Abstract
Pneumocystis jirovecii pneumonia (PCP) is a life-threatening infection in immunocompromised patients. Quantitative real-time PCR (qPCR) is more sensitive than microscopic examination for the detection of P. jirovecii but also detects colonized patients. Hence, its positive predictive value (PPV) needs evaluation. In this 4-year prospective observational study, all immunocompromised patients with acute respiratory symptoms who were investigated for PCP were included, totaling 659 patients (814 bronchoalveolar lavage fluid samples). Patients with negative microscopy but positive qPCR were classified through medical chart review as having retained PCP, possible PCP, or colonization, and their clinical outcomes were compared to those of patients with microscopically proven PCP. Overall, 119 patients were included for analysis, of whom 35, 41, and 43 were classified as having retained PCP, possible PCP, and colonization, respectively. The 35 patients with retained PCP had clinical findings similar to those with microscopically proven PCP but lower fungal loads (P < 0.001) and were mainly non-HIV-infected patients (P < 0.05). Although the mean amplification threshold was higher in colonized patients, it was not possible to determine a discriminant qPCR cutoff. The PPV of qPCR in patients with negative microscopy were 29.4% and 63.8% when considering retained PCP and retained plus possible PCP, respectively. Patients with possible PCP had a higher mortality rate than patients with retained PCP or colonization (63% versus 3% and 16%, respectively); patients who died had not received co-trimoxazole. In conclusion, qPCR is a useful tool to diagnose PCP in non-HIV patients, and treatment might be better targeted through a multicomponent algorithm including both clinical/radiological parameters and qPCR results.
INTRODUCTION
The ascomycete fungus Pneumocystis jirovecii is responsible for Pneumocystis pneumonia (PCP), a life-threatening infection in immunocompromised patients that ranks first among opportunistic pathogens, revealing HIV-positive status when CD4+ T lymphocyte counts fall below 200 cells/μl (1). Moreover, PCP is also of increasing importance in non-HIV immunocompromised patients such as transplant patients, patients with hematological malignancies or solid cancers, and patients receiving corticosteroid therapy (CST) or other immunosuppressive drugs within the framework of connective tissue diseases or chronic inflammatory diseases (2–5), reaching 50% of cases in the most recent series (6, 7). Although chemoprophylaxis guidelines recommend co-trimoxazole in transplant patients for 6 to 12 months following transplantation, there is currently no consensus on chemoprophylaxis in other non-HIV immunocompromised patients, except for granulomatosis with polyangiitis (Wegener's granulomatosis) (8). The prognosis of PCP in non-HIV patients is poorer and the evolution more acute, with a shorter delay between onset of symptoms and hospitalization (6, 9). Therefore, rapid diagnosis is essential.
PCP diagnosis currently relies on the demonstration of trophic forms or cysts after microscopic examination of bronchoalveolar lavage fluid (BALF) using adequate staining methods (May-Grünwald-Giemsa, Gomori-Grocott, or immunofluorescence assay). However, microscopic diagnosis is difficult and requires specific skills, particularly when fungal burdens are low, and thus may be falsely negative. Various PCR targets and methods have been developed (10–12), and quantitative real-time PCR (qPCR) has progressively supplanted conventional PCR. Quantitative PCR is usually used to exclude PCP diagnosis because its negative predictive value (NPV) is nearly 100% (13). However, although the good sensitivity of qPCR allows for diagnosis in patients with low fungal loads, it also leads to the overdetection of P. jirovecii DNA in patients with colonization. Indeed, PCR has contributed to demonstrating the concept of a dynamic reservoir of P. jirovecii, with possible transient asymptomatic carriage, as observed in immunocompetent subjects or in the proximity of infected patients (14). Therefore, a positive PCR without microscopic detection is difficult to interpret (15).
The aim of this study was to evaluate the positive predictive value (PPV) of a qPCR method routinely used in our lab. We retrospectively reviewed all cases with positive BALF P. jirovecii qPCR over a 4-year period (2009 to 2012), focusing on patients with negative or ambiguous microscopy and positive qPCR, with the goal of defining the positive predictive value (PPV) of qPCR in these population of patients, and the benefit for care management.
MATERIALS AND METHODS
Patients and BALF samples.
All BALF samples with P. jirovecii detection from January 2009 to December 2012 were included. During this 4-year period, 814 BALF samples from 659 immunocompromised patients (785 episodes) were analyzed for P. jirovecii. As our lab does not perform systematic diagnostic panels, this examination was guided by clinical and/or radiological signs in patients at risk. BALF was collected during the course of routine care management, as previously described (16); thus, no particular consent was obtained. The samples were sent to laboratories for the detection of various infectious agents (bacteria, fungi, viruses) at the discretion of the clinician.
Microscopic examination for P. jirovecii.
BALF samples were digested with Digest-EUR (Eurobio, Courtaboeuf, France), and 4 to 6 slides were cytocentrifuged and stained with May-Grünwald-Giemsa (MGG) and immunofluorescence assay (IFA) (Monofluokit, Bio-Rad, Marnes-la-Coquette, France). Cyst detection by IFA was graduated, according to the manufacturer's recommendations, and considered ambiguous when <5 cysts were detected.
Molecular diagnosis.
After the centrifugation of 1 ml of BALF, DNA extraction was performed using a 200-μl pellet with the Qiagen DNA minikit tissue (Qiagen, Courtaboeuf, France). The qPCR technique targeted the mitochondrial large subunit rRNA (mtLSU), as described previously (17), and amplification was performed using a Step One plus device (Applied Biosystems, Saint Aubin, France) following the standard amplification protocol for 40 cycles, preceded by 10 min at 50°C for uracil-DNA glycosylase activity. The amplification threshold was fixed at 0.02 to allow cycle threshold (CT) comparison between runs. Adequate controls (positive, negative, and extraction) were included in each run.
