Abstract
Rothia spp. are Gram-positive cocco-bacilli that cause a wide range of serious infections, especially in immunocompromised hosts. Risk factors for Rothia mucilaginosa (previously known as Stomatococcus mucilaginosus) bacteremia include prolonged and profound neutropenia, malignancy, and an indwelling vascular foreign body. Here, we describe 67 adults at the Mayo Clinic in Rochester, MN, from 2002 to 2012 with blood cultures positive for Rothia. Twenty-five of these patients had multiple positive blood cultures, indicating true clinical infection. Among these, 88% (22/25) were neutropenic, and 76% (19/25) had leukemia. Common sources of bacteremia were presumed gut translocation, mucositis, and catheter-related infection. One patient died with Rothia infection. Neutropenic patients were less likely to have a single positive blood culture than were nonneutropenic patients. Antimicrobial susceptibility testing was performed on 21% of the isolates. All of the tested isolates were susceptible to vancomycin and most beta-lactams; however, four of six tested isolates were resistant to oxacillin. There was no difference between the neutropenic and nonneutropenic patients in need of intensive care unit care, mortality, or attributable mortality.
INTRODUCTION
Rothia mucilaginosa, previously known as Stomatococcus mucilaginosus, and other Rothia species (R. dentocariosa, R. aeria, R. nasimurium, and R. amarae) are part of the normal flora of the human oropharynx and upper respiratory tract (1). They are aerobic or facultatively anaerobic nonmotile non-spore-forming Gram-positive cocco-bacilli that can form filamentous branches (1). Rothia spp. are commonly associated with dental caries and periodontal disease (1). Invasive disease does occur, predominantly in immunocompromised hosts, but has rarely been reported in healthy hosts. The clinical syndromes associated with Rothia infection have included bacteremia (2), endocarditis (3), meningitis (4), peritonitis (5), bone and joint infections (1), pneumonia (6), skin and soft tissue infection, endophthalmitis (7), and prosthetic device infection (3). The main risk factors described for invasive disease have been hematological malignancy and severe neutropenia (8); other risk factors include diabetes mellitus, alcoholism, chronic liver disease, and infection with HIV (3). The clinical significance of Rothia spp. isolated from a blood culture is frequently unclear, especially in the setting of a single positive blood culture set with polymicrobial infection, suggesting contamination. To our knowledge, no large systematic evaluation of invasive Rothia infections has been published to date. We sought to describe the epidemiology and clinical significance of Rothia bacteremia in the past decade at our institution and to evaluate the differences in clinical outcomes between neutropenic and nonneutropenic patients. The other aim of our study was to describe the antimicrobial susceptibility pattern of Rothia isolates at our institution.
MATERIALS AND METHODS
We conducted a single-center retrospective cohort study of adult patients with blood cultures positive for Rothia between January 2002 and December 2012 at the Mayo Clinic in Rochester, MN. The protocol was approved by the Mayo Clinic Institutional Review Board. Cases were obtained by querying the microbiology records for Rothia spp. or Stomatococcus spp. grown from blood culture from 1 January 2002 to 31 December 2012 among patients presenting for medical care at the Mayo Clinic. Patients were excluded from analysis if they were younger than 18 years of age or did not consent to the use of their medical records for research purposes.
The medical records of patients included for analysis were manually reviewed for patient demographics, medical comorbidities, antimicrobial exposure within the prior month, clinical outcomes, and microbiological data, including antimicrobial susceptibilities. The source of bloodstream infection was determined through a review of the medical records for a suspected source as documented by the attending infectious diseases physician (preferred) or by the attending physician of the primary service, if infectious diseases consultation was not involved in the patient's care. All documented potential sources were abstracted. If a suspected source was not documented, then the source was categorized as “no source identified.” Attributable mortality was determined by two of the investigators (P.R. and P.K.T.), who reviewed the medical records of all patients who died with Rothia bacteremia. A case of mortality was considered nonattributable if a clear alternative cause of mortality was identified.
