Abstract
Effective and easy-to-use methods for detecting Clostridium difficile spore contamination would be useful for identifying environmental reservoirs and monitoring the effectiveness of room disinfection. Culture-based detection methods are sensitive for detecting C. difficile, but their utility is limited due to the requirement of anaerobic culture conditions and microbiological expertise. We developed a low-cost selective broth medium containing thioglycolic acid and l-cystine, termed C. difficile brucella broth with thioglycolic acid and l-cystine (CDBB-TC), for the detection of C. difficile from environmental specimens under aerobic culture conditions. The sensitivity and specificity of CDBB-TC (under aerobic culture conditions) were compared to those of CDBB (under anaerobic culture conditions) for the recovery of C. difficile from swabs collected from hospital room surfaces. CDBB-TC was significantly more sensitive than CDBB for recovering environmental C. difficile (36/41 [88%] versus 21/41 [51%], respectively; P = 0.006). C. difficile latex agglutination, an enzyme immunoassay for toxins A and B or glutamate dehydrogenase, and a PCR for toxin B genes were all effective as confirmatory tests. For 477 total environmental cultures, the specificity of CDBB-TC versus that of CDBB based upon false-positive yellow-color development of the medium without recovery of C. difficile was 100% (0 false-positive results) versus 96% (18 false-positive results), respectively. False-positive cultures for CDBB were attributable to the growth of anaerobic non-C. difficile organisms that did not grow in CDBB-TC. Our results suggest that CDBB-TC provides a sensitive and selective medium for the recovery of C. difficile organisms from environmental samples, without the need for anaerobic culture conditions.
INTRODUCTION
Contaminated environmental surfaces are an important source of Clostridium difficile transmission (1). However, many studies have demonstrated that environmental disinfection is often suboptimal in health care facilities (1–3). Effective and easy-to-use methods for the detection of spore contamination would be useful for improving environmental disinfection interventions by identifying environmental reservoirs and providing a means to monitor the effectiveness of disinfection (2). Commercial real-time PCR assays are easy to use and widely available. However, commercial PCR assays may not be sufficiently sensitive for detecting low levels of environmental contamination (4). Culture-based detection methods are sensitive for detecting C. difficile, but their utility is hindered by the requirement of anaerobic culture conditions and microbiological expertise. Recently, Curry et al. (5) demonstrated that combining selective anaerobic broth culture preamplification with a confirmatory real-time PCR assay for toxin genes provided a sensitive method for detecting asymptomatic carriers of C. difficile. This approach can be used for the detection of environmental contamination, but its limitations include the need for anaerobic culture conditions and the expense of the PCR assay.
Thioglycolic acid and l-cystine are reducing agents that consume oxygen in medium and facilitate the growth of obligate anaerobes (6). Here, we tested the hypothesis that a modified selective broth culture medium containing thioglycolic acid and l-cystine as reducing agents would provide a sensitive and selective method for the recovery of C. difficile from environmental specimens under aerobic culture conditions. We evaluated the utility of a commercial PCR assay for toxin B genes, an enzyme immunoassay for toxins A and B, an enzyme immunoassay for glutamate dehydrogenase, or a C. difficile latex agglutination assay as confirmatory tests for positive broth specimens. Our goal was to develop an effective assay that would be inexpensive and easy to use.
MATERIALS AND METHODS
C. difficile strains.
Two C. difficile strains were studied. Strain VA 17 is an epidemic restriction endonuclease analysis (REA) type BI strain, and strain VA 11 is an REA J-type strain.
Preparation of spores.
The spores were prepared by growth on Duncan-Strong agar medium, as previously described (7). The spores were stored at 4°C in sterile distilled water until use. The spores were confirmed by phase-contrast microscopy and malachite green staining to be >98% free of vegetative cells or cell debris.
Modified medium for culture of C. difficile in room air.
