Abstract
Encephalitis is a frequently diagnosed condition in cattle with neurological diseases. Many affected animals present with a nonsuppurative inflammatory reaction pattern in the brain. While this pattern supports a viral etiology, the causative pathogen remains unknown in a large proportion of cases. Using viral metagenomics, we identified an astrovirus (bovine astrovirus [BoAstV]-CH13) in the brain of a cow with nonsuppurative encephalitis. Additionally, BoAstV RNA was detected with reverse transcription-PCR and in situ hybridization in about one fourth (5/22 animals) of cattle with nonsuppurative encephalitis of unknown etiology. Viral RNA was found primarily in neurons and at the site of pathology. These findings support the notion that BoAstV infection is a common cause of encephalitis in cattle. Phylogenetically, BoAstV-CH13 was closely related to rare astrovirus isolates from encephalitis cases in animals and a human patient. Future research needs to be directed toward the pathogenic mechanisms, epidemiology, and potential cross-species transmission of these neurotropic astroviruses.
INTRODUCTION
Infectious diseases of the central nervous system (CNS) in cattle livestock have important economic and public health implications. Approximately one fourth of the infectious diseases listed by the World Organization for Animal Health (OIE) as notifiable are caused by neurotropic agents. Some of these agents, such as rabies virus and the bovine spongiform encephalopathy prion, are also threats to humans (1).
Often, the first step toward an etiological diagnosis is postmortem histopathological examination of the brain, followed by procedures to identify the specific pathogen. Once notifiable diseases have been excluded, however, many cases of encephalitis in cattle remain etiologically unresolved and are not subjected to full diagnostic investigations, due to financial and methodological constraints. Viral encephalitis of unknown etiology is a frequent diagnosis among cattle with neurological clinical signs in central Europe; it was found in 10 to 15% of cattle subjected to histological brain examinations as part of the Swiss statutory reporting of rabies and bovine spongiform encephalopathy suspects (2, 3) in past decades. Pathologically, these cases are characterized by nonsuppurative polioencephalomyelitis with neuronal necrosis, as well as perivascular cuffs and meningitis involving mononuclear lymphocytes (4). Such lesions often are most severe in the brainstem but also appear in the cerebellar cortex, the cerebral cortex, or the hippocampus in some animals. Fankhauser first investigated such cases systematically in Switzerland in the 1960s (5) and coined the term European sporadic bovine encephalomyelitis (ESBE). Similar pathologies were reported from other countries as well (6–9). However, attempts to culture and to identify the infectious agents associated with these conditions have yielded largely inconclusive results (10, 11).
In recent years, novel methods for pathogen discovery through viral metagenomic approaches using unbiased high-throughput next-generation sequencing (NGS) have become available (12). These techniques allow massively parallel de novo sequencing of nucleic acids in tissue specimens. Bioinformatics tools are then applied to identify sequence similarities with known pathogens, by comparison with nucleic acid and protein database entries.
In the present study, we sought to identify pathogens associated with nonsuppurative encephalitis of unknown etiology in cattle, using metagenomic methods, reverse transcription (RT)-PCR, and in situ hybridization (ISH). We identified an astrovirus in the brains of a series of cows with nonsuppurative encephalitis. Strikingly, a phylogenetically closely related virus was demonstrated in cattle brains in a very recent study from the United States, which paralleled our work (13). These findings suggest that neurotropic astroviruses are geographically widespread and are a frequent cause of encephalitis in cattle.
MATERIALS AND METHODS
Animals.
Brain tissues were available from the archive of the Division of Neurological Sciences, Vetsuisse Faculty, University of Bern (Bern, Switzerland). Samples included frozen unfixed tissue samples and formalin-fixed paraffin-embedded tissue blocks from the medulla oblongata of 25 cows with lesions indicative of nonsuppurative encephalitis (Table 1). These animals had presented clinical signs of CNS disease, and their brains had been submitted to a diagnostic service for postmortem neuropathological investigations. Similarly, processed brain tissue samples from animals without lesions or reported neurological clinical signs (n = 26), with encephalitis of known etiology (malignant catarrhal fever; n = 5), or with bacterial encephalitis (n = 2) served as negative controls.
TABLE 1.