The Charlson comorbidity index score was used to assess the severity of underlying diseases (9). Analyses of microbiologic characteristics, including species identification and antimicrobial susceptibility, were performed on all the isolates recovered from blood culture. Analyses of clinical characteristics were performed on those with blood cultures positive for Rothia spp. from more than one set of blood cultures to minimize the inclusion of potential contaminants. A descriptive analysis was performed, as was an analysis to compare patients who were neutropenic (absolute neutrophil count, ≤1,000/μl) at the time of bacteremia to those who were not neutropenic and to compare those with monomicrobial infection to those with polymicrobial infection. Two-tailed Fisher's exact and Kruskal-Wallis tests were used for comparisons of proportions and medians, respectively, with P values of <0.05 considered statistically significant. At our institution, blood samples are cultured using the Bactec instrumented blood culture system (Becton, Dickinson, and Company, Franklin Lakes, NJ, USA). Antimicrobial susceptibilities were determined using agar dilution with isolates from a pure subculture. The clinical microbiology laboratory at the Mayo Clinic uses CLSI breakpoints for Corynebacterium for reporting the susceptibilities of Rothia isolates. Most of the Rothia isolates were identified using morphological and biochemical properties; recent isolates were identified using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS).
RESULTS
After excluding 5 patients younger than 18 years of age, we identified 67 adults from 2002 to 2012 with blood cultures positive for Rothia; for 42 patients, Rothia grew from a single blood culture set and was considered potentially contaminated, and the remaining 25 patients were considered to have a true bloodstream infection (Fig. 1). All 67 patients identified with blood cultures that were positive for Rothia had at least two sets of blood cultures drawn at the same time, with at least one being from the periphery; for all of the cases in which only one set was positive for Rothia, there was a second blood culture set drawn at the same time from which Rothia did not grow. Neutropenic patients were significantly less likely to have a single positive blood culture set than were nonneutropenic patients (41% versus 90%, P < 0.001). The demographic, clinical, and microbiological characteristics and clinical outcomes of the patients considered to have had true bloodstream infection are summarized in Table 1. Twenty-two (88%) patients were neutropenic with a median of 9.5 days of neutropenia (interquartile range, 7 to 20 days) at the time of Rothia bloodstream infection, and 19 (76%) had an underlying diagnosis of leukemia.
FIG 1.
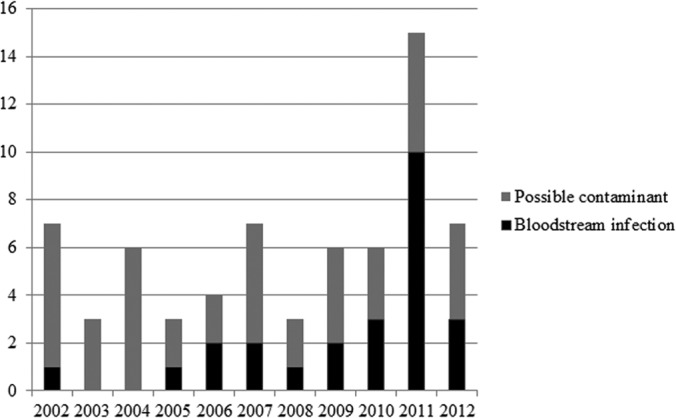
Distribution of patients with Rothia bacteremia per year at the Mayo Clinic in Rochester, MN (2002-2012).
TABLE 1.