Data collection and classification of patients.
The medical charts of all patients with positive P. jirovecii qPCR detection and negative or ambiguous microscopic examination (MGG negative and IFA ambiguous) were retrospectively reviewed by a multidisciplinary team (a parasitologist, an infectious diseases specialist, and an intensive care unit practitioner). The following data were recorded for analysis: age, sex, clinical signs at the time of diagnosis, immune background, and immunosuppression factors, Pneumocystis chemoprophylaxis, chest imaging findings (X-ray or computed tomography [CT] scan when available), any documented pulmonary coinfection or any noninfectious etiology that could possibly result in respiratory failure or imaging findings, specific treatment, and outcome. The patients were classified according to the following criteria.
The diagnosis of PCP was retained when (i) at least three of the four following items were present, cough, fever, dyspnea, and compatible radiography or CT scan, and (ii) a favorable outcome was obtained under co-trimoxazole therapy, provided that no other infectious agent was found and that no simultaneous immuno-allergic etiology explained the respiratory symptoms or (iii) PCP was confirmed by histological examination postmortem.
Possible PCP was defined by (i) the presence of at least two of the four following items, cough, fever, dyspnea, compatible radiography, or CT scan, and (ii) co-trimoxazole treatment was not clearly evaluable because an alternate diagnosis was suspected or simultaneously treated or (iii) the patient died early.
Colonization with P. jirovecii was defined by (i) favorable clinical outcome in the absence of co-trimoxazole treatment or (ii) death due to a duly identified organic cause and not attributable to PCP.
Patients with microscopically proven PCP (i.e., the presence of P. jirovecii trophic forms or cysts detected by MGG and/or IFA) were used as a control group to compare the epidemiologic and diagnostic data.
Statistical analysis.
PPV and NPV were calculated using standard formulas. Qualitative variables were analyzed using the chi-square test or Fisher's exact test. Quantitative variables were compared using the Mann-Whitney test. The statistical analysis was performed with GraphPad Prism 5.0 software.
RESULTS
Threefold more patients were identified with positive PCR than with positive direct examination.
A total of 43 of 659 (6.5%) immunocompromised patients with respiratory symptoms were diagnosed with PCP after microscopic examination on the basis of the presence of trophic forms or cysts on MGG slides and/or IFA slides (Fig. 1). One patient had two distinct episodes of PCP at an approximately 1-year interval and thus was counted twice. In 23 cases, the microscopic slides were very demonstrative, and qPCR was not performed (n = 23). In the 20 remaining cases, qPCR was performed and was always positive (n = 20). In 497 patients, both microscopic examination and qPCR were negative, and the diagnosis of PCP was excluded. In the 119 remaining patients (namely, the PCR-positive [PCR+] group), MGG staining was negative and qPCR was positive, whereas IFA was negative in 103/119 (87%) or ambiguous in 16/119 (13%) patients. The prevalence of a positive qPCR result among patients with a negative direct examination and pulmonary symptoms was 19%.
FIG 1.
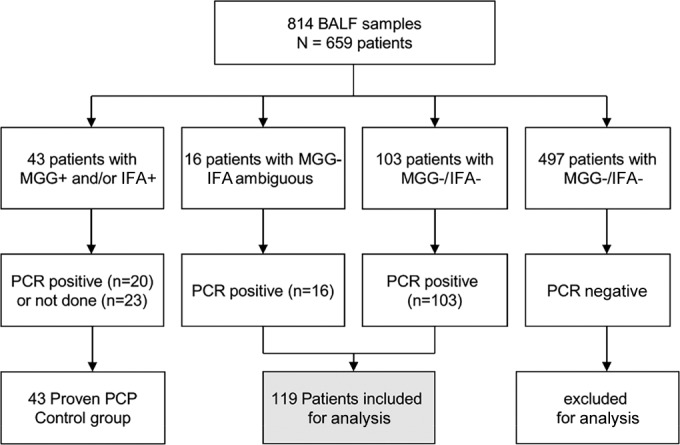
Flowchart of the BALF samples prospectively analyzed over a 4-year period.
Patients with isolated positive PCR have different underlying risk factors than patients with microscopy-proven PCP.
Among the 43 patients with proven PCP, the most frequent risk factor was HIV infection (47%), followed by corticosteroid therapy (CST) with or without combined immunosuppressive drug (21%), solid cancer (16%), hematological malignancy (9%), and transplantation (5%) (Table 1). Conversely, in patients with a negative direct examination and positive qPCR, the most frequently observed risk factors were CST (30%), hematological malignancy (29%), and solid cancer (20%); HIV infection accounted for only 4% of the cases (Table 1). Taken together, the HIV-infected patients were more likely to be diagnosed with PCP by direct examination (P < 0.0001) than the non-HIV patients, whereas the patients with hematological malignancy most likely had a positive qPCR and negative direct examination (P < 0.05). Other risk factors did not differ between the groups (Table 1).
TABLE 1.
