of one BD Bactec Plus aerobic/F bottle and one BD Bactec lytic anaerobic/F bottle (BD Diagnostics). All bottles were sent promptly to the laboratory within 1 h and incubated on the Bactec FX (BD Diagnostics) continuously monitored blood culture system. The bottles were incubated and monitored for 5 days before being called negative. Positive blood cultures were removed immediately from the instrument, and a Gram stain was performed. The peptide nucleic acid fluorescence in situ hybridization (PNA-FISH) Enterococcus faecalis-other Enterococcus dual probe and the PNA-FISH Candida albicans-Candida glabrata dual probe were used to rapidly identify Gram-positive cocci and yeast, respectively. All other pathogens were subcultured to the appropriate medium depending upon the Gram stain results and the type of bottle from which they were recovered. The organisms were subsequently identified by a variety of phenotypic methods, including the Phoenix automated microbiology system (BD Diagnostics), classical biochemical analyses, cell wall fatty acid analysis using gas liquid chromatography (Sherlock microbial identification system), and, rarely, 16S rRNA gene sequencing.
RESULTS
Optimization of PCR and desalting steps for sensitivity in the presence of high levels of nontarget DNA.
Due to very high numbers of white blood cells in the 5-ml blood specimens, the BAC assay was optimized in the presence of high levels of human DNA (up to ∼12 μg per PCR). The optimal PCR conditions were determined using a systematic matrix analysis that varied primer, Mg++, and polymerase concentrations, annealing time, and temperature. The optimum concentrations for each component were chosen as those that gave the maximum amplicon yields as determined by capillary electrophoresis. The results showed that increasing primer and polymerase concentrations simultaneously to 750 μM and 2.2 units per reaction, respectively, resulted in a PCR yield in a 12-μg DNA background of 86% (range, 56% to 120%) of the yield when 1 μg of human DNA was present (data not shown). Changing the other parameters resulted in negligible improvements to the previously reported PCR formulations and thermocycling conditions (25).
Prior to mass spectrometry, the amplicons were desalted by anion-exchange chromatography using an automated platform (30). In addition to salts, which interfere with mass spectrometry, this procedure removes >98% of human DNA background in samples containing 12 μg human DNA per PCR (data not shown). We discovered that during primary amine anion exchange on irregularly shaped porous microparticle clusters, short PCR amplicons bound to submicron-sized pores on the surface and within the porous cluster. The dual anion-exchange and size exclusion properties of the particles prevented the larger-sized human genomic DNA from binding, effectively enriching the sample for PCR amplicons and improving detection by ESI-MS.
Levels of human DNA in 5 ml of blood.
In order to define the limit of human DNA background tolerated, healthy volunteer blood samples were spiked at the limit of detection (LOD) with multiple microorganisms and analyzed in the presence of a range of human DNA concentrations. The total amount of DNA extracted from 5 ml of blood has been capped to deliver no more than 12 μg per PCR and post-PCR amplicon enrichment reaction. Human DNA in whole-blood samples comes primarily from white blood cells. There is approximately 72 μg of total human DNA in approximately 12 × 106 cells. The white blood cell counts from the population intended for use showed that 90% of patients fell into a range between 0 and 16 × 106 cells/ml (Fig. 2). Most patients had between 6 × 106 and 12 × 106 white blood cells per ml; 15.1, 14.3, and 14.3% of the subjects had 6 × 106, 8 × 106, and 12 × 106 white blood cells/ml, respectively; 90% of the patients had ≤16 × 106 white blood cells/ml. To understand the capability of the assay to withstand higher levels of human DNA, 5-ml whole-blood samples were spiked at the assay LOD of 16 CFU/ml of Enterococcus faecium (a vancomycin-resistant Enterococcus [VRE] species) and 4 CFU/ml of C. albicans with 5 × 106, 7 × 106, 8 × 106, 10 × 106, 12 × 106, 23 × 106, and 40 × 106 white blood cells/ml. PCR/ESI-MS correctly identified the spiked organisms at all white blood cell levels (data not shown). These results suggest that microbial DNA was detected and correctly identified in patients with the highest white blood cell counts observed in this study.
FIG 2.

Frequency distribution plot for white blood cell counts obtained from patients. Each bin has a width of 2 × 106 white blood cells/ml. The y axis represents the frequency of each bin relative to the total number of samples, and the x axis shows the range of white blood cell counts. The bracket at the top indicates that 90% of samples fall into the range from 0 to 16 × 106 cells/ml. The black regions of the bars correspond to the specimens that were positive by PCR/ESI-MS.
Limits of detection for relevant organisms.
To determine the analytical sensitivity of the PCR/ESI-MS system with the 5-ml blood preparation system, we determined the limits of detection for Klebsiella pneumoniae (blaKPC+), E. faecium (vanA+ vanB+), Staphylococcus aureus (mecA+), and C. albicans. Each of the primer pairs in the assay targets one or more of these organisms. Aliquots of uninfected blood were spiked with a dilution series of microbes in 2-fold steps. The LOD was taken as the last dilution step at which at least 19 of 20 replicates (95%) were positive. The LODs for each organism (inclusive of their resistance markers) were 16 CFU/ml for S. aureus, 16 CFU/ml for K. pneumoniae, 16 CFU/ml for E. faecium, and4 CFU/ml for C. albicans.
Clinical specimens.