Demographic and clinical characteristics and clinical outcomes of patients with Rothia bloodstream infections at Mayo Clinic, Rochester, MN (2002–2012)
| Variable | Dataa for: |
P value | ||
|---|---|---|---|---|
| All patients (n = 25) | Neutropenic patients (n = 22) | Nonneutropenic patients (n = 3) | ||
| Age (median [range]) (yr) | 55 (22–78) | 60 (22–78) | 30 (28–48) | 0.03 |
| Male sex | 19 (76) | 18 (82) | 1 (33) | 0.2 |
| Comorbidities | ||||
| Myocardial infarction | 2 (8) | 2 (9) | 0 (0) | 1.0 |
| Congestive heart failure | 1 (4) | 0 (0) | 1 (33) | 0.12 |
| Peripheral vascular disease | 1 (4) | 1 (5) | 0 (0) | 1.0 |
| Chronic lung disease | 1 (4) | 1 (5) | 0 (0) | 1.0 |
| Connective tissue disease | 1 (4) | 1 (5) | 0 (0) | 1.0 |
| Diabetes mellitus | 2 (8) | 2 (9) | 0 (0) | 1.0 |
| Solid tumor | 3 (12) | 3 (14) | 0 (0) | 1.0 |
| Leukemia | 19 (76) | 19 (86) | 0 (0) | 0.009 |
| Lymphoma/multiple myeloma | 4 (16) | 4 (18) | 0 (0) | 1.0 |
| Moderate to severe liver disease | 1 (4) | 1 (5) | 0 (0) | 1.0 |
| Moderate to severe kidney disease | 2 (8) | 2 (9) | 0 (0) | 1.0 |
| Hemodialysis | 1 (4) | 1 (5) | 0 (0) | 1.0 |
| Hematopoietic stem cell transplant | 6 (24) | 6 (27) | 0 (0) | 1.0 |
| Allogeneic | 4 (16) | 4 (18) | 0 (0) | 1.0 |
| Autologous | 2 (8) | 2 (9) | 0 (0) | 1.0 |
| Charlson comorbidity index | ||||
| 0 | 1 (4) | 0 (0) | 1 (33) | 0.002 |
| 1 | 1 (4) | 0 (0) | 1 (33) | |
| 2 | 3 (12) | 3 (14) | 0 (0) | |
| 3 | 3 (12) | 3 (14) | 0 (0) | |
| 4 | 3 (12) | 3 (14) | 0 (0) | |
| 5 | 8 (32) | 8 (36) | 0 (0) | |
| 6 | 1 (4) | 0 (0) | 1 (33) | |
| 7 | 5 (20) | 5 (23) | 0 (0) | |
| Medication use within 30 days prior to bacteremia | ||||
| Corticosteroid | 2 (8) | 2 (9) | 0 (0) | 1.0 |
| Antimicrobial | 25 (100) | 22 (100) | 3 (100) | 1.0 |
| Levofloxacin | 21 (84) | 20 (91) | 1 (33) | 0.06 |
| Penicillin | 3 (12) | 3 (14) | 0 (0) | 1.0 |
| Presence of central venous catheter at the time of bacteremia | 24 (96) | 22 (100) | 2 (67) | 0.12 |
| Source of bloodstream infection | ||||
| Presumed gut translocation with negative CT of abdomen | 12 (48) | 9 (41) | 3 (100) | 0.28 |
| Presumed gut translocation without a CT of abdomen | 1 (4) | 1 (5) | 0 (0) | 1.0 |
| Neutropenic colitis with consistent CT of abdomen | 1 (4) | 1 (5) | 0 (0) | 1.0 |
| Catheter-related bloodstream infection | 8 (32) | 6 (27) | 2 (66) | 0.23 |
| Endocarditis | 1 (4) | 0 (0) | 1 (33) | 0.12 |
| Dental abscess | 2 (8) | 2 (9) | 0 (0) | 1.0 |
| Mucositis | 9 (36) | 9 (41) | 0 (0) | 0.28 |
| No source identified | 5 (20) | 5 (23) | 0 (0) | 1.0 |
| Presence of polymicrobial infection | 9 (36) | 8 (36) | 1 (33) | 1.0 |
| Antimicrobial susceptibility test performed | 6 (24) | 6 (27) | 0 (0) | 1.0 |
| Duration of hospitalization (mean [range]) (days) | 21 (1–53) | 24 (1–53) | 12 (6–21) | 0.32 |
| Need for ICU care | 11 (44) | 10 (45) | 1 (33) | 1.0 |
| Complications | ||||
| Septic shock | 3 (12) | 3 (14) | 0 (0) | 1.0 |
| Respiratory failure | 3 (12) | 3 (14) | 0 (0) | 1.0 |
| Liver failure | 1 (4) | 1 (3) | 0 (0) | 1.0 |
| Renal failure | 3 (12) | 2 (9) | 1 (33) | 0.33 |
| Altered mental status | 2 (8) | 2 (9) | 0 (0) | 1.0 |
| Attributable mortality | 1 (4) | 1 (3) | 0 (0) | 1.0 |
| Antimicrobial treatment | ||||
| Cefepime | 9 (36) | 9 (41) | 0 (0) | 0.28 |
| Ceftriaxone | 1 (4) | 1 (4) | 0 (0) | 1.0 |
| Piperacillin-tazobactam | 5 (20) | 5 (23) | 0 (0) | 1.0 |
| Ertapenem | 3 (12) | 3 (14) | 0 (0) | 1.0 |
| Meropenem | 4 (16) | 4 (18) | 0 (0) | 1.0 |
| Daptomycin | 3 (12) | 2 (9) | 1 (33) | 0.33 |
| Linezolid | 2 (8) | 2 (9) | 0 (0) | 1.0 |
| Vancomycin | 20 (80) | 18 (82) | 2 (67) | 0.50 |
| Duration of antimicrobial treatment (median [range]) (days) | 14 (2–42) | 14 (2–28) | 30 (10–42) | 0.17 |
Data are no. (%) unless stated otherwise.