Underlying risk factors in patients with proven PCPa and in patients with positive PCR and negative direct examination
| Risk factor | MGG+- and/or IFA+-proven PCP (n = 43) (n [%])b | PCR+ group (n = 119) |
P valuec | |||
|---|---|---|---|---|---|---|
| Retained PCP (n = 35) (n [%]) | Possible PCP (n = 41) (n [%]) | Colonization (n = 43) (n [%]) | Total (n = 119) (n [%]) | |||
| HIV | 20 (47) | 4 (12) | 0 | 1 (2) | 5 (4) | <0.0001 |
| Hematological malignancy | 4 (9) | 13 (37) | 9 (22) | 12 (28) | 34 (29) | 0.0112 |
| Corticosteroid with or without other ISDd | 9 (21) | 5 (14) | 15 (37) | 16 (37) | 36 (30) | 0.321 (NS) |
| Solid cancer | 7 (16) | 8 (23) | 14 (34) | 2e (5) | 24 (20) | 0.657 (NS) |
| Transplantation | 2 (5) | 5 (14) | 3 (7) | 4f (9) | 12 (10) | 0.357 (NS) |
| Other | 1 (2) | 0 | 0 | 8g (19) | 8 (7) | 0.451 (NS) |
PCP, Pneumocystis pneumonia.
MGG, May-Grünwald-Giemsa staining; IFA, immunofluorescence assay.
The P value was calculated using Fisher's exact test for each risk factor. NS, not significant.
ISD, immunosuppressive drug.
Significant difference (P < 0.01) compared to the retained PCP or possible PCP groups.
One transplant patient was also HIV+.
Miscellaneous underlying conditions consisting of 1 rheumatoid arthritis treated with abatacept, 1 chronic alcoholism, 1 proteinosis, 3 chronic obstructive pulmonary disease, and 2 cirrhosis cases; P < 0.001 compared to the retained PCP and possible PCP groups.
Approximately one-third of the patients who were negative for Pneumocystis by microscopy but positive by qPCR developed PCP.
Among the 119 patients of the PCR+ group, PCP was retained as the final diagnosis in 35 (29%) patients; 34 were successfully treated by co-trimoxazole, and PCP was confirmed at autopsy in the last case. A total of 43 (36%) patients were considered to be colonized and presented less frequently with solid cancer than the patients with retained PCP (P < 0.01) but more frequently with mild risk factors, such as cirrhosis or chronic obstructive pulmonary disease (Table 1). These colonized patients were usually not treated (Table 2); 4 of them died from acute evolution of their underlying disease or another documented infection, and the attributability of P. jirovecii was not acknowledged.
TABLE 2.
Patient characteristics according to the result of Pneumocystis jirovecii direct examination
| Characteristica | MGG+-and/or IFA+-proven PCPb (n = 43) | PCR+ group (n = 119) |
P valuec | ||
|---|---|---|---|---|---|
| Retained PCP (n = 35) | Possible PCP (n = 41) | Colonization (n = 43) | |||
| Age (mean ± SEM) (yr) | 55 ± 2 | 62 ± 2 | 68 ± 2 | 62 ± 2 | <0.001 |
| Sex ratio (no. M/no. F) | 1.39 | 1.5 | 1.9 | 2.07 | 0.782 (NS) |
| Hypoxemia (n [%]) | 29d (67) | 26 (74) | 32 (78) | 30 (70) | 0.129 (NS) |
| Radiological findings (n [%]) | |||||
| Diffuse interstitial infiltrate | 27 (63) | 22 (63) | 28 (68) | 14 (33) | <0.01 |
| GGO | 22 (51) | 20 (57) | 26 (63) | 18 (42) | <0.01 |
| Nodules | 3 (7) | 6 (17) | 7 (17) | 8 (18) | 0.356 (NS) |
| Condensations | 3 (7) | 3 (9) | 8 (19.5) | 8 (18) | 0.329 (NS) |
| Other | 3 (7) | 5 (14) | 13 (32) | 17 (39) | <0.01 |
| Coinfectione (n [%]) | 3 (7) | 1 (3) | 12 (29) | 20 (46) | <0.0001 |
| Bacteria | 2 | 0 | 9 | 4 | |
| Virus | 1 | 1 | 3 | 11 | |
| Aspergillus | 1 | 0 | 1 | 8 | |
| IFA ambiguous | 0 | 9 (26) | 4 (10) | 3 (7) | <0.01 |
| Co-trimoxazole treatment (n [%]) | 43 (100) | 35 (100) | 32f (78) | 5g (12) | <0.0001 |
| Mortality (<1 mo) (n [%]) | 8 (19) | 1 (3) | 26h (63) | 7i (16) | <0.0001 |
M, male; F, female; GGO, ground-glass opacities.
MGG, May-Grünwald-Giemsa staining; IFA, immunofluorescence assay; PCP, Pneumocystis pneumonia.
Qualitative variables were analyzed using Fisher's exact test, and quantitative variables were analyzed using the Mann-Whitney test.
Unavailable data in 5 cases.
Multiple codetection of infectious agents was observed in some patients.
One patient received pentamidine after co-trimoxazole because of allergy.
Two additional patients started prophylaxis in light of the PCR results (one kidney transplant patient and one HIV+ patient).
Of whom 9 had not been treated.
Of whom 2 were treated.
In 41 (34%) patients, PCP was considered possible but was not formally retained. Thirty-two (78%) of these patients were treated, but the effect of co-trimoxazole was not clearly evaluable because (i) therapy with potentially deleterious pulmonary side effects (e.g., methotrexate or amiodarone [Cordarone; Wyeth Pharmaceuticals, Inc.]) was simultaneously removed, which might have contributed to clinical improvement (5 patients), (ii) the patient died early after the onset of co-trimoxazole (7 cases) or before co-trimoxazole therapy (9 cases), or (iii) another infectious agent was simultaneously detected (12 cases) (Table 2). The PPV of qPCR within the PCR+ group was 29.4% (35/119 patients) and increased to 63.8% (76/119) when definite PCP and possible PCP were combined.
Mean amplification cycle threshold (CT) differs between groups of patients according to final diagnosis and immune background.