Details on cows with nonsuppurative encephalitis and results of BoAstV-CH13 detection by RT-PCR and in situ hybridization
| Study type and case no. | Yra | Cantonb | Age (yr) | Assay resultsc |
|
|---|---|---|---|---|---|
| RT-PCR | ISH | ||||
| NGS | |||||
| 42145 | 2006 | VD | 6 | − | ND |
| 45641 | 2012 | LU | 10 | − | ND |
| 45664 | 2012 | FR | 1.5 | + | + |
| Retrospective | |||||
| 23871 | 1995 | FR | 2 | + | + |
| 23985 | 1995 | BE | 2.5 | + | + |
| 24250 | 1995 | SO | 5 | − | ND |
| 24586 | 1996 | FR | 4 | − | ND |
| 26731 | 1998 | NE | 5 | − | ND |
| 26875 | 1998 | BE | 1.5 | + | + |
| 27020 | 1998 | BE | 3 | − | ND |
| 32270 | 2000 | NE | 2 | − | ND |
| 32450 | 2000 | BE | 7 | − | ND |
| 33181 | 2001 | VD | 1.5 | − | ND |
| 34421 | 2001 | VD | 3 | − | ND |
| 34510 | 2002 | BE | 2 | − | ND |
| 35154 | 2002 | SO | 4 | − | ND |
| 35946 | 2003 | BE | 11 | − | ND |
| 36108 | 2003 | BE | 5 | − | ND |
| 36166 | 2003 | BE | 5 | − | ND |
| 36768 | 2004 | FR | 3.5 | − | ND |
| 37514 | 2004 | BE | 2 | + | ND |
| 42268 | 2006 | SH | 6 | − | ND |
| 42535 | 2006 | AR | 4 | − | ND |
| 42797 | 2007 | FR | 1.5 | − | ND |
| 42799 | 2007 | FR | 7 | + | + |
Year of diagnosis.
VD, Vaude; LU, Luzern; FR, Fribourg; BE, Bern; SO, Solothurn; NE, Neuchâtel; SH, Schaffhausen; AR, Appenzell Ausserrhoden.
−, negative result; +, positive result; ND, not done.
Nucleic acid extraction.
Frozen brains of cattle were sampled at the level of the medulla oblongata. DNA was isolated with the Nucleon Bacc2 kit (GE Healthcare) and total RNA was isolated with TRIzol reagent (Life Technologies), according to the manufacturers' instructions.
Next-generation sequencing.
The TruSeq DNA sample preparation kit (Illumina) was used to construct a paired-end DNA library from three animals with nonsuppurative encephalitis of unknown etiology (animals 42145, 45641, and 45664). One lane of paired-end reads (2 × 100 bp) in the Illumina HiSeq2000 sequencing system was collected, which corresponded to 328,130,674 (animal 42145), 288,193,743 (animal 45641), and 237,982,650 (animal 45664) reads. RNA libraries were constructed from the same three animals with the Illumina TruSeq stranded total RNA kit (Life Technologies), and rRNA was selectively depleted with the RiboMinus kit (Life Technologies). One lane of single reads (100 bp) per sample in the Illumina HiSeq2000 system was collected, resulting in 200,390,431 (sample 42145), 193,056,376 (sample 45641), and 178,153,829 (sample 45664) reads.
Sequence analysis.
Reads of the DNA and RNA libraries were mapped to the cattle reference genome (UMD3.1 assembly) with bowtie2 (version 2.0.0beta6) and the spliced alignment program TopHat2 (version 2.0.4), respectively, with default parameters. Unmapped DNA and RNA reads were collected and subjected to de novo assembly with SPAdes-2.4.0 (k-mer sizes of 33, 55, 67, 81, 91, 93, 95, 97, and 99). To identify sequences of viral origin, assembled sequences of the DNA and RNA libraries were compared with entries of the complete NCBI viral protein database (of 1 May 2013) by using BLASTx (version 2.2.27+). Matches with E values of <0.001 were further filtered for similarity (≥50%) and alignment length (≥200 amino acids). The resulting sequence contigs were then compared to the entire BLASTx nonredundant protein database. Contigs with strong similarities to known cattle and bacterial proteins were removed.
Sanger sequencing.
Total RNA extracts were reverse-transcribed with Superscript III RT polymerase (Life Technologies). The resulting cDNA was amplified with the Expand Plus PCR kit (Roche) and primer combinations that resulted in overlapping amplicons of ∼1,500 bp (see Table S1 in the supplemental material). The 5′ and 3′ ends of the viral genome were sequenced by rapid amplification of cDNA ends (RACE) (GeneRacer kit and 5′ RACE kit; Life Technologies). All amplicons were cloned into the pCR4 vector (Life Technologies) and sequenced.
RT-PCR.
Total RNA tissue extracts were reverse transcribed with oligo(dT) primers (Life Technologies). The resulting cDNA was PCR amplified with GoTaq green mastermix (Promega) and astrovirus-specific primers MA2 and MA4, as described by Mittelholzer et al. (14).