The base medium that was modified for the purposes of this study was C. difficile brucella broth (CDBB). Under anaerobic conditions, CDBB was previously shown to stimulate the germination and outgrowth of C. difficile spores at a rate comparable to that with cycloserine-cefoxitin-fructose broth, and an agar formulation was as sensitive and selective as cycloserine-cefoxitin-fructose agar for the recovery of C. difficile from stool specimens (7). The modified broth culture medium devised for the growth of C. difficile in room air was termed C. difficile brucella broth with thioglycolic acid and l-cystine (CDBB-TC). Table 1 shows the ingredients for CDBB-TC. For CDBB-TC, brucella broth powder (Sigma-Aldrich, St. Louis, MO), vitamin K1, hemin, sodium bicarbonate, agar, mannitol, neutral red solution prepared in absolute ethanol, thioglycolic acid (mercaptoacetic acid), l-cystine, and agar were prepared in 1,000 ml of distilled water (7). Mannitol and neutral red were added in order to distinguish C. difficile colonies by the typical change in color to yellow associated with the fermentation of mannitol. The medium was adjusted to pH 7.6, autoclaved at 121°C for 15 min, and cooled to 50°C in a water bath. Sterile solutions of cycloserine, cefoxitin, sodium taurocholate, and lysozyme were prepared in distilled water and added to the base broth. The sensitivity of the medium decreased modestly after 2 weeks of storage in polystyrene tubes but was restored by brief boiling. The base medium was thus boiled prior to use if >2 weeks had passed since the medium was prepared; antibiotic-containing medium was not boiled. Lysozyme was included in the culture medium because some studies have suggested that it may stimulate spore germination and increase the recovery of environmental C. difficile spores (8).
TABLE 1.
Formulation of Clostridium difficile brucella broth with thioglycolic acid and l-cysteine
| Ingredient | Quantity/liter |
|---|---|
| Brucella broth | 28.0 g |
| Vitamin K1 solution (1 mg/ml) | 1.0 ml |
| Hemin solution (5 mg/ml) | 1.0 ml |
| Sodium bicarbonate solution (20 mg/ml) | 5.0 ml |
| d-Mannitol | 6.0 g |
| Neutral red solution (1%) | 5.0 ml |
| Sodium taurocholate | 0.5 g |
| Lysozyme | 5.0 mg |
| d-Cycloserine | 500.0 mg |
| Cefoxitin | 16.0 mg |
| Agar | 1.0 g |
| Thioglycolic acid (mercaptoacetic acid) | 1.0 g |
| l-Cystine | 1.0 g |
For environmental cultures, 10 ml of CDBB-TC was added to 15-ml conical polystyrene centrifuge tubes (Thermo Fisher Scientific, Rochester, NY). BD BBL CultureSwabs (Becton, Dickinson, Cockeysville, MD) were applied to the surfaces, and the swab heads were broken off and submersed in the medium. The tubes were capped and placed inside an incubator at 37°C (room temperature) for 72 h.
Germination and outgrowth of C. difficile in CDBB versus CDBB-TC.
To compare the rate and extent of C. difficile spore germination in CDBB-TC versus CDBB, spores of the two test strains (104 CFU/ml) were added to CDBB (anaerobic chamber) or CDBB-TC (room air incubator) and incubated for 24 h at 37°C. The aliquots were removed at 0.5, 4, 6, and 24 h diluted 1:1 in either phosphate-buffered saline (PBS) or absolute ethanol, serially diluted in PBS, and plated on prereduced C. difficile brucella agar (CDBA) plates inside a Whitley Workstation MG-1000 anaerobic chamber (Microbiology International, Frederick, MD) to enumerate the CFU. The ethanol shock method provides a measurement of the spore concentration, whereas the samples diluted in PBS provide a measurement of the total C. difficile count (i.e., spores and vegetative cells). All experiments were performed in triplicate.
Comparison of sensitivity and selectivity of CDBB-TC and CDBB in the laboratory.