As we used a multiple-copy gene as the PCR target, the quantification of P. jirovecii with regard to copies/ml of sample was not reliable; thus, we chose to express results as the mean number of amplification cycles necessary to reach the qPCR positivity threshold (CT). CT was significantly much lower in patients with a positive direct examination (proven PCP) than in patients with definite PCP but negative direct examination (23.3 ± 1.3 and 34.9 ± 0.3, respectively, P < 0.001) (Fig. 2A), reflecting higher fungal loads. Regarding the underlying risk factors, the mean CT obtained for HIV-infected patients with proven or retained PCP was lower than for non-HIV patients (24.4 ± 1.9 versus 32.4 ± 0.7, P < 0.001) (Fig. 2B). Within the PCR+ group, a significantly lower CT was observed in patients with retained PCP than in colonized patients (34.1 ± 0.5 versus 36.1 ± 0.3, P < 0.001). Similarly, the mean CT obtained for patients with possible PCP (34.6 ± 0.5) was lower than for patients with P. jirovecii colonization (P < 0.05) (Fig. 2). The distribution of CT results was large and similar between the three subgroups, making it difficult to set a CT to diagnose PCP with 100% specificity. When considering a CT at 32, the overall sensitivity would be 51%, but only 8/35 patients with a negative direct examination would have been diagnosed. A CT at 35 would yield an overall sensitivity of 75% but would detect only 21/35 microscopy-negative PCP cases (60%).
FIG 2.
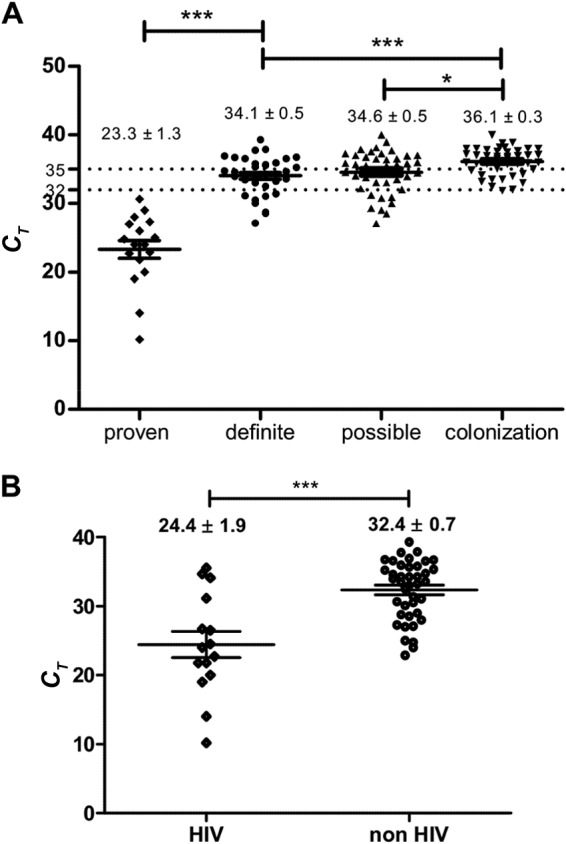
Results of qPCR (CT of amplification) according to final diagnosis (A) or HIV status (B), expressed as the mean ± SEM. The values were compared using t tests. Statistically significant differences are marked as *, P < 0.05 or ***, P < 0.001.
Clinical setting and outcome differ between groups of patients.
The patients with microscopy-proven PCP differed from the whole PCR+ group with respect to age, frequency of coinfection, and outcome but shared similar clinical characteristics as patients with retained or possible PCP (Table 2). Differences were also observed within the PCR+ group. (i) Patients with possible PCP were significantly older than colonized patients (P < 0.05) and had higher mortality than colonized patients and PCP patients (P < 0.001), (ii) colonized patients more frequently had atypical imaging findings than patients with possible or retained PCP and were less likely to present with diffuse interstitial infiltrates (P < 0.01), (iii) coinfections were most frequently documented in patients with possible PCP and in colonized patients than in others, but this was a criterion for excluding a formal PCP diagnosis in the PCR+ group and thus cannot be taken into consideration. As the management of respiratory symptoms in patients with possible PCP is difficult in routine practice, we explored characteristics that could guide decision to treat. Despite nonsignificant statistical tests, it is noteworthy that 56% of the untreated patients had another infectious agent detected, which explained their respiratory symptoms, whereas only 22% of the treated patients had a documented coinfection (P = 0.097, Table 3). Conversely, the treated patients were more likely to have an alternate noninfectious diagnosis supporting the pulmonary symptoms and imaging findings than the untreated patients (72% versus 44%, respectively). A trend toward a higher proportion of patients with typical imaging findings (bilateral infiltrates) was also observed in treated patients. Survival was null in the untreated patients (P < 0.05), yielding an odds ratio of 15.74 (95% confidence interval [CI], 0.84 to 294) for death in cases of a lack of treatment. The mean survival duration was short (8 ± 1.1 days).
TABLE 3.
Characteristics of patients with possible PCPa according to onset of treatment
| Characteristicb | Possible PCP (n = 41) |
P value | |
|---|---|---|---|
| Treated (n = 32) | Untreatedc (n = 9) | ||
| Coinfection (n [%]) | 7 (22) | 5 (56) | 0.097 |
| Other possible noninfectious etiology (n [%]) | 23 (72) | 4 (44) | 0.12 |
| Typical imaging findings (n [%]) | 23 (72) | 4 (44) | 0.231 |
| qPCR result (mean ± SEM) (CT) | 34.8 ± 0.5 | 33.7 ± 1.3 | 0.404 |
| Survival (n [%]) | 15 (47) | 0 (0) | 0.016d |
| Survival time (mean ± SEM) (days) | 11 ± 1.7 | 8 ± 1.4 | 0.244 |
PCP, Pneumocystis pneumonia.