In situ hybridization.
Two digoxigenin (DIG)-labeled antisense RNA probes were generated with the DIG RNA-labeling kit (Roche Applied Science). Probe A is complementary to a 167-nucleotide (nt) sequence at the 5′ end of open reading frame 2 (ORF2) and probe B to a 172-nt sequence in the center of ORF2. In situ detection of viral RNA in formalin-fixed paraffin-embedded tissue slides was conducted essentially as described previously (15) (for details, see Materials and Methods in the supplemental material).
Phylogenetic analysis.
The predicted full-length capsid protein amino acid sequence of the astrovirus obtained in this study and sequences of representative Astroviridae members were imported into MEGA5 software (16). After alignment of the sequence data set, a maximum-likelihood tree was reconstructed with 1,000 bootstrap replicates. The reliability of the topology used in that initial tree was assessed by generating a neighbor-joining tree with the amino acid p-distances and nonparametric bootstrap analysis. Sliding window identity analysis was performed with SimPlot (17), using a window size of 200 nt with 20-nt steps and the Hamming distance model. Identity plots were generated with GraphPad Prism (version 5.03).
Nucleotide sequence accession number.
The nucleotide sequence of bovine astrovirus (BoAstV)-CH13 was submitted to GenBank under accession number KM035759.
RESULTS
Virus identification.
Tissue samples from three cows with nonsuppurative encephalitis of unknown etiology were selected for DNA and RNA library preparation and NGS. These animals were representative of different age groups and geographic origins (Table 1). The bioinformatics pipeline that we applied to the NGS data set resulted in sequence similarities to known viral proteins in all three cases. Two contigs, derived from the DNA libraries of animals 42145 and 45641, matched proteins of human betaretrovirus and mouse mammary tumor virus (see Table S2 in the supplemental material). When we compared these contigs with nucleotide sequences in the BLAST database, both revealed a high degree of similarity to bovine endogenous retrovirus sequences and to some genomic sequences of cattle. Moreover, the absence of complementary sequences in the RNA libraries indicates that virus replication did not occur. It is therefore likely that these sequences are remnants of ancient exogenous retroviral infections of the host germ line (18, 19).
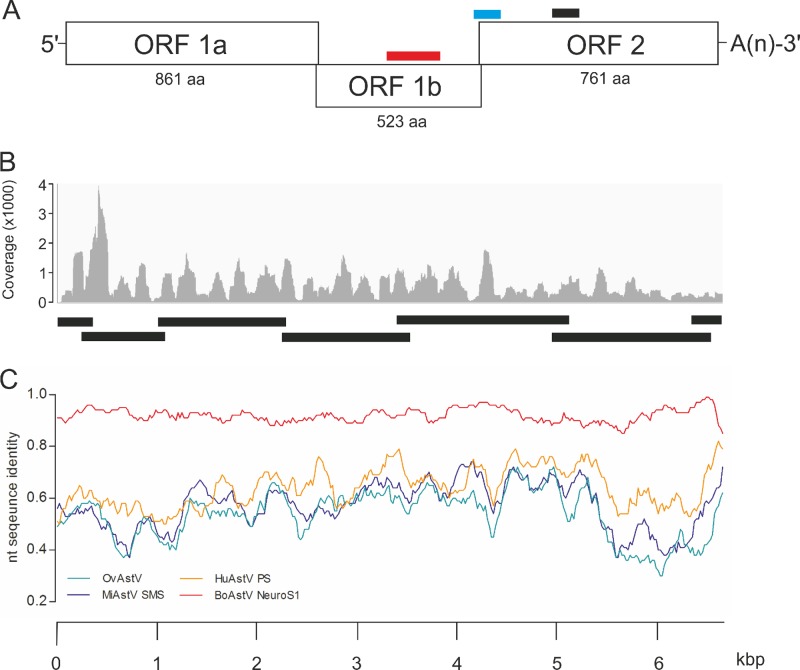
For the third animal (animal 45664), we identified one contig of 6,450 bp in the RNA library that matched protein entries of different virus species of the family Astroviridae, with amino acid sequence similarities between 50% and 69% (see Table S2 in the supplemental material). Astroviruses have a positive-sense, single-stranded RNA genome that varies between ∼6.2 kb and ∼7.7 kb in size (20). Their genomic RNA comprises short 5′ and 3′ untranslated regions (UTRs), three partially overlapping open reading frames (ORF1a, ORF1b, and ORF2), and a poly(A) tail (Fig. 1A). These features were also present in the 6,450-bp contig. Among the protein sequence matches for case 45664 were translation products of astrovirus ORF1a (protease), ORF1b (RNA-dependent RNA polymerase), and ORF2 (capsid protein) (see Table S2 in the supplemental material). The average nucleotide coverage of this contig was 625 ± 476 nt (mean ± standard deviation [SD]) (Fig. 1B). The sequence assembled in silico was confirmed by conventional Sanger sequencing of overlapping cDNA fragments, and the 5′ and 3′ ends were determined by RACE (Fig. 1C), resulting in an RNA sequence of 6,530 nt. Taken together, the similarities in size and organization between this RNA sequence and the genome of Astroviridae indicated the presence of an astrovirus in the brain of cow 45664, which we tentatively named BoAstV-CH13.