To demonstrate the accuracy of organism identification in clinical specimens, we tested 331 prospectively collected deidentified blood specimens from consenting patients from the Johns Hopkins Medical Center emergency department under institutional review board (IRB) approval (JHU IRB no. NA_00013251). The PCR/ESI-MS results from an analysis of 5 ml of EDTA-blood using the BAC assay were compared with the results from standard clinical microbiology cultures. An analysis of 331 subjects with suspected bloodstream infections (Table 1) yielded 35 PCR/ESI-MS-positive specimens (10.6%) compared to 18 positive specimens by culture (5.4%). There were 15 samples that were positive by both methods. One specimen, sample 1,070, was polymicrobial. Culture identified two organisms, while PCR/ESI-MS identified three. Overall, for the 16 cases for which culture reported an organism, PCR identified the same organism in 15 instances, for an accuracy of identification of 94%. The one mismatch was a case (sample 1,346) for which culture failed to identify the organism in the primary culture used for comparison and PCR/ESI-MS identified it as Escherichia coli. The culture of a subsequent blood draw yielded E. coli, but this was not included in the analysis.
TABLE 1.
Positive detections by culture or PCR/ESI-MS
| Sample no. | Culture result | PCR/ESI-MS result | Q score | Level (GE/ml)a |
|---|---|---|---|---|
| IBIS0838 | Klebsiella pneumoniae | K. pneumoniae | 0.98 | 332 |
| IBIS0917 | Staphylococcus species, coagulase negative | Staphylococcus epidermidis, mecA+ | 0.97 | 40 |
| IBIS1088 | Escherichia coli | E. coli | 0.99 | 220 |
| IBIS1090 | E. coli | E. coli | 0.96 | 12 |
| IBIS1126 | K. pneumoniae | K. pneumoniae | 0.99 | 124 |
| IBIS1168 | Streptococcus group G | Streptococcus dysgalactiae | 0.98 | 60 |
| IBIS1170 | E. coli | E. coli | 0.98 | 544 |
| Vancomycin-resistant Enterococcus faecium | E. faecium, vanA+ | 0.96 | 120 | |
| Not detected | Candida glabrata | 0.97 | 204 | |
| IBIS1185 | Staphylococcus species, coagulase negative | S. epidermidis, mecA+ | 0.99 | 444 |
| IBIS1195 | E. coli | E. coli | 0.96 | 40 |
| IBIS1230 | Staphylococcus aureus | S. aureus | 0.98 | 172 |
| IBIS1296 | S. aureus | S. aureus | 0.99 | 80 |
| IBIS1346 | Reported as positive, but no organism listed | E. coli | 0.97 | 12 |
| IBIS1366 | S. aureus | S. aureus | 0.97 | 36 |
| IBIS1407 | S. aureus | S. aureus | 0.99 | 764 |
| IBIS1414 | K. pneumoniae | K. pneumoniae | 0.98 | 16 |
| IBIS0834 | Viridans Streptococcus group | Negative | Not detected | NA |
| IBIS1016 | E. coli | Negative | Not detected | NA |
| IBIS1051 | Streptococcus pneumoniae | Negative | Not detected | NA |
| IBIS0840 | Negative | Bacteroides fragilis | 0.97 | 72 |
| IBIS0852 | Negative | Finegoldia magna | 0.90 | 20 |
| IBIS0868 | Negative | f. magna | 0.88 | 16 |
| IBIS0869 | Negative | Bartonella henselae | 0.96 | 160 |
| IBIS0933 | Negative | Acinetobacter baumannii | 0.98 | 148 |
| IBIS0885 | Negative | K. pneumoniae | 0.98 | 72 |
| IBIS0965 | Negative | S. aureus | 0.97 | 16 |
| IBIS1000 | Negative | E. coli | 0.98 | 292 |
| IBIS1006 | Negative | E. coli | 0.97 | 100 |
| IBIS1023 | Negative | E. coli | 0.95 | 20 |
| IBIS1083 | Negative | Serratia marcescens | 0.99 | 172 |
| IBIS1097 | Negative | E. coli | 0.98 | 192 |
| Negative | Pseudomonas aeruginosa | 0.98 | 560 | |
| IBIS1093 | Negative | Enterobacter cloacae complex | 0.98 | 76 |
| IBIS1109 | Negative | E. coli | 0.99 | 32 |
| IBIS1181 | Negative | Viridans/Mitis group Streptococcus | 0.96 | 104 |
| IBIS1170 | Negative | C. glabrata | 0.97 | 204 |
| IBIS0800 | Negative | K. pneumoniae | 0.96 | 32 |
| IBIS1121 | Negative | Enterococcus faecalis | 0.98 | 28 |
| IBIS1365 | Negative | K. pneumoniae | 0.99 | 36 |
| IBIS1381 | Negative | E. faecalis | 0.98 | 16 |
| IBIS1416 | Negative | E. coli | 0.98 | 36 |
GE, genome copy; NA, not applicable.
Using culture as the comparator method, PCR/ESI-MS was 83% sensitive and 94% specific (Table 2). When PCR/ESI-MS-positive but culture-negative specimens were confirmed by repeat PCR/ESI-MS testing of additional replicate specimens (Table 3) and the confirmed detections were considered true positives, sensitivity increased to 91% and specificity to 99% (see section below). In one specimen (sample 1,070), two organisms were identified by culture; both were correctly identified in direct testing by PCR/ESI-MS. PCR/ESI-MS also detected a high level of Candida glabrata in the same sample that culture failed to identify.
TABLE 2.