CT, cycle threshold.
Underlying risk factors consisted of 3 corticosteroid therapy cases, 4 hematological malignancies, 1 solid-organ transplant, and 1 solid cancer.
P < 0.05.
Survival is poorer for patients with negative IFA.
There was a trend toward lower CT values in BALF samples with ambiguous IFA results than in BALF samples with negative IFA, though this was not statistically significant (Table 4). An ambiguous IFA result was observed in 9 and 4 patients with retained and possible PCP, respectively, but also in 3 untreated colonized patients who survived. However, IFA results, even though ambiguous, might have been frequently taken into account, as 87% of the patients received co-trimoxazole, whereas only 56% with negative IFA were treated (P < 0.05). A fatal outcome was less frequently observed in the patients with ambiguous IFA than in the patients with negative IFA (P < 0.05), despite similar underlying risk factors between these groups (Table 4).
TABLE 4.
PCR quantification, decision to treat, clinical outcome, and underlying risk factors according to the results of IFA in patients with positive PCR but negative direct examination (n = 119)
| Characteristic | IFAa ambiguous (n = 16) | IFA negative (n = 103) | P value |
|---|---|---|---|
| qPCR result (mean ± SEM) (CT) | 33.7 ± 0.9 | 35.2 ± 0.3 | 0.075 (NSb) |
| Treatment (n [%]) | 14 (87) | 58 (56) | 0.025c |
| Mortality (n [%]) | 1 (6) | 34 (33) | 0.037c |
| Risk factor [n (no. fatal cases)] | |||
| HIV | 2 (0) | 3 (0) | 0.133 (NS) |
| Hematological malignancy | 4 (0) | 30 (11) | 1 (NS) |
| Corticosteroids | 5 (0) | 31 (11) | 1 (NS) |
| Cancer | 3 (1) | 21 (10) | 1 (NS) |
| Transplantation | 2 (0) | 10 (2) | 0.664 (NS) |
| Other | 0 | 8 (0) | 0.596 (NS) |
IFA, immunofluorescence assay.
NS, not significant.
P < 0.05.
DISCUSSION
PCP is an opportunistic infection of growing importance in non-HIV patients (18), as illustrated again in this study. HIV-noninfected patients accounted for 53% of PCP diagnosed by microscopic examination and 69% (54/78) of PCP cases when we pooled the 35 patients with isolated positive qPCR results. It is well acknowledged that the diagnosis of PCP is particularly tricky in these patients, who can develop rapidly progressive PCP even with low parasite loads (6). In agreement with already published data (1, 19), higher parasite loads were detected in the HIV-infected patients than the non-HIV patients (P < 0.001), emphasizing the need for sensitive detection methods. In particular, patients with hematological malignancies were more likely to have a negative direct examination, and 13/17 (76%) were diagnosed only by qPCR, whereas only 4/24 (17%) HIV+ patients had an isolated positive qPCR (Table 1). PCR was also the only positive marker for PCP in 5/14 (36%) CST patients, in 5/7 (71%) solid-organ transplant patients, and in 8/15 (53%) cancer patients.
Patients with a retained PCP diagnosis and patients with microscopically proven PCP did not differ significantly regarding clinical signs or outcome, thus bolstering the retrospective classification of cases.
The group of colonized patients differed from the others, since they presented with lower mean CT values and a greater heterogeneity of imaging findings, most likely explained by frequent coinfections. The observation of ambiguous IFA results for 3 colonized patients who were not treated but still recovered also indicates that this test is not completely reliable for diagnosing PCP.
Patients with possible PCP are obviously a challenging group. They had higher mortality rates than colonized patients and patients with retained PCP diagnosis. These data suggest that even if P. jirovecii was not recognized as the main cause of disease, it might play an important role as a morbidity cofactor in patients with a severe pulmonary background (15, 20, 21). Indeed, death occurred within a context of septic shock and/or multiorgan failure in 9 cases, of acute respiratory distress in 12, or of diffuse lung tumor infiltrate in 5 cases. It appears that the clinicians were more likely to disregard co-trimoxazole onset if another infectious etiology was documented simultaneously than when the patient was receiving drugs with possible toxic pulmonary side effects (yet the drug was immediately withdrawn). The mean CT values were similar in the treated and untreated patients and thus most likely did not influence the clinicians with regard to the patients' care management (Table 3). The observation that mortality was 100% in the 9 patients with possible PCP who did not receive co-trimoxazole raises questions on whether P. jirovecii contributed to their deaths, despite the fact that five of them were documented with another infection. As stated by Asai et al. (22), the severity and rapid progression of PCP in non-HIV patients should not be underestimated, even in cases of moderate or mild respiratory failure at admission, and a delay in diagnosis or treatment rapidly increases mortality.
The care management of colonized patients is debatable. On the one hand, the immediate outcome is generally good without specific treatment, as observed in this study. On the other hand, some studies have shown that these patients had poorer survival at 1 year if left untreated (23) and that colonization might be a first step toward PCP within a few months (24). It has been proposed that patients with inflammatory rheumatic diseases or systemic autoimmune diseases should be screened for P. jirovecii colonization and treated prior to the prescription of immunosuppressive therapy (25, 26). Moreover, independently of the potential self-benefit of co-trimoxazole treatment for patients with P. jirovecii detection, a growing number of studies have stressed the risk of colonized patients infecting other immunocompromised patients during hospital care or consultations, as recently shown in renal transplant patients (5, 27) (28, 29). Therefore, future strategies could also consider treatment, at least in targeted patients or hospital care units, to prevent airborne dissemination (14, 30).