FIG 1.
Identification of the novel bovine astrovirus BoAstV-CH13 in the brain tissue of a cow with nonsuppurative encephalitis. (A) The viral genome is composed of 3 open reading frames (ORFs), short 5′ and 3′ untranslated regions (UTRs), and a poly(A) tail [-A(n)]. ORF1a and ORF1b encode nonstructural proteins, and ORF2 encodes structural proteins. The numbers of amino acids (aa) in each translation product are shown. Bars, target sequences for ISH (blue, probe A; black, probe B) and RT-PCR (red). (B) Assembly of NGS reads revealed a contig of 6,540 nt that included the entire coding region of the viral genome. The graph presents the average coverage of this contig for each nucleotide. Bars, fragments that were confirmed by Sanger sequencing. (C) Whole-genome sequences of BoAstV-CH13 (reference), ovine astrovirus (OvAstV), and astroviruses isolated from brain tissues of mink (MiAstV-SMS), a human patient (HuAstV-PS), and a steer in the United States (BoAstV-NeuroS1) were compared by sliding window pairwise identity plots. Scale, nucleotide positions within the astrovirus genome.
Retrospective study.
To determine the extent to which BoAstV-CH13 is associated with nonsuppurative encephalitis, we analyzed brainstem tissues from additional cows with nonsuppurative encephalitis of unknown etiology (n = 22) (Table 1) and from a negative-control group (n = 33) by RT-PCR targeting a region in the RNA-dependent RNA polymerase domain of ORF1b (Fig. 1A). An amplicon of the expected size of 427 bp was observed for five animals in the encephalitis group and none in the control group. These results suggest that BoAstV-CH13 is associated with approximately one quarter of the cases of nonsuppurative encephalitis of unknown etiology.
In situ detection of BoAstV-CH13.
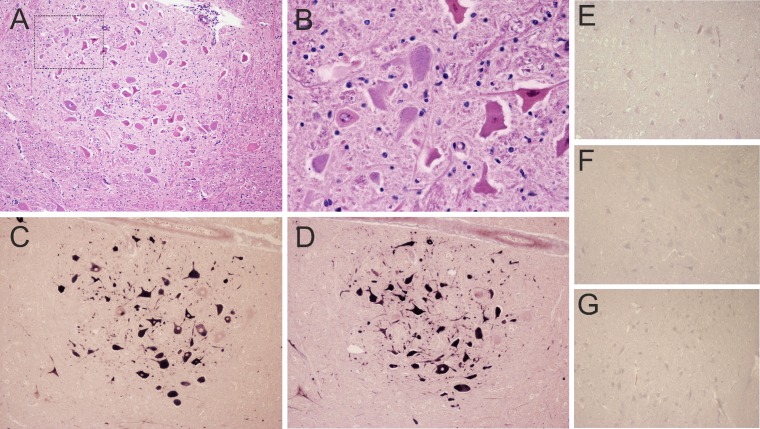
The RT-PCR results were then confirmed by detecting the BoAstV-CH13 genome in situ in affected brain tissues. We constructed two different BoAstV-CH13-specific DIG-labeled RNA probes for nonradioactive ISH (Fig. 1A). Paraffin-embedded tissue slides of the medulla oblongata of RT-PCR-positive animals with nonsuppurative encephalitis (n = 5, including animal 45664) and those of negative-control animals (n = 8) were investigated by ISH. With both ISH probes, there was strong cytoplasmic labeling of the neurons in the animals that were positive by RT-PCR (n = 5) (Table 1). Overall, the presence of viral RNA correlated well with the localization of the pathological lesions, especially with neuronal necrosis and chromatolysis (Fig. 2A to D). As expected, negative-control samples remained unlabeled with both probes, and BoAstV-CH13-positive samples did not show labeling when an unrelated DIG-labeled RNA probe was used (Fig. 2E to G).