In the laboratory, we compared the sensitivity of CDBB-TC under aerobic conditions with that of CDBB under anaerobic conditions inside the anaerobic chamber. Ten-microliter aliquots of serial dilutions of spores of the two test strains in sterile water were applied to polyester swabs (Fisher Scientific, Rochester, NY) that were inoculated into 15 ml of CDBB-TC or prereduced CDBB in 20-ml plastic conical tubes (Fisher Scientific). Agitation was kept to a minimum. After 72 h of incubation at 37°C, specimens with a color change from red to yellow were plated onto prereduced CDBA plates inside the anaerobic chamber to determine if C. difficile was present. The experiments were repeated 9 times.
To assess selectivity, Clostridium sporogenes (strain ATCC 11437), Clostridium perfringens (strain ATCC 131124), and Bacillus subtilis (ATCC 6051) spores and facultative organisms, including Enterococcus faecium (strain C68) (8), methicillin-resistant Staphylococcus aureus (clinical isolate), Staphylococcus warneri (ATCC 14990), and Candida glabrata (ATCC 90030), were incubated in CDBB, CDBB-TC (incubated in room air incubator), and CDBB-TC (incubated inside the anaerobic chamber) at 37°C for 72 h. The samples that underwent a color change from red to yellow were plated onto blood plates to assess the growth of the organisms.
Comparison of sensitivity and selectivity of CDBB-TC and CDBB for detecting environmental contamination on hospital wards.
We evaluated the sensitivity and selectivity of CDBB-TC versus CDBB for detecting environmental contamination. BD BBL CultureSwabs (Becton, Dickinson) with 2 swab prongs were applied to surfaces in C. difficile infection (CDI) patient rooms and on portable equipment. The swabs were premoistened in Dey-Engley neutralizing broth (Remel Products, Lenexa, KS) and applied to a 5 by 10 cm area of the surfaces. One prong was transferred to the anaerobic chamber and inoculated into prereduced CDBB inside the anaerobic chamber, and the other was inoculated into CDBB-TC in room air and placed in an incubator in room air. The cultures were incubated at 37°C for 72 h. All specimens that underwent a color change from red to yellow were plated onto prereduced CDBA inside the anaerobic chamber and incubated for 72 h. Yellow colonies with the typical appearance were streaked for isolation onto blood plates and were confirmed to be C. difficile on the basis of the typical odor and appearance of the colonies and by a positive reaction using a C. difficile latex agglutination assay. For a subset of 18 positive CDBB-TC specimens, confirmatory testing was performed on an aliquot from the bottom of the tube using a commercial PCR assay (Xpert C. difficile; Cepheid, Sunnyvale, CA), enzyme immunoassays (EIA) for glutamate dehydrogenase and toxins A and B, and a C. difficile latex agglutination assay (Microgen Bioproducts, Camberley, United Kingdom) for the detection of C. difficile somatic antigens.
To evaluate false-positive cultures (i.e., cultures that turned yellow but did not grow C. difficile when plated on CDBA), colonies from CDBA that were not consistent with C. difficile based on color and morphology were transferred to blood plates and identified by using the RapID ANA II system (Remel Products, Lenexa, KS) for obligate anaerobes or the Vitek 2 system (bioMérieux, Durham, NC) for facultative organisms; if cultures that turned yellow did not yield growth on CDBA, the medium was plated on blood plates to assess growth. Organisms that were identified were then inoculated into CDBB and CDBB-TC to reassess growth and color change.
Effect of thioglycolic acid on stimulation of germination of C. difficile spores.