The most common sources of Rothia bloodstream infection were presumed gut translocation (n = 13, 52%), catheter-related infection (n = 8, 25%), and mucositis (n = 9, 36%). No source of bacteremia was identified for 5 (20%) of the patients.
A comparison of the clinical characteristics and outcomes of patients with monomicrobial infection (n = 16) and those with polymicrobial infection (n = 9) identified no qualitative or statistically significant differences between the two groups for any of the variables collected, including median age (56 years versus 53 years, P = 0.93), recent corticosteroid use (6% versus 11%, P = 1.0), presence of neutropenia (88% versus 89%, P = 1.0), ability to perform susceptibility testing (20% versus 33%, P = 0.63), median duration of hospital stay (20 days versus 21 days, P = 0.32), median duration of antimicrobial treatment (14 days versus 14 days, P = 0.57), need for intensive care unit (ICU) care (44% versus 44%, P = 1.0), or attributable mortality (0% versus 11%, P = 0.36). Of the nine polymicrobial Rothia bloodstream infections, five cases had additional growth of coagulase-negative staphylococci (three attributed to central line-related bloodstream infection, one attributed to presumed gut translocation, and one for which a source was not identified), one case had additional growth of viridans group streptococci attributed to presumed gut translocation, one case had additional growth of Enterococcus faecium for which a source was not identified, one case had additional growth of Candida dubliniensis attributed to central line-related bloodstream infection, and one case had additional growth of Clostridium inoculum attributed to presumed gut translocation.
We identified one patient with a Rothia bloodstream infection who died, potentially as a result of the infection. The patient was a 51-year-old man who developed acute abdominal pain, fever, and hypotension in the setting of profound neutropenia 4 days after allogeneic peripheral blood stem cell transplantation for multiple myeloma. Intravenous vancomycin, cefepime, metronidazole, fluconazole, and acyclovir were started empirically, and the patient was transferred to the ICU, where he required ventilatory support, vasopressor support, and dialysis. The blood culture bottles from all the sets were positive for vancomycin-susceptible Enterococcus faecium and Rothia mucilaginosa resistant to oxacillin (no other antimicrobial susceptibility tests were performed) without any significant differential time to positivity between peripherally drawn cultures and those drawn from the central venous catheter. He was felt to be too unstable to transport for computed tomography (CT) imaging of his abdomen. Despite maximal support, the patient developed cardiac arrest and died within 48 h of his initial presentation. An autopsy was not performed.
We also identified a 28-year-old woman with Rothia prosthetic aortic valve endocarditis (monomicrobial) who had a history of intravenous drug use and prior native tricuspid and aortic valve endocarditis with methicillin-susceptible Staphylococcus aureus. The diagnosis was confirmed with a transesophageal echocardiogram. There was not adequate growth to perform susceptibility testing, and the patient was treated with a 6-week course of intravenous ceftriaxone and vancomycin and with oral rifampin; 1 year after the completion of therapy, there was clinical resolution of the infection and no evidence of relapsed disease.
All of the 25 clinical bloodstream infections involved Rothia mucilaginosa, although one of the isolates from a patient with a single positive blood culture set (possible contaminant) involved Rothia dentocariosa. Due to poor growth of the organism on Mueller-Hinton agar (supplemented with 5% lysed horse blood), we were able to perform susceptibility testing on only 14 (21%) of the total 67 isolates and on 6 (24%) of the 25 isolates from true bloodstream infection. There was no significant association between the ability to grow the organism for susceptibilities and whether or not the patient received penicillin (40% versus 20%, P = 0.29), levofloxacin (25% versus 18%, P = 0.55), cefepime (9% versus 24%, P = 0.43), or vancomycin (14% versus 23%, P = 0.72) within 30 days prior to bacteremia. All tested isolates were susceptible to penicillin (9/9), ceftriaxone (8/8), ertapenem (2/2), meropenem (8/8), and vancomycin (13/13). Four isolates were resistant to oxacillin (4/6), but concomitant penicillin susceptibility testing was not performed on them.