Concordance of culture results with PCR/ESI-MS on a per-sample basisa
| PCR/ESI-MS result | Culture result |
Culture and repeated PCR/ESI-MS |
||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | Positive | Negative | Total | |
| Positive (no.) | 15 | 20 | 35 | 32 | 3 | 35 |
| Negative (no.) | 3 | 293 | 296 | 3 | 293 | 296 |
| Total (no.) | 18 | 313 | 331 | 35 | 296 | 331 |
| Sensitivity (%) | 83 | 91 | ||||
| Specificity (%) | 94 | 99 | ||||
Culture results are from direct comparison of culture and PCR/ESI-MS, and the culture and repeated PCR/ESI-MS results are from comparison of culture plus PCR/ESI-MS when replicated as a comparator method.
TABLE 3.
Replicate testing of PCR/ESI-MS-positive samples that were negative by culture
| PCR/ESI-MS replicate 1 | Q score | Level (GE/ml) | PCR/ESI-MS replicate 2 | Q score | Level (GE/ml) |
|---|---|---|---|---|---|
| Acinetobacter baumannii | 0.98 | 148 | A. baumannii | 0.98 | 88 |
| Bacteroides fragilis | 0.97 | 72 | B. fragilis | 0.9 | 16 |
| Bartonella henselae | 0.96 | 160 | B. henselae | 0.98 | 128 |
| Candida glabrata | 0.97 | 204 | C. glabrata | 0.97 | 276 |
| Enterobacter cloacae complex | 0.98 | 76 | E. cloacae complex | 0.98 | 88 |
| Enterococcus faecalis | 0.98 | 28 | No result | NA | NA |
| E. faecalis | 0.98 | 16 | No result | NA | NA |
| Escherichia coli | 0.98 | 292 | E. coli | 0.99 | 236 |
| E. coli | 0.97 | 100 | E. coli | 0.99 | 108 |
| E. coli | 0.95 | 20 | E. coli | 0.98 | 24 |
| E. coli | 0.98 | 192 | E. coli | 0.98 | 172 |
| E. coli | 0.99 | 32 | E. coli | 0.98 | 40 |
| E. coli | 0.98 | 36 | E. coli | 0.96 | 32 |
| Finegoldia magna | 0.90 | 20 | f. magna | 0.99 | 32 |
| f. magna | 0.88 | 16 | f. magna | 0.98 | 24 |
| Klebsiella pneumoniae | 0.98 | 72 | K. pneumoniae | 0.98 | 92 |
| K. pneumoniae | 0.96 | 32 | No result | NA | NA |
| Pseudomonas aeruginosa | 0.98 | 560 | P. aeruginosa | 0.99 | 656 |
| Serratia marcescens | 0.99 | 172 | S. marcescens | 0.98 | 164 |
| Staphylococcus aureus | 0.97 | 16 | S. aureus | 0.99 | 32 |
| Viridans/Mitis group Streptococcus | 0.96 | 104 | Viridans/Mitis group Streptococcus | 0.97 | 80 |
Sequencing is not an appropriate comparator method for PCR/ESI-MS.
To determine whether sequencing can be used as a comparator method for PCR/ESI-MS, we attempted to sequence the extracted DNA that was used for PCR/ESI-MS analysis using primers and protocols specified by the CLSI guidance document for the identification of bacteria and candida. We examined the specimens from the patient population with suspected bloodstream infections and also extracted a second set of samples from orthopedic tissues suspected of being infected (kindly provided by Garth Ehrlich [32]). The extracted blood specimens had a higher concentration of human DNA (270 ng/μl) than did the tissue specimens (27 ng/μl) but a substantially smaller amount of infecting bacterial DNA by PCR/ESI-MS (Fig. 3). Only two of the 35 PCR/ESI-MS-positive specimens (including 15 that were also culture positive) from patients with suspected bloodstream infections were confirmed by sequencing. Sequencing confirmed that the organisms identified by PCR/ESI-MS in 27 of 36 (75%) tissue specimens were present. No specimens were positive by sequencing and negative by PCR/ESI-MS.
FIG 3.

Microbial and human DNA loads define the functional limits of 16S sequence analysis of clinical specimens. Blood sample extracts were obtained using the 5-ml DNA isolation protocol from whole-blood specimens collected from patients suspected to have bacteremia. For tissues, 25-mg specimens were collected from patients suspected of sterile-site bacterial/candidal infection. All PCR/ESI-MS-positive samples were further analyzed by Sanger sequencing. The samples are plotted with respect to their total DNA load (y axis) and bacterial DNA detection level by PCR/ESI-MS (x axis). The horizontal dashed line indicates a 0.4-μg DNA load threshold above which only one tissue sample is present and below which only one blood sample is represented. The vertical solid line indicates the detection level of approximately 40 bacterial genomes per PCR, the threshold above which sequencing was successful.
An examination of the data in Fig. 3 suggests that the success of sequencing is dependent on both the concentration of the target bacterial DNA and level of human DNA. Overall, 84% of samples with <0.4 μg of background DNA per PCR sequencing reaction and >40 genomes per PCR well were positive by sequencing. In contrast, only 8% of the PCR/ESI-MS-positive samples containing >0.4 μg DNA (all but one of the blood samples tested) were positive by sequencing. Thus, in the absence of a method to remove or reduce human DNA, 16S Sanger sequencing of nucleic acid extracts from whole-blood specimens is not a suitable method for detecting and identifying infecting bacteria or fungi, and it cannot be used as a comparator method for PCR/ESI-MS of blood specimens.
Repeated analytical testing by PCR/ESI-MS.