FIG 2.
Histopathological lesions and BoAstV-CH13 RNA detection in the facial nucleus in the medulla oblongata of a cow with nonsuppurative encephalitis. (A) Affected animals exhibited gliosis and neuronal necrosis. (B) High magnification of the marked area in panel A, showing necrotic neurons. (C to E) In situ hybridization for BoAstV-CH13 with probe A (C), probe B (D), and an unrelated control probe (E). (F and G) Negative-control sections of a healthy cow, analyzed with probe A (F) and probe B (G). Dark-blue labeling, presence of viral RNA. Magnification, ×10 (A and C to G) or ×40 (B).
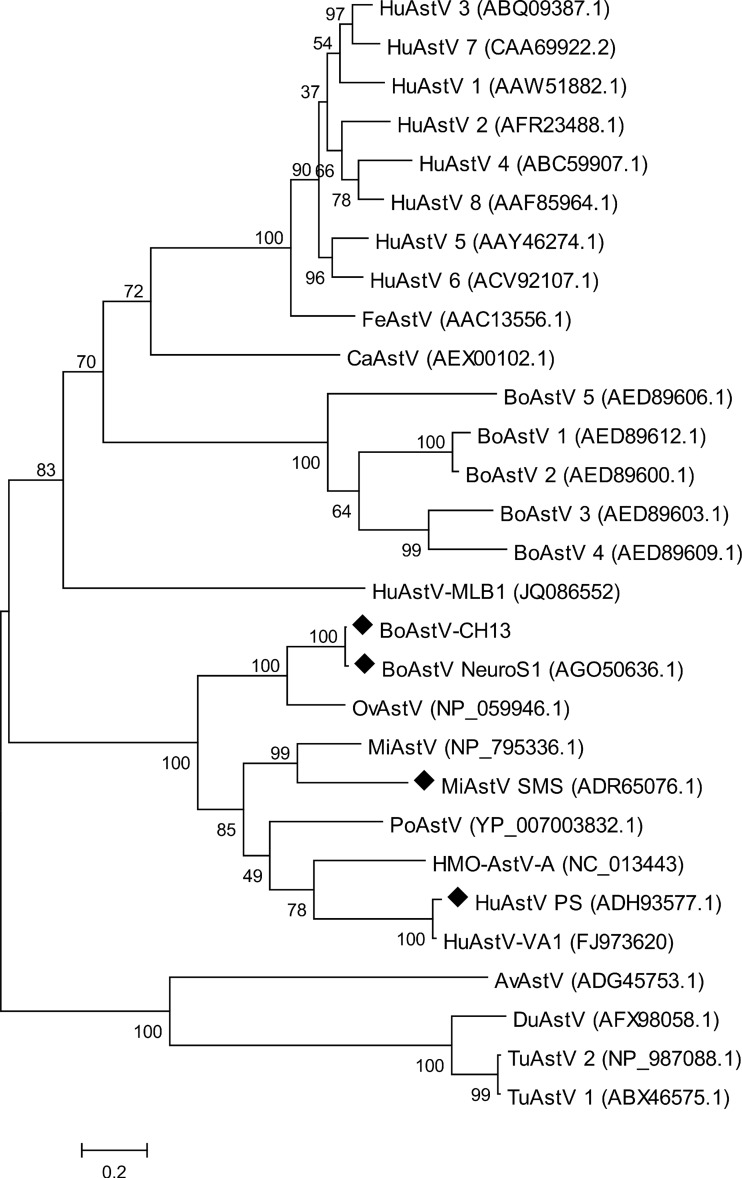
Phylogenetic analysis.
Finally, we assessed the genetic relationship between BoAstV-CH13 and previously characterized Astroviridae isolates in a maximum-likelihood tree, based on the capsid protein amino acid sequences. Although most of the astrovirus isolates were derived from fecal specimens from humans and animals, three viruses had been isolated from brain tissues and were associated with encephalitis. Interestingly, BoAstV-CH13 was not closely related to other bovine isolates but rooted from the same branch as these brain isolates (Fig. 3). The closest relationships observed, however, were with an ovine astrovirus (OvAstV) that had been isolated from feces of sheep with diarrhea (21) and with the recent brain isolate from cattle (BoAstV-NeuroS1 [13]). BoAstV-NeuroS1 protein sequences were not identified by the bioinformatics pipeline because they were not available in GenBank at that time (see Table S2 in the supplemental material). Subsequent BLASTx analysis revealed protein sequence similarities between BoAstV-CH13 and BoAstV-NeuroS1 of 98% for ORF1a, 98% for ORF1b, and 97% for ORF2. Moreover, sliding window pairwise identity plots of full genome sequences resulted in overall nucleotide identity of 92% (Fig. 1C). There were striking differences in the length and composition of the 3′ UTR between BoAstV-CH13 (85 nt) and BoAstV-NeuroS1 (26 nt), whereas the 5′ UTR was 96% identical and composed of 52 nt in both isolates.
