Abstract
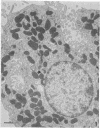
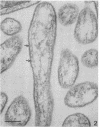
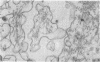
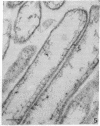
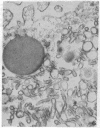
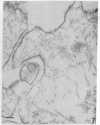
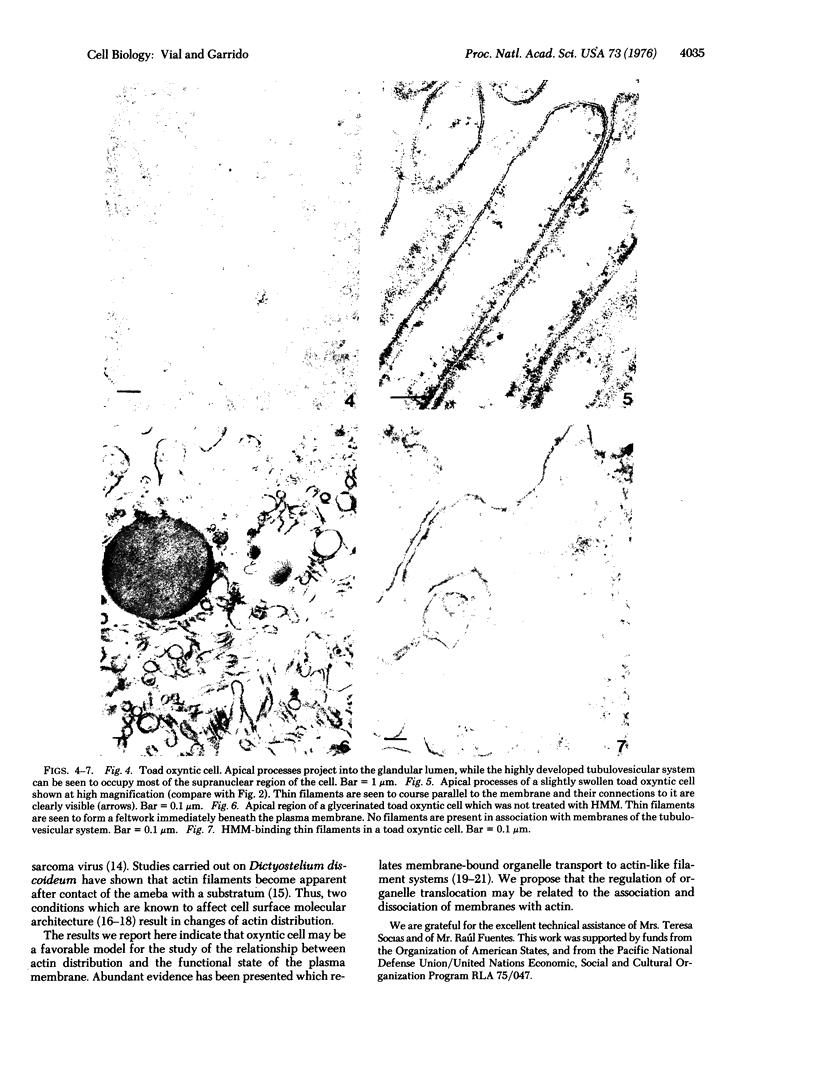
The secretory pole of vertebrate oxyntic cells possesses two distinct membrane systems: the apical plasma membrane which presents numerous infoldings, microvilli and processes, and a complex tubulovesicular system located in close proximity to the plasma membrane. These two membrane systems are generally believed to be interconvertible in relation to the functional state of the cell. To determine the role that filaments may play in the interconversion process, the secretory pole of rat and toad oxyntic cells was examined by electron microscopy under conditions designed to demonstrate filamentous structures, i.e., slight cellular swelling and incubation with heavy meromyosin. Filaments 50-80 A in diameter are present in close association with the plasma membrane to which they are connected by regularly spaced bridges. Heavy meroxyosin-treated material reveals "decorated" filaments in topographically corresponding locations. No filaments are seen in association with membranes of the tubulovesicular system. These findings suggest that association with actin-like filaments is a step in the translocation of membranes from the tubulovesicular system to the plasma membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger M. M., Martin G. S. Agglutination of cells transformed by Rous sarcoma virus by wheat germ agglutinin and concanavalin A. Nat New Biol. 1972 May 3;237(70):9–12. doi: 10.1038/newbio237009a0. [DOI] [PubMed] [Google Scholar]
- Clarke M., Schatten G., Mazia D., Spudich J. A. Visualization of actin fibers associated with the cell membrane in amoebae of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1975 May;72(5):1758–1762. doi: 10.1073/pnas.72.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido J. Ultrastructural labeling of cell surface lectin receptors during the cell cycle. Exp Cell Res. 1975 Aug;94(1):159–175. doi: 10.1016/0014-4827(75)90543-1. [DOI] [PubMed] [Google Scholar]
- Ito S., Schofield G. C. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974 Nov;63(2 Pt 1):364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller M., Doljanski F. Agglutination of normal and rous sarcoma virus-transformed chick embryo cells by concanavalin A and wheat germ agglutinin. Nat New Biol. 1972 Feb 9;235(58):184–185. doi: 10.1038/newbio235184a0. [DOI] [PubMed] [Google Scholar]