Many studies have shown that molecular methods will find bacterial and candidal DNA in blood extracts for which cultures are negative (19, 20, 28, 29). In the absence of another technology sharing both the sensitivity and the breadth of coverage of the BAC BSI assay, it is challenging to prove that PCR/ESI-MS identifications are correct in the absence of a confirming culture result. To partially address this issue, we retrieved frozen remnant blood samples of the PCR/ESI-MS-positive/culture-negative patient specimens and retested them on a different instrument with a different operator. The results are shown in Table 3. In 18 of 21 cases for which there was sufficient volume of blood to retest, the results were identical in replicate testing in terms of the organism identified and similar in terms of the level of microbial DNA detected, providing supporting evidence that the organism DNA reported by PCR/ESI-MS was indeed present in these samples and not the result of postcollection contamination. The three specimens that were negative upon retesting had yielded relatively low-level signals on the initial test. In order to be counted as a detection by PCR/ESI-MS, a critical number of spectra with positive peaks for specific organisms must be achieved. Representative spectra from the specimens that were PCR/ESI-MS positive in duplicate independent tests are shown in Fig. 4. The species identification with this method results from the completion of a succession of well-defined tasks that are themselves submitted to stepwise stringent quality control requirements, and hence repeatable identifications strongly indicate that these specimens contain the initially reported microbial DNA.
FIG 4.
Quantitative bacterial loads in whole blood determined by various methods. Q, interquartile range; I, range; S, ±1 standard deviation; |, cutoff; diamond, median. The PCR/ESI-MS values are those reported in Table 1 corrected for the dilution factors in sample preparation and converted to genome copies per ml of blood to be consistent with the reported values for quantitative PCR. The analytical LOD for PCR/ESI-MS is indicated by the vertical dashed line.
Expected levels of bacterial DNA in blood from patients with bloodstream infections.
In order to estimate the true concentration of pathogen DNA available in whole blood for molecular analysis from patients with bloodstream infections, we analyzed the data available in the literature. Organism-specific quantitative PCR has been used to measure the bacterial DNA present in whole-blood specimens from patients with sepsis, pneumonia, or suspected bloodstream infections (13, 33–48). Each of these studies analyzed whole-blood specimens from patients with suspected or confirmed infections rather than spiked samples for which the genome-to-viable cell ratio is expected to be close to 1:1. Of 16 such publications, 15 used a calibrated real-time PCR method focused on single organisms, and one publication reported a quantitative 16S broad-range method (37). The investigators calibrated their PCRs by using either an analytically prepared DNA reference standard of a single-copy gene (reporting results as bacterial genome copies/ml) or quantified spikes of cultured microbes (reporting results as CFU equivalents/ml of blood). Although the results of these experiments revealed some variability between different microorganisms, specific PCR methods, and different patient populations, the central values (median and mean) for bacteria were typically between 1 × 103 and 1 × 104 genome copies per ml (Fig. 5). Thus, in subjects with suspected or confirmed bloodstream infections, the amount of bacterial nucleic acid available for detection by molecular methods is approximately 2 to 3 orders of magnitude higher than what one might expect from the literature on culture-based quantitative microbiology. This explains the apparent inconsistency between the reported analytical LODs of PCR/ESI-MS, which are approximately 16 CFU/ml for freshly spiked blood, the reported concentrations of viable microbial cells in septic blood (averaging between 1 and 10 CFU/ml), and the approximate clinical sensitivity of PCR/ESI-MS of 83 to 91%. The results reported here are consistent with quantitative PCR analysis of patient specimens (Fig. 5).
FIG 5.
Spectra from representative PCRs. The spectra for primer pairs 348 (left) and 349 (right) are reported for sample 1,083 (Serratia marcescens detected in replicates 1 and 2, first and second rows) and for sample 933 (Acinetobacter baumannii detected in replicates 1 and 2, third and fourth rows). For each primer pair, the conserved horizontal scale emphasizes the reproducibility of the detections between replicates, as can be verified by the vertical alignment of the peaks corresponding to the internal positive control of the assay (calibrant, [gray]) and to the detected species (blue). The vertical scales are normalized to the highest peak present in the corresponding well. The spectra shown are for only two primer pairs; the reported species detections are further supported by similar detections of the corresponding amplicons using four additional primer pairs.
DISCUSSION
In order for molecular assays to be optimally useful for diagnosing patients with suspected systemic infections, the assay must (i) accurately and sensitively identify bacterial and candidal species present in blood, (ii) provide rapid results, (iii) detect the most important genetic mediators of antimicrobial drug resistance, and (iv) be carried out with a workflow and throughput suitable for a hospital laboratory. To meet these objectives, we developed a PCR/ESI-MS hardware platform and research assay with improved clinical sensitivity that facilitates workflow in a clinical laboratory environment. The semiautomated workflow described in Fig. 1 allows the first sample to obtain a result in approximately 6 h for the first batch of 6 specimens. The modular nature of the workflow permits additional rounds of samples to begin processing through the front-end lysis and extraction steps while the later modules are still finishing the previous set of samples. This allows for samples that enter the testing laboratory later in the day to be incorporated into the testing workflow throughout the day. Improved sensitivity was achieved by extracting nucleic acids from a 5-ml volume of blood, developing an automated specimen preparation technology to accommodate this volume, and optimizing the entire remaining system to be tolerant to the high levels of human DNA arising from human white blood cells. Unlike protocols that separate human white blood cells from the bacteria or those that separate bacterial DNA from human DNA after lysis, the procedure we developed retains all potential compartments of bacterial DNA, including cell-associated bacteria, free bacteria and free bacterial DNA, and it avoids the introduction of steps that would complicate the workflow and increase costs.