FIG 3.
Maximum-likelihood tree constructed from aligned amino acid sequences of the full-length capsid protein, showing the phylogenetic relationships between BoAstV-CH13 and other representative astroviruses. GenBank accession numbers are given in parentheses. HuAstV, human astrovirus; FeAstV, feline astrovirus; CaAstV, canine astrovirus; BoAstV, bovine astrovirus; OvAstV, ovine astrovirus; PoAstV, porcine astrovirus; MiAstV, mink astrovirus; AvAstV, avian astrovrius (avian nephritis virus); DuAstV, duck astrovirus; TuAstV, turkey astrovirus; HMO-AstV, human-mink-ovine-like astrovirus. ◆, isolates from brains of neurologically diseased mammals.
DISCUSSION
Using viral metagenomics, we identified a novel type of astrovirus, BoAstV-CH13, in the brain of a cow that presented clinical signs and pathological lesions consistent with nonsuppurative encephalitis, a neurological condition for which related pathogens have remained unknown in many cases. We found BoAstV RNA in the brains of 5 of 22 additional cows with nonsuppurative encephalitis of unknown etiology. ISH confirmed the presence of viral RNA at the site of the disease-associated pathology, notably in neurons.
Taken together, these findings provide biologically plausible evidence for a causal relationship between BoAstV-CH13 and nonsuppurative encephalitis in cattle. However, final proof of causation requires the fulfillment of Koch's postulates, namely, virus isolation in culture and induction of the disease in experimentally infected animals. Preliminary attempts to propagate BoAstV-CH13 in colorectal adenocarcinoma cells (Caco-2), which are permissive to human astrovirus (HuAstV), and in bovine kidney cells (MDBK) were not successful. This work is currently being extended to other in vitro systems, and in vivo infection studies are being considered.
Phylogenetic comparison of the BoAstV-CH13 capsid protein revealed close relationships with three isolates that were previously associated with encephalitis in humans and animals. Specifically, the first isolate (HuAstV-PS) was reported from a boy with X-linked agammaglobulinemia (22). The second was a mink astrovirus (MiAstV) isolate (MiAstV-SMS) that Blomstrom and colleagues identified in the brains of farmed mink affected by shaking mink syndrome (23). This disease emerged in Scandinavia between 2000 and 2002 (23, 24). The final isolate (BoAstV-NeuroS1) was detected very recently by Li et al. in a steer and three additional cows with encephalitis in the United States (13). Pathological lesions in these animals were consistent with nonsuppurative polioencephalomyelitis and were reminiscent of lesions in the European cases of nonsuppurative encephalitis.
The study by Li et al. (13) and the present study were conducted in parallel, using different experimental configurations and samples from different geographic locations. With our NGS technology and bioinformatics pipeline, we were able to assemble the entire coding region of the viral genome with ∼600-fold coverage after a single NGS run, without the need for additional sample preparation such as nuclease treatment to enrich viral nucleic acids. This result underscores the sensitivity and potency of our approach for pathogen detection from clinical specimens. BoAstV-NeuroS1 and BoAstV-CH13 showed 92% identity of nucleotide sequences, which indicates that these viruses are closely related, if not identical. The significance of the differences observed in the 3′ UTR of the viral genomes remains speculative at this stage. It is likely that the 3′ UTR sequences play a role in viral genomic RNA replication, similar to other positive-strand RNA viruses, although this remains to be determined for astroviruses (25).
The fact that BoAstVs were detected in nervous tissues of cattle by two groups independently adds to the robustness of the data and increases the level of confidence in the clinical significance of BoAstV brain infections. Moreover, it implies that astrovirus encephalitis may be geographically widespread in livestock cattle. Indeed, brain pathologies similar to those observed in US and Swiss cattle have been reported from many countries (6–9, 11, 26). Our data also show that the virus has been present in the Swiss cattle population at least since 1995, and they suggest that heifers in the age range of 1.5 to 2.5 years are particularly affected. It will be interesting to examine, prospectively and retrospectively, additional cases of nonsuppurative encephalitis of different geographic origins and ages, to assess more precisely the prevalence of astrovirus-related encephalitis.