To investigate potential explanations for the increased recovery of environmental C. difficile by CDBB-TC compared with that by CDBB, we tested the hypothesis that thioglycolic acid stimulates the germination of C. difficile spores, with the degree of germination based on the pH. Spore germination was compared in sterile water (control) versus sterile water supplemented with 1 mg/ml thioglycolic acid alone or in combination with 1 mg/ml lysozyme and at pH 5 versus 7.6. To assess germination, spores (106 CFU) were added to 1 ml of each solution and incubated in room air at 22°C for 1 h, and then 100-μl aliquots were subjected to heat shock at 80°C for 5 min in a water bath (activated spores are killed at 80°C, whereas nongerminated spores are not). After heat shock, the samples were serially diluted and plated onto prereduced CDBA in the anaerobic chamber at 37°C for 72 h, and the counts were calculated. All experiments were repeated in triplicate.
Data analysis.
Fisher's exact test was used to compare the proportions of cultures positive for C. difficile and non-C. difficile breakthrough growth.
RESULTS
Germination and outgrowth of C. difficile in CDBB versus CDBB-TC.
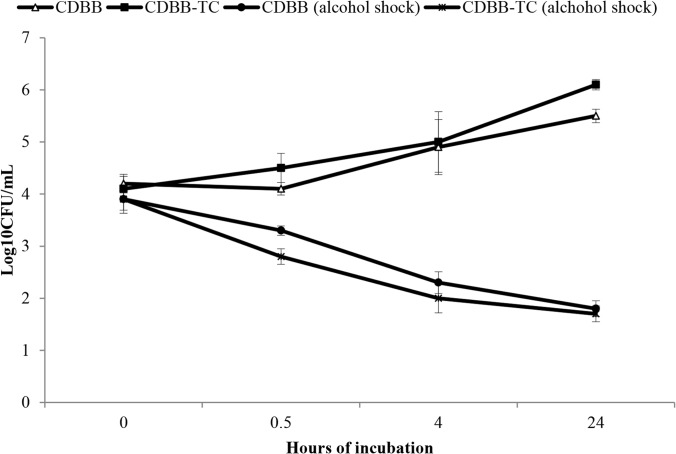
As shown in Fig. 1, the rate and extent of C. difficile spore germination and outgrowth were similar in CDBB-TC and CDBB (Fig. 1). Approximately 90% of the spores (1 log) germinated within 30 min, based upon susceptibility to alcohol.
FIG 1.
Germination and outgrowth of C. difficile spores in C. difficile brucella broth with thioglycolic acid and l-cystine (CDBB-TC) under aerobic culture conditions versus C. difficile brucella broth (CDBB) under anaerobic culture conditions. At time zero, 104 CFU/ml of spores were added to the medium and incubated at 37°C. The aliquots were removed at the specified time points and diluted 1:1 in either phosphate-buffered saline (PBS) or absolute ethanol, serially diluted in PBS, and plated on prereduced selective plates inside an anaerobic chamber. The ethanol-shock method provides a measurement of the spore concentration, whereas the samples diluted in PBS provide a measurement of the total C. difficile count. The data shown are for C. difficile strain VA 17, an epidemic restriction endonuclease analysis (REA) type BI strain; similar results were obtained for C. difficile strain VA 11. Error bars indicate standard errors.
Comparison of sensitivity and selectivity of CDBB-TC and CDBB in the laboratory.
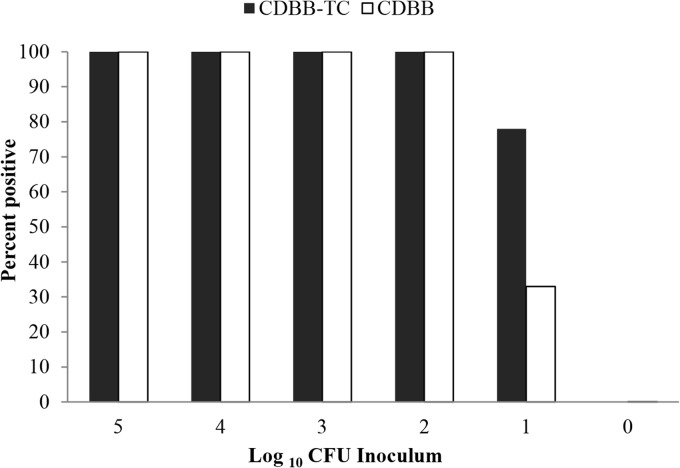
CDBB-TC was equivalent to CDBB for the recovery of C. difficile spores from inoculated swabs (Fig. 2). CDBB-TC and CDBB consistently yielded positive results from swabs inoculated with ≥2 log10 CFU of spores. Positive results were obtained from a subset of swabs inoculated with 1 log10 CFU of spores; 3 of 9 (33%) runs were positive for CDBB versus 7 of 9 (78%) for CDBB-TC (P = 0.15). Positive cultures for CDBB were yellow throughout the medium tube, whereas positive CDBB-TC cultures were yellow at the bottom of the tube.