Despite numerous studies reported in the literature, the place of Pneumocystis PCR is not clearly stated; a positive qPCR result in the absence of a positive IFA is not considered a definite criterion of infection, and patients fulfilling these diagnostic characteristics are usually not included (though treated) in epidemiological studies or recorded in reporting systems (7). In view of the present study, we think that they should be included, provided that improvement is obtained by co-trimoxazole and alternate diagnosis is lacking.
To date, most studies have aimed at defining a qPCR threshold based on IFA results that would distinguish infection and colonization (23, 31–34), but such an approach simply provides the threshold at which there is a good correlation between microscopic and molecular techniques, which has little relevance for diagnosing PCP in non-HIV patients. Indeed, it appears difficult to set a limit, and recent works suggest the definition of two thresholds: a “high” threshold that would diagnose PCP with 100% specificity and a “low” threshold that would indicate colonization with maximal specificity (6, 23, 32, 35, 36). In fact, there are cases that inevitably remain in the gray zone; their diagnosis is uncertain, leading to suboptimal management. Additionally, quantification data are not comparable among studies and techniques (single-copy or multiple-copy genes). Another important pitfall that complicates the setting of a quantification threshold is that Pneumocystis is a polymorphic microorganism developing in its host as a trophic form (single genome) and cysts (multiple genomes), with an unknown ratio of these stages. Furthermore, accurate quantification in copy number/ml does not reflect the real number of cysts, which are presumed to be the result of the reproductive cycle during infection. In our study, the CT values from patients with retained PCP, possible PCP, and colonization completely overlapped, thus illustrating the difficulty of fungal quantification and the meaninglessness of a quantification threshold in microscopy-negative BALF.
To our knowledge, this 4-year study includes the most important homogenous series of BALF samples from immunocompromised patients prospectively investigated within the framework of the routine investigation of acute respiratory symptoms. There are, however, some limitations because the data were analyzed retrospectively. The study was not designed to analyze prognoses and the severities of underlying background conditions, which can play an important role in the clinical expression of PCP and thus should be considered. Conversely, the strength of this study relies on the homogenous classification of cases a posteriori using sharply defined criteria, by a multidisciplinary team, and without knowledge of the level of positivity of qPCR results. Therefore, it can be assumed that the PPV of qPCR was not overestimated.
The originality of this work is not only that it provides data on diagnostic performance but also that it opens an innovative way of interpreting a positive qPCR result according to a patient's characteristics. As a qPCR threshold does not appear to be an adequate solution for guiding co-trimoxazole treatment, we suggest that a patient profile based on multiple parameters, such as clinical and radiological parameters, underlying background, immunosuppressive therapies, age, comorbidities, or coinfections (37), should be taken into account in the building of a decision tree. Moreover, the association of other biological parameters, such as serum dosage of β-1,3-d glucan, should be included for evaluation, as promising results have been reported for small series in combination with qPCR (35, 38, 39). This approach could benefit from the experience gained in the field of aspergillosis. Indeed, the diagnosis of invasive aspergillosis, which has been long underestimated in nonneutropenic patients (40), now relies on the association of multiple biological and clinical parameters (41) to classify patients as having certain, probable, or possible aspergillosis. More recently, a similar strategy was proposed to determine whether patients with cystic fibrosis and Aspergillus colonization should be treated (42). Such a process for the management of patients with Pneumocystis-positive qPCR is needed and should rely on a future prospective multicenter study.
Footnotes
Published ahead of print 9 July 2014
REFERENCES
- 1.Thomas C, Jr, Limper A. 2004. Pneumocystis pneumonia. N. Engl. J. Med. 350:2487–2498. 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 2.Sepkowitz KA. 2002. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin. Infect. Dis. 34:1098–1107. 10.1086/339548. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa J, Harigai M, Nagasaka K, Nakamura T, Miyasaka N. 2005. Prediction of and prophylaxis against Pneumocystis pneumonia in patients with connective tissue diseases undergoing medium- or high-dose corticosteroid therapy. Mod. Rheumatol. 15:91–96. 10.3109/PL00021707. [DOI] [PubMed] [Google Scholar]
- 4.Tasaka S, Tokuda H. 2012. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J. Infect. Chemother. 18:793–806. 10.1007/s10156-012-0453-0. [DOI] [PubMed] [Google Scholar]