DISCUSSION
We conducted a retrospective review of all adult patients with blood cultures positive for Rothia in a single academic institution during the past decade and have described the epidemiology and clinical characteristics of Rothia bacteremia. In addition, we performed a comparative analysis of clinical outcomes between neutropenic and nonneutropenic patients and between monomicrobial and polymicrobial infections. Rothia bloodstream infections occurred often in patients with significant medical comorbidities, most commonly hematologic malignancy. A majority of the patients were exposed to at least one antimicrobial agent (predominantly a fluoroquinolone) within the month preceding the infection. Most of these patients had an indwelling central venous catheter at the time of bacteremia, most likely related to the need for central venous access for the administration of chemotherapeutic agents (for patients with hematologic malignancy). There was no apparent temporal change in the incidence of bacteremia in the past decade, although a transient increase was noted in 2011; the reason for this transient increase is not clear, as there were no notable changes in patient management or concomitant increases in central line-related infections elsewhere in our institution. In this study, Rothia mucilaginosa caused all of the clinical bloodstream infections. Gut translocation was the most commonly identified source of Rothia bloodstream infection, although central line-related infections and mucositis were also common. There were no differences detected in clinical characteristics or clinical outcomes between those with monomicrobial and those with polymicrobial Rothia bloodstream infection.
When encountering Rothia bacteremia in clinical practice, many clinicians are faced with the challenge of deciding whether it represents a true bloodstream infection or contamination; 63% of the Rothia isolates in our study were potential contaminants since they grew from a single positive blood culture. Transient Rothia bacteremia has been reported in the literature, and its clinical significance remains unknown (10). In our study, neutropenic patients were less likely to have single blood culture set positivity than were nonneutropenic patients. This indicates that when Rothia bacteremia is identified in neutropenic patients, it is likely to represent true infection. We did find that the vast majority of neutropenic patients had an underlying diagnosis of leukemia and had prolonged and profound neutropenia at the time they were diagnosed with Rothia bacteremia. Potential reasons for this predilection in patients with leukemia include a higher preponderance of mucositis due to the chemotherapeutic agents used to treat the underlying disease and prolonged duration of chemotherapy-induced neutropenia. Furthermore, the vast majority of patients undergoing chemotherapy for leukemia at our institution receive levofloxacin prophylaxis during neutropenia, so there may be a shift in the oral and gastrointestinal flora away from aerobic Gram-negative bacilli and toward other pathogens such as Rothia. The presence of polymicrobial Rothia bloodstream infection did not appear to be associated with higher-risk clinical characteristics or portend less favorable clinical outcomes than those with monomicrobial infection. We did not record whether the other organisms were identified in more than one blood culture set, raising the possibility that they were contaminants, but the Rothia species were identified in more than one blood culture set, which argues against its presence being a result of contamination.