FIG 2.
Comparison of sensitivities of C. difficile brucella broth with thioglycolic acid and l-cystine (CDBB-TC) versus C. difficile brucella broth (CDBB) in the laboratory. Ten-microliter aliquots of serial dilutions of spores of two test strains were applied to polyester swabs that were inoculated into 15 ml of CDBB-TC or prereduced CDBB and incubated for 72 h at 37°C. Specimens with a color change from red to yellow were cultured to confirm the growth of C. difficile.
None of the organisms used to assess selectivity (C. sporogenes, C. perfringens, B. subtilis, E. faecium, methicillin-resistant S. aureus, S. warneri, or C. glabrata) grew in CDBB-TC incubated in room air. However, the C. sporogenes and C. perfringens strains grew in CDBB and in CDBB-TC that was incubated inside the anaerobic chamber.
Comparison of sensitivity and selectivity of CDBB-TC and CDBB for detecting environmental contamination on hospital wards.
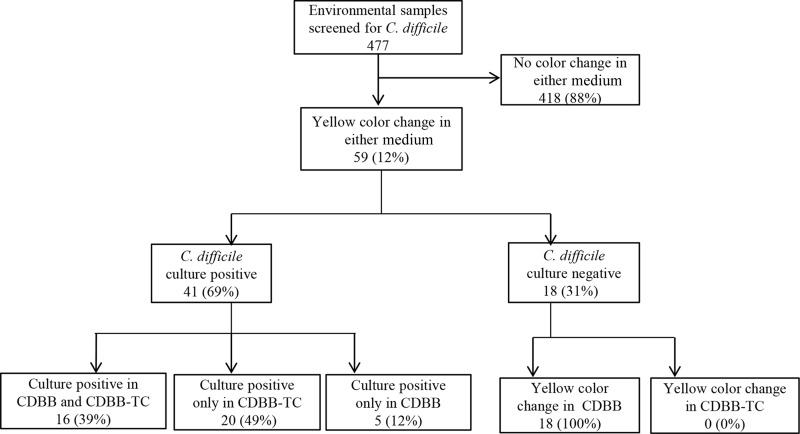
Environmental swabs were collected from 477 total surfaces (345 from surfaces in CDI patient rooms and 132 from portable equipment). As shown in Fig. 3, 41 (9%) of the cultures were positive by CDBB-TC and/or CDBB based on a subculture onto CDBA yielding C. difficile: 16 of the cultures were positive from both culture media, 20 were positive from CDBB-TC only, and 5 were positive from CDBB only. For environmental samples that were negative by one culture medium but positive by the other, confirmatory testing of the negative samples by C. difficile latex agglutination assay, EIA for glutamate dehydrogenase and toxins A and B, and PCR for toxin B genes was consistently negative.
FIG 3.
Comparison of sensitivity and selectivity of C. difficile brucella broth with thioglycolic acid and l-cysteine (CDBB-TC) versus C. difficile brucella broth (CDBB) for the detection of environmental contamination on hospital wards. Swabs were applied to a 5 by 10 cm area of surfaces and incubated at 37°C for 72 h. All specimens with a change from red to yellow were cultured for C. difficile. On the right are cultures negative for C. difficile but positive for other organisms that grew when reinoculated into CDBB with a change in color to yellow (i.e., false-positive cultures for C. difficile).