- 5.Fritzsche C, Riebold D, Fuehrer A, Mitzner A, Klammt S, Mueller-Hilke B, Reisinger EC. 2013. Pneumocystis jirovecii colonization among renal transplant recipients. Nephrology 18:382–387. 10.1111/nep.12054. [DOI] [PubMed] [Google Scholar]
- 6.Alanio A, Desoubeaux G, Sarfati C, Hamane S, Bergeron A, Azoulay E, Molina JM, Derouin F, Menotti J. 2011. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin. Microbiol. Infect. 17:1531–1537. 10.1111/j.1469-0691.2010.03400.x. [DOI] [PubMed] [Google Scholar]
- 7.Magne D, Angoulvant A, Botterel F, Bouges-Michel C, Bougnoux ME, Bouree P, Chochillon C, Cornet M, Dannaoui E, Fekkar A, Galeazzi G, Yera H, Sarfati C, Roux P. 2011. Pneumocystosis: a network survey in the Paris area 2003–2008. Eur. J. Clin. Microbiol. Infect. Dis. 30:673–675. 10.1007/s10096-010-1139-0. [DOI] [PubMed] [Google Scholar]
- 8.Green H, Paul M, Vidal L, Leibovici L. 2007. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin. Proc. 82:1052–1059. 10.4065/82.9.1052. [DOI] [PubMed] [Google Scholar]
- 9.Limper AH, Offord KP, Smith TF, Martin WJ., 2nd 1989. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 140:1204–1209. [DOI] [PubMed] [Google Scholar]
- 10.Wakefield AE, Pixley FJ, Banerji S, Sinclair K, Miller RF, Moxon ER, Hopkin JM. 1990. Detection of Pneumocystis carinii with DNA amplification. Lancet 336:451–453. 10.1016/0140-6736(90)92008-6. [DOI] [PubMed] [Google Scholar]
- 11.Fan LC, Lu HW, Cheng KB, Li HP, Xu JF. 2013. Evaluation of PCR in bronchoalveolar lavage fluid for diagnosis of Pneumocystis jirovecii pneumonia: a bivariate meta-analysis and systematic review. PLoS One 8:e73099. 10.1371/journal.pone.0073099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robberts FJ, Liebowitz LD, Chalkley LJ. 2007. Polymerase chain reaction detection of Pneumocystis jiroveci: evaluation of 9 assays. Diagn. Microbiol. Infect. Dis. 58:385–392. 10.1016/j.diagmicrobio.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Durand-Joly I, Chabe M, Soula F, Delhaes L, Camus D, Dei-Cas E. 2005. Molecular diagnosis of Pneumocystis pneumonia. FEMS Immunol. Med. Microbiol. 45:405–410. 10.1016/j.femsim.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Choukri F, Menotti J, Sarfati C, Lucet JC, Nevez G, Garin YJ, Derouin F, Totet A. 2010. Quantification and spread of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis pneumonia. Clin. Infect. Dis. 51:259–265. 10.1086/653933. [DOI] [PubMed] [Google Scholar]
- 15.Morris A, Norris KA. 2012. Colonization by Pneumocystis jirovecii and its role in disease. Clin. Microbiol. Rev. 25:297–317. 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jouneau S, Poineuf JS, Minjolle S, Tattevin P, Uhel F, Kerjouan M, Le Ho H, Desrues B. 2013. Which patients should be tested for viruses on bronchoalveolar lavage fluid? Eur. J. Clin. Microbiol. Infect. Dis. 32:671–677. 10.1007/s10096-012-1791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meliani L, Develoux M, Marteau-Miltgen M, Magne D, Barbu V, Poirot JL, Roux P. 2003. Real time quantitative PCR assay for Pneumocystis jirovecii detection. J. Eukaryot. Microbiol. 50(Suppl):651. [DOI] [PubMed] [Google Scholar]
- 18.Azoulay E, Bergeron A, Chevret S, Bele N, Schlemmer B, Menotti J. 2009. Polymerase chain reaction for diagnosing pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest 135:655–661. 10.1378/chest.08-1309. [DOI] [PubMed] [Google Scholar]
- 19.Hauser PM, Bille J, Lass-Florl C, Geltner C, Feldmesser M, Levi M, Patel H, Muggia V, Alexander B, Hughes M, Follett SA, Cui X, Leung F, Morgan G, Moody A, Perlin DS, Denning DW. 2011. Multicenter, prospective clinical evaluation of respiratory samples from subjects at risk for Pneumocystis jirovecii infection by use of a commercial real-time PCR assay. J. Clin. Microbiol. 49:1872–1878. 10.1128/JCM.02390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.To KK, Hung IF, Xu T, Poon RW, Ip WC, Li PT, Li CP, Lau SK, Yam WC, Chan KH, Yuen KY. 2013. Clinical significance of Pneumocystis jiroveci in patients with active tuberculosis. Diagn. Microbiol. Infect. Dis. 75:260–265. 10.1016/j.diagmicrobio.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Norris KA, Morris A. 2011. Pneumocystis infection and the pathogenesis of chronic obstructive pulmonary disease. Immunol. Res. 50:175–180. 10.1007/s12026-011-8218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asai N, Motojima S, Ohkuni Y, Matsunuma R, Nakasima K, Iwasaki T, Nakashita T, Otsuka Y, Kaneko N. 2012. Non-HIV Pneumocystis pneumonia: do conventional community-acquired pneumonia guidelines under estimate its severity? Multidiscip Respir. Med. 7:2. 10.1186/2049-6958-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botterel F, Cabaret O, Foulet F, Cordonnier C, Costa JM, Bretagne S. 2012. Clinical significance of quantifying Pneumocystis jirovecii DNA by using real-time PCR in bronchoalveolar lavage fluid from immunocompromised patients. J. Clin. Microbiol. 50:227–231. 10.1128/JCM.06036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori S, Cho I, Sugimoto M. 2009. A follow-up study of asymptomatic carriers of Pneumocystis jiroveci during immunosuppressive therapy for rheumatoid arthritis. J. Rheumatol. 36:1600–1605. 10.3899/jrheum.081270. [DOI] [PubMed] [Google Scholar]
- 25.Mekinian A, Durand-Joly I, Hatron PY, Moranne O, Denis G, Dei-Cas E, Morell-Dubois S, Lambert M, Launay D, Delhaes L, Hachulla E, Queyrel V. 2011. Pneumocystis jirovecii colonization in patients with systemic autoimmune diseases: prevalence, risk factors of colonization and outcome. Rheumatology 50:569–577. 10.1093/rheumatology/keq314. [DOI] [PubMed] [Google Scholar]