The etiology of disease in BoAstV-CH13-negative animals with encephalitis remains unknown. It is likely that other viral pathogens, which were not recognized in our study, are involved. Only three cases were subjected to NGS, however, and our bioinformatics pipeline was relatively stringent. Consequently, viral sequences more divergent from known viruses might have been missed. These shortcomings need to be addressed by subjecting additional cases to viral metagenomics and by refining the NGS methodologies, including the bioinformatics pipeline.
Although BoAstVs have been detected in the feces of cattle, their involvement in enteric and other diseases has not been well documented. Here, we provide further evidence that astroviruses have a role outside the enteric system and are a likely cause of one of the most frequently observed neurological conditions in cattle. In humans, astrovirus infections are a main cause of diarrhea in young children and are an important public health concern (27). With the development of tools for pathogen discovery, previously unknown astroviruses such as HuAstV-MLB (28), HuAstV-VA1 (29), and human-mink-ovine-like astrovirus (HMO-AstV) (30) have been identified. These viruses were more closely related to animal isolates than to human isolates (Fig. 3), indicating that astrovirus infections in humans may emerge periodically from zoonotic transmissions. The mechanisms of disease pathogenesis and epidemiology, as well as a putative zoonotic role of neurotropic astroviruses, currently remain obscure. In this regard, our results provide a starting point for future research, which is needed to address these aspects in more detail.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by the Swiss Federal Food Safety and Veterinary Office.
We thank Michèle Ackermann and Muriel Fragnière for providing excellent technical support and the Next Generation Sequencing Platform of the University of Bern for performing the high-throughput sequencing experiments.
Footnotes
Published ahead of print 2 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01195-14.
REFERENCES
- 1.World Organization for Animal Health. 2004. Manual of diagnostic tests and vaccines for terrestrial animals, 5th ed. World Organization for Animal Health, Paris, France. [Google Scholar]
- 2.Heim D, Fatzer R, Hornlimann B, Vandevelde M. 1997. Frequency of neurologic diseases in cattle. Schweiz. Arch. Tierheilkd. 139:354–362 (In German.) [PubMed] [Google Scholar]
- 3.Fatzer R, Steck F. 1974. Histological differential diagnosis in cattle suspected of rabies. Schweiz. Arch. Tierheilkd. 116:347–356 (In German.) [PubMed] [Google Scholar]
- 4.Vandevelde M, Higgins RJ, Oevermann A. 2012. Veterinary neuropathology, p 57 John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 5.Fankhauser R. 1961. Sporadic meningo-encephalomyelitis in cattle. Schweiz. Arch. Tierheilkd. 103:225–235 (In German.) [Google Scholar]
- 6.Billing J. 1974. Epizoozic investigations on sporadically non-suppurative encephalomyelitis in cattle. Ph.D. thesis, University of Munich, Munich, Germany: (In German.) [Google Scholar]
- 7.Bozzetta E, Caramelli M, Casalone C, Acutis PL, Ru G. 2003. BSE surveillance in Italy: neuropathological findings in cattle in the frame of the passive surveillance programme. J. Vet. Med. A Physiol. Pathol. Clin. Med. 50:48–49. 10.1046/j.1439-0442.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- 8.Munday BL, Mason RW, Cumming R. 1973. Observations on diseases of the central nervous system of cattle in Tasmania. Aust. Vet. J. 49:451–455. 10.1111/j.1751-0813.1973.tb09290.x. [DOI] [PubMed] [Google Scholar]
- 9.Jeffrey M, Wilesmith JW. 1992. Idiopathic brainstem neuronal chromatolysis and hippocampal sclerosis: a novel encephalopathy in clinically suspect cases of bovine spongiform encephalopathy. Vet. Rec. 131:359–362. 10.1136/vr.131.16.359. [DOI] [PubMed] [Google Scholar]