This improved approach resulted in PCR/ESI-MS detecting twice as many positive samples (10.6%) as culture (5.4%) in the 331 samples analyzed from patients suspected of having a blood infection. This is consistent with the well-established observation that approximately half of the truly infected patients are not positive by culture (3, 6, 7), often because samples are taken after patients began antimicrobial drug treatment. The analytical sensitivity for uninfected blood spiked with bacteria was improved about 5-fold compared to that of the previously reported 1-ml sample preparation method (28, 29). More importantly, the higher-volume-sample preparation method integrated with the other sensitivity improvements increased the detection rate for specimens that were blood culture positive from about 50% for the low-blood-volume PCR-ESI-MS assay to 83% for the high-volume research protocol.
Previously published quantitative microbiology studies have shown that the number of recoverable CFU of bacteria in the blood of patients with clinically significant bacteremia is low, typically in the range of <1 to 30 CFU/ml (49–51). However, the CFU measured by quantitative microbiology represents only separable viable organisms that survive the plating process and not dead cells, cells that cannot form colonies, multiple cells in irreducible clumps, or free microbial DNA that may have been liberated from lysed cells in the blood compartment. Thus, the true concentrations of pathogen DNA available in whole blood for molecular analysis in patients with bloodstream infections cannot be inferred from viable cell count (quantitative culture) data. Here, we report that in subjects with suspected or confirmed bloodstream infections, the amount of bacterial nucleic acid available for detection by molecular methods is approximately 2 to 3 orders of magnitude higher than what one might expect from the culture-based quantitative microbiology literature. This estimate of the bacterial DNA load in patient specimens may have clinical utility. Several studies have demonstrated that bacterial load measured by PCR correlates with disease severity and that the bacterial load is predictive of which patients are likely to become severely ill (35, 37, 41, 42, 52, 53). Two studies independently identified a bacterial load of 1 × 103 genome copies per ml of blood as a critical threshold above which patients had an increased risk of developing septic shock with E. coli (37), S. aureus (37), or Streptococcus pneumoniae (42), suggesting that quantitative measurement of the bacterial DNA load in blood may have clinical value. The analytical sensitivity of PCR/ESI-MS (16 CFU/ml) is more than sufficient to detect the concentrations of organisms present in patients with these serious infections.
A highly sensitive molecular test that detects a broad range of bacteria and fungi presents challenges for validation. Although PCR/ESI-MS-positive results can be compared to positive culture results, there is no good way to corroborate a PCR/ESI-MS-positive result when cultures are negative other than to show that PCR/ESI-MS gives the same result when testing additional specimens from the same patient. When replicate testing was performed, 91% of the PCR/ESI-MS results were corroborated. Broad amplification followed by sequencing failed to provide a sufficiently sensitive comparator method for bloodstream infections because it is difficult to amplify and sequence the small amount of targeted bacterial DNA against an overwhelming background of human DNA. Mass spectrometry of unfragmented amplicons is, in comparison to sequencing, relatively unaffected by background genomic DNA but still provides species-specific signatures that can be matched to sequence database-derived signatures for unambiguous identification.
There is a growing body of evidence that rapid and accurate identification of the microbes causing bloodstream infections provide significant clinical and economic value. The use of molecular methods to identify culture-isolated organisms decreases the overall time to identification, resulting in improved patient outcomes and significantly decreasing hospital costs (54–57). This is a strong step forward but requires time for culture and is not useful when cultures from truly infected patients are negative (58, 59). The direct analysis of patient specimens would both increase the number of patients who benefit from the information and further decrease the time to result by avoiding the lag time associated with growing cultures.
ACKNOWLEDGMENTS
This work was funded in part by the Middle Atlantic RCE Program (NIAID/NIH grant 2 U54 AI057168 to R.E.R).
N.J.K. is a independent consultant under contract with Ibis Biosciences; all other authors affiliated with Ibis Biosciences are full-time employees.