If any positive culture with confirmed C. difficile was considered the gold standard, CDBB-TC was significantly more sensitive than CDBB (36/41 [88%] versus 21/41 [51%]; P = 0.006). For 18 culture-positive specimens from CDBB-TC subjected to other confirmatory tests, 18 (100%) aliquots from the bottom of the CDBB-TC tube tested positive by each of the other confirmatory tests, including C. difficile latex agglutination, PCR for toxin B genes, and EIA for glutamate dehydrogenase and toxins A and B. For 8 culture-negative CDBB and 8 culture-negative CDBB-TC tubes, the broth from the bottom of the tubes was negative by the same confirmatory tests.
For the 477 total environmental cultures, the specificities of CDBB-TC and CDBB based upon false-positive yellow-color development of the medium without the recovery of C. difficile were 100% (0 false-positive results) and 96% (18 false-positive results), respectively (P = 0.0001). The 18 false-positive cultures for CDBB were attributable to the growth of non-C. difficile anaerobic organisms that did not grow in CDBB-TC in room air. Fourteen colonies from false-positive cultures that were not consistent with C. difficile were identified as Fusobacterium spp. (n = 6), C. perfringens (n = 3), Clostridium septicum (n = 2), Clostridium tertium (n = 1), Streptococcus constellatus (n = 1), and Bacteroides fragilis (n = 1); 4 additional isolates were unable to be subcultured. The inoculation of these organisms into CDBB or CDBB-TC inside the anaerobic chamber resulted in a yellow color and growth, whereas inoculation into CDBB-TC with incubation in room air did not.
Effect of thioglycolic acid on stimulation of C. difficile spore germination.
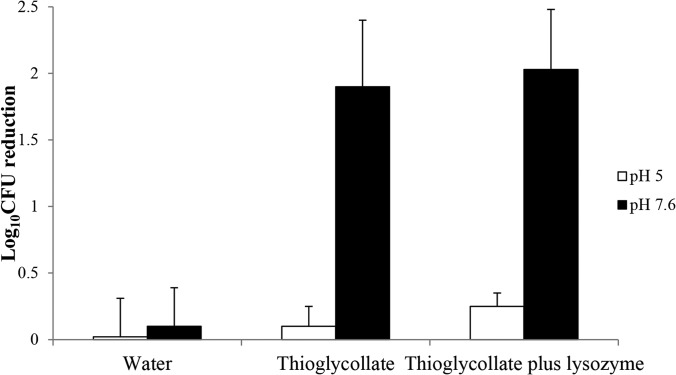
As shown in Fig. 4, minimal germination (based upon a reduction in spores at 80°C) of C. difficile spores occurred in sterile water or in 1 mg/ml thioglycolic acid alone or in combination with 1 mg/ml lysozyme at pH 5 (P ≥ 0.82); however, at pH 7.6, a significant reduction in spores occurred at 80°C in 1 mg/ml thioglycolic acid (P < 0.0001), with no further enhancement by lysozyme (P = 0.74).
FIG 4.
Effect of thioglycolic acid on stimulation of germination of C. difficile spores. Spores (106 CFU) were incubated at 22°C for 1 h in sterile water versus sterile water supplemented with 1 mg/ml thioglycolic acid alone or in combination with 1 mg/ml lysozyme and at pH 5 and 7.6, and aliquots were subjected to heat shock at 80°C for 5 min to determine the log reductions (as activated spores are killed at 80°C, whereas nongerminated spores are not). Error bars indicate standard errors.
DISCUSSION
Our findings demonstrate that a broth medium containing thioglycolic acid and l-cystine provides a sensitive and selective method for the culture of C. difficile from environmental specimens without the need for anaerobic culture conditions. The medium is easy to prepare. Moreover, the requirement for microbiological expertise is minimal because positive broth samples based on color change can be confirmed as C. difficile by latex agglutination assay or EIA for glutamate dehydrogenase, or as toxigenic C. difficile using commercial PCR assays for toxin genes or enzyme immunoassays for toxins.