- 10.Theil D, Fatzer R, Schiller I, Caplazi P, Zurbriggen A, Vandevelde M. 1998. Neuropathological and aetiological studies of sporadic non-suppurative meningoencephalomyelitis of cattle. Vet. Rec. 143:244–249. 10.1136/vr.143.9.244. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez S, Clark EG, Wobeser GA, Janzen ED, Philibert H. 2013. A retrospective study of non-suppurative encephalitis in beef cattle from western Canada. Can. Vet. J. 54:1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 12.Lipkin WI. 2010. Microbe hunting. Microbiol. Mol. Biol. Rev. 74:363–377. 10.1128/MMBR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Diab S, McGraw S, Barr B, Traslavina R, Higgins R, Talbot T, Blanchard P, Rimoldi G, Fahsbender E, Page B, Phan TG, Wang C, Deng X, Pesavento P, Delwart E. 2013. Divergent astrovirus associated with neurologic disease in cattle. Emerg. Infect. Dis. 19:1385–1392. 10.3201/eid1909.130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittelholzer C, Hedlund KO, Englund L, Dietz HH, Svensson L. 2003. Molecular characterization of a novel astrovirus associated with disease in mink. J. Gen. Virol. 84:3087–3094. 10.1099/vir.0.19267-0. [DOI] [PubMed] [Google Scholar]
- 15.Graber HU, Meyer RK, Fatzer R, Vandevelde M, Zurbriggen A. 1995. In situ hybridization and immunohistochemistry for prion protein (PrP) in bovine spongiform encephalopathy (BSE). Zentralbl. Veterinarmed. A 42:453–459. 10.1111/j.1439-0442.1995.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lower R, Lower J, Kurth R. 1996. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc. Natl. Acad. Sci. U. S. A. 93:5177–5184. 10.1073/pnas.93.11.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Etxebarria K, Jugo BM. 2010. Genome-wide detection and characterization of endogenous retroviruses in Bos taurus. J. Virol. 84:10852–10862. 10.1128/JVI.00106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Benedictis P, Schultz-Cherry S, Burnham A, Cattoli G. 2011. Astrovirus infections in humans and animals: molecular biology, genetic diversity, and interspecies transmissions. Infect. Genet. Evol. 11:1529–1544. 10.1016/j.meegid.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonassen CM, Jonassen TT, Sveen TM, Grinde B. 2003. Complete genomic sequences of astroviruses from sheep and turkey: comparison with related viruses. Virus Res. 91:195–201. 10.1016/S0168-1702(02)00269-1. [DOI] [PubMed] [Google Scholar]
- 22.Quan PL, Wagner TA, Briese T, Torgerson TR, Hornig M, Tashmukhamedova A, Firth C, Palacios G, Baisre-De-Leon A, Paddock CD, Hutchison SK, Egholm M, Zaki SR, Goldman JE, Ochs HD, Lipkin WI. 2010. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg. Infect. Dis. 16:918–925. 10.3201/eid1606.091536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blomstrom AL, Widen F, Hammer AS, Belak S, Berg M. 2010. Detection of a novel astrovirus in brain tissue of mink suffering from shaking mink syndrome by use of viral metagenomics. J. Clin. Microbiol. 48:4392–4396. 10.1128/JCM.01040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavier-Widen D, Brojer C, Dietz HH, Englund L, Hammer AS, Hedlund KO, Hard af Segerstad C, Nilsson K, Nowotny N, Puurula V, Thoren P, Uhlhorn H, Weissenbock H, Agren E, Klingeborn B. 2004. Investigations into shaking mink syndrome: an encephalomyelitis of unknown cause in farmed mink (Mustela vison) kits in Scandinavia. J. Vet. Diagn. Invest. 16:305–312. 10.1177/104063870401600408. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Wimmer E, Paul AV. 2009. Cis-acting RNA elements in human and animal plus-strand RNA viruses. Biochim. Biophys. Acta 1789:495–517. 10.1016/j.bbagrm.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyashita M, Stierstorfer B, Schmahl W. 2004. Neuropathological findings in brains of Bavarian cattle clinically suspected of bovine spongiform encephalopathy. J. Vet. Med. B Infect. Dis. Vet. Public Health 51:209–215. 10.1111/j.1439-0450.2004.00755.x. [DOI] [PubMed] [Google Scholar]
- 27.Walter JE, Mitchell DK. 2003. Astrovirus infection in children. Curr. Opin. Infect. Dis. 16:247–253. 10.1097/00001432-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Finkbeiner SR, Le BM, Holtz LR, Storch GA, Wang D. 2010. Detection of newly described astrovirus MLB1 in stool samples from children: response. Emerg. Infect. Dis. 16:169–170. 10.3201/eid1601.091563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkbeiner SR, Li Y, Ruone S, Conrardy C, Gregoricus N, Toney D, Virgin HW, Anderson LJ, Vinje J, Wang D, Tong SX. 2009. Identification of a novel astrovirus (astrovirus VA1) associated with an outbreak of acute gastroenteritis. J. Virol. 83:10836–10839. 10.1128/JVI.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapoor A, Li L, Victoria J, Oderinde B, Mason C, Pandey P, Zaidi SZ, Delwart E. 2009. Multiple novel astrovirus species in human stool. J. Gen. Virol. 90:2965–2972. 10.1099/vir.0.014449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.