Footnotes
Published ahead of print 20 June 2014
REFERENCES
- 1.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34:1589–1596. 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41:580–637. 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL, International Surviving Sepsis Campaign Guidelines Committee, American Association of Critical-Care Nurses, American College of Chest Physicians, American College of Emergency Physicians, Canadian Critical Care Society, European Society of Clinical Microbiology and Infectious Diseases, European Society of Intensive Care Medicine, European Respiratory Society, International Sepsis Forum, Japanese Association for Acute Medicine, Japanese Society of Intensive Care Medicine, Society of Critical Care Medicine, Society of Hospital Medicine, Surgical Infection Society, World Federation of Societies of Intensive and Critical Care Medicine 2008. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 36:296–327. 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 4.Kuti EL, Patel AA, Coleman CI. 2008. Impact of inappropriate antibiotic therapy on mortality in patients with ventilator-associated pneumonia and blood stream infection: a meta-analysis. J. Crit. Care. 23:91–100. 10.1016/j.jcrc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH. 2008. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin. Infect. Dis. 47(Suppl 1):S3–S13. 10.1086/590061. [DOI] [PubMed] [Google Scholar]
- 6.Fenollar F, Raoult D. 2007. Molecular diagnosis of bloodstream infections caused by non-cultivable bacteria. Int. J. Antimicrob. Agents 30(Suppl 1):S7–S15. 10.1016/j.ijantimicag.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. 1995. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 273:117–123. [PubMed] [Google Scholar]
- 8.Lamy B, Roy P, Carret G, Flandrois JP, Delignette-Muller ML. 2002. What is the relevance of obtaining multiple blood samples for culture? A comprehensive model to optimize the strategy for diagnosing bacteremia. CID. 35:842–850. 10.1086/342383. [DOI] [PubMed] [Google Scholar]
- 9.Reimer LG, Wilson ML, Weinstein MP. 1997. Update on detection of bacteremia and fungemia. Clin. Microbiol. Rev. 10:444–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein MP. 1996. Current blood culture methods and systems: clinical concepts, technology, and interpretation of results. Clin. Infect. Dis. 23:40–46. 10.1093/clinids/23.1.40. [DOI] [PubMed] [Google Scholar]
- 11.Morata P, Queipo-Ortuño MI, de Dios Colmenero J. 1998. Strategy for optimizing DNA amplification in a peripheral blood PCR assay used for diagnosis of human brucellosis. J. Clin. Microbiol. 36:2443–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cogswell FB, Bantar CE, Hughes TG, Gu Y, Philipp MT. 1996. Host DNA can interfere with detection of Borrelia burgdorferi in skin biopsy specimens by PCR. J. Clin. Microbiol. 34:980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters RP, van Agtmael MA, Gierveld S, Danner SA, Groeneveld AB, Vandenbroucke-Grauls CM, Savelkoul PH. 2007. Quantitative detection of Staphylococcus aureus and Enterococcus faecalis DNA in blood to diagnose bacteremia in patients in the intensive care unit. J. Clin. Microbiol. 45:3641–3646. 10.1128/JCM.01056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handschur M, Karlic H, Hertel C, Pfeilstöcker M, Haslberger AG. 2009. Preanalytic removal of human DNA eliminates false signals in general 16S rDNA PCR monitoring of bacterial pathogens in blood. Comp. Immunol. Microbiol. Infect. Dis. 32:207–219. 10.1016/j.cimid.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Gebert S, Siegel D, Wellinghausen N. 2008. Rapid detection of pathogens in blood culture bottles by real-time PCR in conjunction with the pre-analytic tool MolYsis. J. Infect. 57:307–316. 10.1016/j.jinf.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Hansen WL, Bruggeman CA, Wolffs PF. 2009. Evaluation of new preanalysis sample treatment tools and DNA isolation protocols to improve bacterial pathogen detection in whole blood. J. Clin. Microbiol. 47:2629–2631. 10.1128/JCM.00821-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wellinghausen N, Kochem AJ, Disqué C, Mühl H, Gebert S, Winter J, Matten J, Sakka SG. 2009. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. J. Clin. Microbiol. 47:2759–2765. 10.1128/JCM.00567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachse S, Straube E, Lehmann M, Bauer M, Russwurm S, Schmidt KH. 2009. Truncated human cytidylate-phosphate-deoxyguanylate-binding protein for improved nucleic acid amplification technique-based detection of bacterial species in human samples. J. Clin. Microbiol. 47:1050–1057. 10.1128/JCM.02242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann LE, Alvarez J, Hunfeld KP, Goglio A, Kost GJ, Louie RF, Raglio A, Regueiro BJ, Wissing H, Stüber F. 2009. Potential clinical utility of polymerase chain reaction in microbiological testing for sepsis. Crit. Care Med. 37:3085–3090. 10.1097/CCM.0b013e3181b033d7. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber J, Nierhaus A, Braune SA, de Heer G, Kluge S. 2013. Comparison of three different commercial PCR assays for the detection of pathogens in critically ill sepsis patients. Med. Klin. Intensivmed. Notfmed. 108:311–318. 10.1007/s00063-013-0227-1. [DOI] [PubMed] [Google Scholar]
- 21.Ecker DJ, Sampath R, Li H, Massire C, Matthews HE, Toleno D, Hall TA, Blyn LB, Eshoo MW, Ranken R, Hofstadler SA, Tang YW. 2010. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev. Mol. Diagn. 10:399–415. 10.1586/erm.10.24. [DOI] [PubMed] [Google Scholar]