The fact that C. difficile was recovered more frequently from environmental swabs inoculated into CDBB-TC than into CDBB was unexpected. In contrast, Wilcox and Dave (9) recently reported that lysozyme enhanced the recovery of C. difficile from environmental samples, but preexposure to alkaline thioglycolate did not further improve recovery. Our results suggest that a potential explanation for the increased recovery of environmental spores is the stimulation of germination by thioglycolic acid. At pH 7.6 but not pH 5, thioglycolic acid stimulated the germination of C. difficile spores based upon the susceptibility of spores to killing by heating to 80°C. Others have demonstrated that pH may influence the germination of C. difficile spores, with an optimum pH range for germination being 6.5 to 7.5 (10). It is possible that thioglycolic acid stimulates the germination of a fraction of spores from the environment that exist in a superdormant state (i.e., spores that do not germinate in response to common germinants) (11). Alternatively, some studies have suggested that thioglycolic acid exposure may sensitize spores to the activity of lysozyme, presumably by rupturing disulfide bonds and increasing the penetration of lysozyme to the site of action (12–14).
The finding that CDBB-TC was more selective than CDBB was also unexpected. The selectivity of the medium is an advantage, because extra work and expenses are required in the evaluation of false-positive cultures that turn yellow due to the growth of organisms other than C. difficile. A variety of anaerobic organisms grew in CDBB and caused a color change to yellow; these organisms did not grow in CDBB-TC incubated in room air but did grow in CDBB-TC incubated inside the anaerobic chamber. We postulate that some of these organisms may be more fastidious than C. difficile in their requirement for obligate anaerobic conditions (i.e., CDBB-TC incubated in room air may be sufficiently anaerobic to grow C. difficile but not the other Clostridium spp. that grew only under anaerobic conditions).
In addition to being easy to prepare, CDBB-TC is relatively inexpensive. Based upon the current pricing of ingredients from the listed distributors, the costs for one culture would be $2.99, including the medium ingredients ($0.50/10 ml), BD BBL CultureSwabs ($2.00/swab), and polystyrene tubes ($0.49/tube). The addition of a confirmatory test to be used on tubes showing a change in color to yellow would increase costs a modest degree. If the C. difficile latex agglutination test were used, it would add $5.24 for each confirmatory test. If all of the 8% of CDBB-TC tubes that exhibited a color change in the current study had been confirmed using the latex agglutination test, the total cost per 100 environmental cultures would have been $340.92 (versus $299 if no confirmatory testing were done).
Our study has some limitations. The medium is qualitative and does not provide an assessment of the burden of contamination. The sensitivity of the swabs for recovering spores may be reduced in comparison to that for sponges (15). Environmental samples were collected from one institution only. Additional larger studies are needed in multiple institutions. Finally, we tested only one commercial real-time PCR assay. There may be variability in the sensitivities of the different nucleic amplification tests (16).
ACKNOWLEDGMENTS
This work was supported by a Merit Review grant from the Department of Veterans Affairs to C.J.D. and by a grant from the Agency for Healthcare Research and Quality (R18 HS20004-01A1) to C.J.D.