- Koenig C., Vial J. D. A histochemical study of adenosine triphosphatase in the toad (Bufo spinulosus) gastric mucosa. J Histochem Cytochem. 1970 May;18(5):340–353. doi: 10.1177/18.5.340. [DOI] [PubMed] [Google Scholar]
- LOWEY S., HOLTZER A. The homogeneity and molecular weights of the meromyosins and their relative proportions in myosin. Biochim Biophys Acta. 1959 Aug;34:470–484. doi: 10.1016/0006-3002(59)90300-2. [DOI] [PubMed] [Google Scholar]
- Mooseker M. S., Tilney L. G. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol. 1975 Dec;67(3):725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. L., Dubin M. W. The occurrence of actinlike filaments in association with migrating pigment granules in frog retinal pigment epithelium. J Cell Biol. 1975 Mar;64(3):705–710. doi: 10.1083/jcb.64.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrego H., Navia E., Vial J. D. Effect of puromycin on the membrane changes of the stimulated oxyntic cell. Exp Cell Res. 1966 Sep;43(2):351–357. doi: 10.1016/0014-4827(66)90062-0. [DOI] [PubMed] [Google Scholar]
- Painter R. G., Sheetz M., Singer S. J. Detection and ultrastructural localization of human smooth muscle myosin-like molecules in human non-muscle cells by specific antibodies. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1359–1363. doi: 10.1073/pnas.72.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevitz B. A., Hepler P. K. Identification of actin in situ at the ectoplasm-endoplasm interface of Nitella. Microfilament-chloroplast association. J Cell Biol. 1975 Apr;65(1):29–38. doi: 10.1083/jcb.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun L. I. Polarized intracellular particle transport: saltatory movements and cytoplasmic streaming. Int Rev Cytol. 1972;32:93–137. doi: 10.1016/s0074-7696(08)60339-3. [DOI] [PubMed] [Google Scholar]
- Romrell L. J., Coppe M. R., Munro D. R., Ito S. Isolation and separation of highly enriched fractions of viable mouse gastric parietal cells by velocity sedimentation. J Cell Biol. 1975 May;65(2):428–438. doi: 10.1083/jcb.65.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas L., Sananes L., Michelangeli F. Relation between water flux and content in frog gastric mucosa. Am J Physiol. 1971 May;220(5):1282–1288. doi: 10.1152/ajplegacy.1971.220.5.1282. [DOI] [PubMed] [Google Scholar]
- Wickus G., Gruenstein E., Robbins P. W., Rich A. Decrease in membrane-associated actin of fibroblasts after transformation by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):746–749. doi: 10.1073/pnas.72.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]