- 22.Kaleta EJ, Clark AE, Cherkaoui A, Wysocki VH, Ingram EL, Schrenzel J, Wolk DM. 2011. Comparative analysis of PCR-electrospray ionization/mass spectrometry (MS) and MALDI-TOF/MS for the identification of bacteria and yeast from positive blood culture bottles. Clin. Chem. 57:1057–1067. 10.1373/clinchem.2011.161968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaleta EJ, Clark AE, Johnson DR, Gamage DC, Wysocki VH, Cherkaoui A, Schrenzel J, Wolk DM. 2011. Use of PCR coupled with electrospray ionization mass spectrometry for rapid identification of bacterial and yeast bloodstream pathogens from blood culture bottles. J. Clin. Microbiol. 49:345–353. 10.1128/JCM.00936-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzgar D, Frinder M, Lovari R, Toleno D, Massire C, Blyn LB, Ranken R, Carolan HE, Hall TA, Moore D, Hansen CJ, Sampath R, Ecker DJ. 2013. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J. Clin. Microbiol. 51:2670–2678. 10.1128/JCM.00966-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eshoo MW, Crowder CD, Li H, Matthews HE, Meng S, Sefers SE, Sampath R, Stratton CW, Blyn LB, Ecker DJ, Tang YW. 2010. Detection and identification of Ehrlichia species in blood by use of PCR and electrospray ionization mass spectrometry. J. Clin. Microbiol. 48:472–478. 10.1128/JCM.01669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowder CD, Matthews H, Schutzer S, Rounds MA, Luft BJ, Nolte O, Campbell SR, Phillipson CA, Li F, Sampath R, Ecker DJ, Eshoo MW. 2010. Genotypic variation and mixtures of Lyme Borrelia in Ixodes ticks from North America and Europe. PLoS One 5:e10650. 10.1371/journal.pone.0010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrell JJ, Sampath R, Bonomo RA. 2011. Identifying pathogens in “culture negative” infections: a case series exploring PCR and electrospray ionization mass spectrometry for microbial identification; poster 1022 Annual Meeting of the Infectious Diseases Society of America, Boston, MA, 20 to 23 October 2011. [Google Scholar]
- 28.Laffler TG, Cummins LL, McClain CM, Quinn CD, Toro MA, Carolan HE, Toleno DM, Rounds MA, Eshoo M, Stratton CW, Sampath R, Blyn LB, Ecker DJ, Tang YW. 2013. Enhanced diagnostic yields of bacteremia and candidemia in blood specimens by PCR/electrospray ionization mass spectrometry. J. Clin. Microbiol. 51:3535–3541. 10.1128/JCM.00876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordana-Lluch E, Carolan HE, Giménez M, Sampath R, Ecker DJ, Quesada MD, Modol JM, Arméstar F, Blyn LB, Cummins LL, Ausina V, Martró E. 2013. Rapid diagnosis of bloodstream infections with PCR followed by mass spectrometry. PLoS One 8:e62108. 10.1371/journal.pone.0062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofstadler SA, Sampath R, Blyn LB, Eshoo MW, Hall TA, Jiang Y, Drader JJ, Hannis JC, Sannes-Lowery KA, Cummins LL, Libby B, Walcott DJ, Schink A, Massire C, Ranken R, Gutierrez J, Manalili S, Ivy C, Melton R, Levene H, Barrett-Wilt G, Feng L, Zapp V, White N, Samant V, McNeil JA, Knize D, Robbins D, Rudnik K, Desai A, Moradi E, Ecker DJ. 2005. TIGER: the universal biosensor. Int. J. Mass Spectrom. 242:23–41. 10.1016/j.ijms.2004.09.014. [DOI] [Google Scholar]
- 31.CLSI. 2008. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing; approved guideline. CLSI Document MM18-A Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Bowers DR, Hunter AS, Jacobs DM, Kuper KM, Musick WL, Perez KK, Shah DN, Schilling AN. 2013. Significant publications on infectious diseases pharmacotherapy in 2012. Am. J. Health Syst. Pharm. 70:1930–1940. 10.2146/ajhp130129. [DOI] [PubMed] [Google Scholar]
- 33.Chuang YC, Chang SC, Wang WK. 2012. Using the rate of bacterial clearance determined by real-time polymerase chain reaction as a timely surrogate marker to evaluate the appropriateness of antibiotic usage in critical patients with Acinetobacter baumannii bacteremia. Crit. Care Med. 40:2273–2280. 10.1097/CCM.0b013e3182515190. [DOI] [PubMed] [Google Scholar]
- 34.Agampodi SB, Matthias MA, Moreno AC, Vinetz JM. 2012. Utility of quantitative polymerase chain reaction in leptospirosis diagnosis: association of level of leptospiremia and clinical manifestations in Sri Lanka. Clin. Infect. Dis. 54:1249–1255. 10.1093/cid/cis035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darton T, Guiver M, Naylor S, Jack DL, Kaczmarski EB, Borrow R, Read RC. 2009. Severity of meningococcal disease associated with genomic bacterial load. Clin. Infect. Dis. 48:587–594. 10.1086/596707. [DOI] [PubMed] [Google Scholar]
- 36.Massi MN, Shirakawa T, Gotoh A, Bishnu A, Hatta M, Kawabata M. 2005. Quantitative detection of Salmonella enterica serovar Typhi from blood of suspected typhoid fever patients by real-time PCR. Int. J. Med. Microbiol. 295:117–120. 10.1016/j.ijmm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Kirkbright S, Fatovich D, Kee C, Kay I, Flexman J, Pryce TM, Waterer GW. 2011. Quantitative rt-PCR holds promise as a screening tool for patients with severe sepsis. Emerg. Med. Australas. 23:502–506. 10.1111/j.1742-6723.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- 38.Reier-Nilsen T, Farstad T, Nakstad B, Lauvrak V, Steinbakk M. 2009. Comparison of broad range 16S rDNA PCR and conventional blood culture for diagnosis of sepsis in the newborn: a case control study. BMC Pediatr. 9:5. 10.1186/1471-2431-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuang YC, Chang SC, Wang WK. 2010. High and increasing Oxa-51 DNA load predict mortality in Acinetobacter baumannii bacteremia: implication for pathogenesis and evaluation of therapy. PLoS One 5:e14133. 10.1371/journal.pone.0014133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reller ME, Clemens EG, Schachterle SE, Mtove GA, Sullivan DJ, Dumler JS. 2011. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. J. Clin. Microbiol. 49:3245–3249. 10.1128/JCM.00940-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho YC, Chang SC, Lin SR, Wang WK. 2009. High levels of mecA DNA detected by a quantitative real-time PCR assay are associated with mortality in patients with methicillin-resistant Staphylococcus aureus bacteremia. J. Clin. Microbiol. 47:1443–1451. 10.1128/JCM.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rello J, Lisboa T, Lujan M, Gallego M, Kee C, Kay I, Lopez D, Waterer GW, DNA-Neumococo Study Group 2009. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest 136:832–840. 10.1378/chest.09-0258. [DOI] [PubMed] [Google Scholar]