Rothia (Stomatococcus mucilaginosa) bacteremia in neutropenic patients was first described in the 1990s (8, 11, 12). Ascher et al. described 10 patients with Rothia mucilaginosa bacteremia, 5 of whom had more than one positive blood culture. Among these 5 patients, 3 were neutropenic and had malignancies; all of them had an indwelling vascular foreign body. Most patients recovered with vancomycin (11). Henwick et al. characterized 8 cases of Rothia mucilaginosa bacteremia in children with cancer; 6 of them had leukemia, 7 had profound neutropenia, 4 had mucositis, and 5 had central venous catheters. Despite the prompt initiation of antibiotics, the rate of complications in this cohort were high (eg, septic shock [50%], pneumonia, altered mental status, meningitis, and acute respiratory distress syndrome). All the isolates were susceptible to vancomycin, but 50% were penicillin resistant and 29% were methicillin resistant (12). Fanourgiakis et al. described 8 patients with Rothia mucilaginosa bacteremia, the majority of whom (7/8) had hematological malignancies (6 leukemia); 1 had breast cancer. All these patients had profound neutropenia and chemotherapy-induced disruption in the oral or gut mucosal barrier. All of the patients were on quinolone prophylaxis at the time of bacteremia; 5 of 6 tested isolates were quinolone resistant (13). One patient in our study expired potentially as a result of Rothia infection, despite organism identification, prompt appropriate antimicrobial initiation, and intense supportive management. He possessed many of the previously described risk factors for Rothia infection, demonstrating the pathogenicity of Rothia despite it normally being considered a benign and colonizing organism. However, the case description suggests that the patient may have died due to catastrophic gut wall breach with resultant polymicrobial bloodstream infection rather than from the pathogenicity of Rothia bloodstream infection itself. The mortality rates attributable to Rothia infections have varied in the literature according to age, immune status, and site of infection (14–16). Immunocompromised patients are more likely to develop severe complications, including death, from Rothia infections. To our knowledge, ours is the largest published cohort of patients with Rothia bacteremia. In prior publications, Rothia mucilaginosa isolates were generally susceptible to most beta-lactam antimicrobials (penicillin, ampicillin, imipenem, cefotaxime), rifampin, and vancomycin (17). However, isolates with partial resistance to penicillin have been described in the past (17). In one study, the incidences of penicillin and methicillin resistance among isolates were 50% and 29%, respectively (12). Antimicrobial susceptibility testing was not performed on most of the Rothia isolates in our study, owing to their poor growth in vitro, even when the growth medium was supplemented with 5% lysed horse blood. When testing was possible, all of the isolates from our study were susceptible to penicillin, ceftriaxone, meropenem, and vancomycin; however, four of six isolates were resistant to oxacillin. The reasons for this pattern of susceptibility are not clear.
The reason for the preponderance of cases in our study receiving vancomycin as part of dual therapy is likely because most of the patients had Rothia bloodstream infection in the setting of febrile neutropenia. Vancomycin is a recommended empirical antimicrobial agent (in combination with an antipseudomonal beta-lactam antimicrobial) for the treatment of neutropenic fever with Gram-positive bloodstream infection (18). Upon clearance of the bloodstream infection and identification of the causative organism, clinicians may have been inclined to continue with vancomycin, especially if susceptibility data were not available. The results of our study suggest that the addition of vancomycin to neutropenic fever therapy when Gram-positive bloodstream infection is identified would provide appropriate coverage for Rothia infections and that ceftriaxone is likely to be an effective definitive antimicrobial agent in a clinical setting when patients have clinically improved but antimicrobial susceptibility results are not available.
Further research is needed to develop microbiologic techniques to improve our ability to provide antimicrobial susceptibility results in cases of Rothia infection. Additionally, the potential role of fluoroquinolone prophylaxis in shifting oral and gastrointestinal flora in patients undergoing chemotherapy for hematologic malignancy needs further exploration.
In conclusion, members of the genus Rothia, despite their low virulence, have established themselves as significant pathogens, especially in patients with hematological malignancies and neutropenia. Mucositis and central venous catheters are common predisposing factors, and both of these factors are related to treatment for hematologic malignancy. Neutropenic patients are more likely to have true bloodstream infection as evidenced by multiple positive blood culture sets and monomicrobial infection. There was no significant difference in clinical outcomes between the neutropenic and nonneutropenic patients. At this time, there are limited data available on the antimicrobial susceptibility patterns of Rothia; however, isolates are generally susceptible to vancomycin and beta-lactam antimicrobials, except oxacillin.
ACKNOWLEDGMENT
We have no relevant financial disclosures or funding to declare.