Footnotes
Published ahead of print 25 June 2014
REFERENCES
- 1.Donskey CJ. 2013. Does improving surface cleaning and disinfection reduce health care-associated infections? Am. J. Infect. Control 41(5 Suppl):S12–S19. 10.1016/j.ajic.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Sitzlar B, Deshpande A, Fertelli D, Kundrapu S, Sethi AK, Donskey CJ. 2013. An environmental disinfection odyssey: evaluation of sequential interventions to improve disinfection of Clostridium difficile isolation rooms. Infect. Control Hosp. Epidemiol. 34:459–465. 10.1086/670217. [DOI] [PubMed] [Google Scholar]
- 3.Carling PC, Huang SS. 2013. Improving healthcare environmental cleaning and disinfection: current and evolving issues. Infect. Control Hosp. Epidemiol. 34:507–513. 10.1086/670222. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande A, Kundrapu S, Sunkesula VC, Cadnum JL, Fertelli D, Donskey CJ. 2013. Evaluation of a commercial real-time polymerase chain reaction assay for detection of environmental contamination with Clostridium difficile. J. Hosp. Infect. 85:76–78. 10.1016/j.jhin.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Curry SR, Schlackman JL, Hamilton TM, Henderson TK, Brown NT, Marsh JW, Shutt KA, Brooks MM, Pasculle AW, Muto CA, Harrison LH. 2011. Perirectal swab surveillance for Clostridium difficile by use of selective broth preamplification and real-time PCR detection of tcdB. J. Clin. Microbiol. 49:3788–3793. 10.1128/JCM.00679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Summanen P, Baron EJ, Citron DM, Strong CA, Wexler HM, Finegold SM. 1993. Appendix C, p 166 In Hoffman S. (ed), Wadsworth anaerobic bacteriology manual, 5th ed. Star Publishing Company, Belmont, CA. [Google Scholar]
- 7.Nerandzic MM, Donskey CJ. 2009. Effective and reduced-cost modified selective medium for isolation of Clostridium difficile. J. Clin. Microbiol. 47:397–400. 10.1128/JCM.01591-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carias LL, Rudin SD, Donskey CJ, Rice LB. 1998. Genetic linkage and cotransfer of a novel, vanB-containing transposon (Tn5382) and a low-affinity penicillin-binding protein 5 gene in a clinical vancomycin-resistant Enterococcus faecium isolate. J. Bacteriol. 180:4426–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilcox MH, Dave J. 2000. Value of lysozyme agar incorporation and alkaline thioglycollate exposure for the environmental recovery of Clostridium difficile. J. Hosp. Infect. 44:65–69. 10.1053/jhin.1999.0253. [DOI] [PubMed] [Google Scholar]
- 10.Nerandzic MM, Donskey CJ. 2013. Activate to eradicate: inhibition of Clostridium difficile spore outgrowth by the synergistic effects of osmotic activation and nisin. PLoS One 8:e54740. 10.1371/journal.pone.0054740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeldon LJ, Worthington T, Hilton AC, Elliott TS, Lambert PA. 2008. Physical and chemical factors influencing the germination of Clostridium difficile spores. J. Appl. Microbiol. 105;2223–30. 10.1111/j.1365-2672.2008.03965.x. [DOI] [PubMed] [Google Scholar]
- 12.Gould GW, Hitchins AD. 1963. Sensitization of bacterial spores to lysozyme and to hydrogen peroxide with agents which rupture disulphide bonds. J. Gen. Microbiol. 33:413–423. 10.1099/00221287-33-3-413. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura S, Yamakawa K, Izumi J, Nakashio S, Nishida S. 1985. Germinability and heat resistance of spores of Clostridium difficile strains. Microbiol. Immunol. 29:113–118. 10.1111/j.1348-0421.1985.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 14.Ionesco H. 1978. Initiation of germination of Clostridium difficile spores by lysozyme. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D. 287:659–661. [PubMed] [Google Scholar]
- 15.Otter JA, Havill NL, Adams NM, Cooper T, Tauman A, Boyce JM. 2009. Environmental sampling for Clostridium difficile: swabs or sponges? Am. J. Infect. Control. 37:517–518. 10.1016/j.ajic.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Sunkesula VC, Kundrapu S, Muganda C, Sethi AK, Donskey CJ. 2013. Does empirical Clostridium difficile infection (CDI) therapy result in false-negative CDI diagnostic test results? Clin. Infect. Dis. 57:494–500. 10.1093/cid/cit286. [DOI] [PubMed] [Google Scholar]