- 43.Navarro E, Segura JC, Castaño MJ, Solera J. 2006. Use of real-time quantitative polymerase chain reaction to monitor the evolution of Brucella melitensis DNA load during therapy and post-therapy follow-up in patients with brucellosis. Clin. Infect. Dis. 42:1266–1273. 10.1086/503035. [DOI] [PubMed] [Google Scholar]
- 44.Peters RP, de Boer RF, Schuurman T, Gierveld S, Kooistra-Smid M, van Agtmael MA, Vandenbroucke-Grauls CM, Persoons MC, Savelkoul PH. 2009. Streptococcus pneumoniae DNA load in blood as a marker of infection in patients with community-acquired pneumonia. J. Clin. Microbiol. 47:3308–3312. 10.1128/JCM.01071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kee C, Palladino S, Kay I, Pryce TM, Murray R, Rello J, Gallego M, Lujan M, Muñoz-Almagro C, Waterer GW. 2008. Feasibility of real-time polymerase chain reaction in whole blood to identify Streptococcus pneumoniae in patients with community-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 61:72–75. 10.1016/j.diagmicrobio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Kee C, Fatovich DM, Palladino S, Kay ID, Pryce TM, Flexman J, Murray R, Waterer GW. 2010. Specificity of a quantitative real-time polymerase chain reaction assay for the detection of invasive pneumococcal disease: identifying Streptococcus pneumoniae using quantitative polymerase chain reaction. Chest 137:243–244. 10.1378/chest.09-2185. [DOI] [PubMed] [Google Scholar]
- 47.van Haeften R, Palladino S, Kay I, Keil T, Heath C, Waterer GW. 2003. A quantitative LightCycler PCR to detect Streptococcus pneumoniae in blood and CSF. Diagn. Microbiol. Infect. Dis. 47:407–414. 10.1016/S0732-8893(03)00129-9. [DOI] [PubMed] [Google Scholar]
- 48.Carrol ED, Guiver M, Nkhoma S, Mankhambo LA, Marsh J, Balmer P, Banda DL, Jeffers G, IPD Study Group. White SA, Molyneux EM, Molyneux ME, Smyth RL, Hart CA. 2007. High pneumococcal DNA loads are associated with mortality in Malawian children with invasive pneumococcal disease. The Pediatric Infectious Disease J. 26:416–422. 10.1097/01.inf.0000260253.22994.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Werner AS, Cobbs CG, Kaye D, Hook EW. 1967. Studies on the bacteremia of bacterial endocarditis. JAMA 202:199–203. 10.1001/jama.1967.03130160073013. [DOI] [PubMed] [Google Scholar]
- 50.Kreger BE, Craven DE, Carling PC, McCabe WR. 1980. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am. J. Med. 68:332–343. [DOI] [PubMed] [Google Scholar]
- 51.Henry NK, McLimans CA, Wright AJ, Thompson RL, Wilson WR, Washington JA., Jr 1983. Microbiological and clinical evaluation of the isolator lysis-centrifugation blood culture tube. J. Clin. Microbiol. 17:864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waterer G, Rello J. 2011. Why should we measure bacterial load when treating community-acquired pneumonia? Curr. Opin. Infect. Dis. 24:137–141. 10.1097/QCO.0b013e328343b70d. [DOI] [PubMed] [Google Scholar]
- 53.Øvstebø R, Brandtzaeg P, Brusletto B, Haug KBF, Lande K, Høiby EA, Kierulf P. 2004. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J. Clin. Microbiol. 42:2980–2987. 10.1128/JCM.42.7.2980-2987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez KK, Olsen RJ, Musick WL, Cernoch PL, Davis JR, Land GA, Peterson LE, Musser JM. 2013. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch. Pathol. Lab. Med. 137:1247–1254. [DOI] [PubMed] [Google Scholar]
- 55.Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, Collins CD, Nagel JL. 2013. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin. Infect. Dis. 57:1237–1245. 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 56.Davies J, Gordon CL, Tong SYC, Baird RW, Davis JS. 2012. Impact of results of a rapid Staphylococcus aureus diagnostic test on prescribing of antibiotics for patients with clustered Gram-positive cocci in blood cultures. J. Clin. Microbiol. 50:2056–2058. 10.1128/JCM.06773-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sango A, McCarter YS, Johnson D, Ferreira J, Guzman N, Jankowski CA. 2013. Stewardship approach for optimizing antimicrobial therapy through use of a rapid microarray assay on blood cultures positive for Enterococcus species. J. Clin. Microbiol. 51:4008–4011. 10.1128/JCM.01951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farrell JJ, Sampath R, Ecker D, Bonomo RA. 2013. “Salvage microbiology”: detection of bacteria directly from clinical specimens following initiation of antimicrobial treatment. PLoS One 8:e66349. 10.1371/journal.pone.0066349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farrell JJ, Tsung AJ, Flier L, Martinez DL, Beam SB, Chen C, Lowery KS, Sampath R, Bonomo RA. 2013. PCR and electrospray ionizationmass spectrometry (PCR/ESI-MS): detection of persistent Enterococcus faecalis in cerebrospinal fluid following treatment of post-operative ventriculitis. J. Clin. Microbiol. 51:3464–3466. 10.1128/JCM.01343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]