Footnotes
Published ahead of print 20 June 2014
REFERENCES
- 1.Trivedi MN, Malhotra P. Rothia prosthetic knee joint infection. J. Microbiol. Immunol. Infect. in press. 10.1016/j.jmii.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Vaccher S, Cordiali R, Osimani P, Manso E, de Benedictis FM. 2007. Bacteremia caused by Rothia mucilaginosa in a patient with Shwachman-Diamond syndrome. Infection 35:209–210. 10.1007/s15010-007-6284-8. [DOI] [PubMed] [Google Scholar]
- 3.Bruminhent J, Tokarczyk MJ, Jungkind D, Desimone JA., Jr 2013. Rothia mucilaginosa prosthetic device infections: a case of prosthetic valve endocarditis. J. Clin. Microbiol. 51:1629–1632. 10.1128/JCM.03173-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AB, Harker-Murray P, Ferrieri P, Schleiss MR, Tolar J. 2008. Bacterial meningitis from Rothia mucilaginosa in patients with malignancy or undergoing hematopoietic stem cell transplantation. Pediatr. Blood Cancer 50:673–676. 10.1002/pbc.21286. [DOI] [PubMed] [Google Scholar]
- 5.Keng TC, Ng KP, Tan LP, Chong YB, Wong CM, Lim SK. 2012. Rothia dentocariosa repeat and relapsing peritoneal dialysis-related peritonitis: a case report and literature review. Ren. Fail. 34:804–806. 10.3109/0886022X.2012.678208. [DOI] [PubMed] [Google Scholar]
- 6.Cho EJ, Sung H, Park SJ, Kim MN, Lee SO. 2013. Rothia mucilaginosa pneumonia diagnosed by quantitative cultures and intracellular organisms of bronchoalveolar lavage in a lymphoma patient. Ann. Lab. Med. 33:145–149. 10.3343/alm.2013.33.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partner AM, Bhattacharya S, Scott RA, Stavrou P. 2006. Rothia genus endophthalmitis following penetrating injury in a child. Eye 20:502–503. 10.1038/sj.eye.6701902. [DOI] [PubMed] [Google Scholar]
- 8.McWhinney PH, Kibbler CC, Gillespie SH, Patel S, Morrison D, Hoffbrand AV, Prentice HG. 1992. Stomatococcus mucilaginosus: an emerging pathogen in neutropenic patients. Clin. Infect. Dis. 14:641–646. 10.1093/clinids/14.3.641. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Kaufhold A, Reinert RR, Kern W. 1992. Bacteremia caused by Stomatococcus mucilaginosus: report of seven cases and review of the literature. Infection 20:213–220. 10.1007/BF02033062. [DOI] [PubMed] [Google Scholar]
- 11.Ascher DP, Zbick C, White C, Fischer GW. 1991. Infections due to Stomatococcus mucilaginosus: 10 cases and review. Rev. Infect. Dis. 13:1048–1052. 10.1093/clinids/13.6.1048. [DOI] [PubMed] [Google Scholar]
- 12.Henwick S, Koehler M, Patrick CC. 1993. Complications of bacteremia due to Stomatococcus mucilaginosus in neutropenic children. Clin. Infect. Dis. 17:667–671. 10.1093/clinids/17.4.667. [DOI] [PubMed] [Google Scholar]
- 13.Fanourgiakis P, Georgala A, Vekemans M, Daneau D, Heymans C, Aoun M. 2003. Bacteremia due to Stomatococcus mucilaginosus in neutropenic patients in the setting of a cancer institute. Clin. Microbiol. Infect. 9:1068–1072 14616756. 10.1046/j.1469-0691.2003.00772.x. [DOI] [PubMed] [Google Scholar]
- 14.Chavan RS, Pannaraj PS, Luna RA, Szabo S, Adesina A, Versalovic J, Krance RA, Kennedy-Nasser AA. 2013. Significant morbidity and mortality attributable to Rothia mucilaginosa infections in children with hematological malignancies or following hematopoietic stem cell transplantation. Pediatr. Hematol. Oncol. 30:445–454. 10.3109/08880018.2013.783893. [DOI] [PubMed] [Google Scholar]
- 15.Goldman M, Chaudhary UB, Greist A, Fausel CA. 1998. Central nervous system infections due to Stomatococcus mucilaginosus in immunocompromised hosts. Clin. Infect. Dis. 27:1241–1246. 10.1086/515001. [DOI] [PubMed] [Google Scholar]
- 16.Korsholm TL, Haahr V, Prag J. 2007. Eight cases of lower respiratory tract infection caused by Stomatococcus mucilaginosus. Scand. J. Infect. Dis. 39:913–917. 10.1080/00365540701387064. [DOI] [PubMed] [Google Scholar]
- 17.von Eiff C, Herrmann M, Peters G. 1995. Antimicrobial susceptibilities of Stomatococcus mucilaginosus and of Micrococcus spp. Antimicrob. Agents Chemother. 39:268–270. 10.1128/AAC.39.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 52:427–431. 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]