- 26.Wissmann G, Morilla R, Martin-Garrido I, Friaza V, Respaldiza N, Povedano J, Praena-Fernandez JM, Montes-Cano MA, Medrano FJ, Goldani LZ, de la Horra C, Varela JM, Calderon EJ. 2011. Pneumocystis jirovecii colonization in patients treated with infliximab. Eur. J. Clin. Invest. 41:343–348. 10.1111/j.1365-2362.2010.02415.x. [DOI] [PubMed] [Google Scholar]
- 27.Mori S, Sugimoto M. 2012. Pneumocystis jirovecii infection: an emerging threat to patients with rheumatoid arthritis. Rheumatology 51:2120–2130. 10.1093/rheumatology/kes244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damiani C, Choukri F, Le Gal S, Menotti J, Sarfati C, Nevez G, Derouin F, Totet A. 2012. Possible nosocomial transmission of Pneumocystis jirovecii. Emerg. Infect. Dis. 18:877–878. 10.3201/eid1805.111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Gal S, Damiani C, Rouille A, Grall A, Treguer L, Virmaux M, Moalic E, Quinio D, Moal MC, Berthou C, Saliou P, Le Meur Y, Totet A, Nevez G. 2012. A cluster of Pneumocystis infections among renal transplant recipients: molecular evidence of colonized patients as potential infectious sources of Pneumocystis jirovecii. Clin. Infect. Dis. 54:e62–71. 10.1093/cid/cir996. [DOI] [PubMed] [Google Scholar]
- 30.Menotti J, Emmanuel A, Bouchekouk C, Chabe M, Choukri F, Pottier M, Sarfati C, Aliout el M, Derouin F. 2013. Evidence of airborne excretion of Pneumocystis carinii during infection in immunocompetent rats. Lung involvement and antibody response. PLoS One 8:e62155. 10.1371/journal.pone.0062155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhlethaler K, Bogli-Stuber K, Wasmer S, von Garnier C, Dumont P, Rauch A, Muhlemann K, Garzoni C. 2012. Quantitative PCR to diagnose Pneumocystis pneumonia in immunocompromised non-HIV patients. Eur. Respir. J. 39:971–978. 10.1183/09031936.00095811. [DOI] [PubMed] [Google Scholar]
- 32.Fillaux J, Malvy S, Alvarez M, Fabre R, Cassaing S, Marchou B, Linas MD, Berry A. 2008. Accuracy of a routine real-time PCR assay for the diagnosis of Pneumocystis jirovecii pneumonia. J. Microbiol. Methods 75:258–261. 10.1016/j.mimet.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Larsen HH, Masur H, Kovacs JA, Gill VJ, Silcott VA, Kogulan P, Maenza J, Smith M, Lucey DR, Fischer SH. 2002. Development and evaluation of a quantitative, touch-down, real-time PCR assay for diagnosing Pneumocystis carinii pneumonia. J. Clin. Microbiol. 40:490–494. 10.1128/JCM.40.2.490-494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chumpitazi BF, Flori P, Kern JB, Brenier-Pinchart MP, Hincky-Vitrat V, Brion JP, Thiebaut-Bertrand A, Minet C, Maubon D, Pelloux H. 2011. Characteristics and clinical relevance of the quantitative touchdown major surface glycoprotein polymerase chain reaction in the diagnosis of Pneumocystis pneumonia. Med. Mycol. 49:704–713. 10.3109/13693786.2011.566894. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura Y, Ito Y, Iinuma Y, Yasuma K, Yamamoto M, Matsushima A, Nagao M, Takakura S, Ichiyama S. 2012. Quantitative real-time PCR and the (1->3)-beta-d-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin. Microbiol. Infect. 18:591–597. 10.1111/j.1469-0691.2011.03605.x. [DOI] [PubMed] [Google Scholar]
- 36.Maillet M, Maubon D, Brion JP, Francois P, Molina L, Stahl JP, Epaulard O, Bosseray A, Pavese P. 2013. Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients. Eur. J. Clin. Microbiol. Infect. Dis. 33:331–336. 10.1007/s10096-013-1960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fillatre P, Chevrier S, Revest M, Gacouin A, Jouneau S, Leroy H, Robert-Gangneux F, Minjolle S, Le Tulzo Y, Tattevin P. 2013. Human herpes virus co-infection is associated with mortality in HIV-negative patients with Pneumocystis jirovecii pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 32:189–194. 10.1007/s10096-012-1730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A. 2013. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1->3)-beta-d-glucan for differential diagnosis of pneumocystis pneumonia and Pneumocystis colonization. J. Clin. Microbiol. 51:3380–3388. 10.1128/JCM.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damiani C, Le Gal S, Lejeune D, Brahimi N, Virmaux M, Nevez G, Totet A. 2011. Serum (1->3)-beta-d-glucan levels in primary infection and pulmonary colonization with Pneumocystis jirovecii. J. Clin. Microbiol. 49:2000–2002. 10.1128/JCM.00249-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, Chevrier S, Meunier C, Lebert C, Aupee M, Caulet-Maugendre S, Faucheux M, Lelong B, Leray E, Guiguen C, Gangneux JP. 2006. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin. Infect. Dis. 43:577–584. 10.1086/505870. [DOI] [PubMed] [Google Scholar]
- 41.Marchetti O, Lamoth F, Mikulska M, Viscoli C, Verweij P, Bretagne S. 2012. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 47:846–854. 10.1038/bmt.2011.178. [DOI] [PubMed] [Google Scholar]
- 42.Baxter CG, Dunn G, Jones AM, Webb K, Gore R, Richardson MD, Denning DW. 2013. Novel immunologic classification of aspergillosis in adult cystic fibrosis. J. Allergy Clin. Immunol. 132:560–566 e510. 10.1016/j.jaci.2013.04.007. [DOI] [PubMed] [Google Scholar]


