Abstract
The recovery of Clostridium difficile spores from hospital surfaces was assessed using rayon swabs, flocked swabs, and contact plates. The contact plate method was less laborious, achieved higher recovery percentages, and detected spores at lower inocula than swabs. Rayon swabs were the least efficient method. However, further studies are required in health care settings.
TEXT
Clostridium difficile is a spore-forming anaerobic nosocomial pathogen associated with mild to life-threatening diarrhea or colitis (1, 2). C. difficile colonization increases with the length of hospital stay following environmental exposure to spores or contact with an infected person (3). Contamination and survival of C. difficile spores on hospital inanimate surfaces have been reported (4, 5) and shown to be associated with cross-transmission (6–8).
Inactivation and eradication of Clostridium difficile spores are a challenge, and several relatively new techniques have been investigated, such as hydrogen peroxide vapor, UV radiation, or gaseous plasma systems (5, 6, 9, 10). Validation of such techniques should use optimal recovery methods to better quantify bacterial spore killing or eradication.
A variety of methods have been used to detect C. difficile from the hospital environment with variable results (11, 12). Here we report an evaluation of different methods to detect and recover C. difficile spores from materials commonly found in the hospital environment.
(Preliminary data arising from this study were presented as a poster at the European Congress of Clinical Microbiology and Infectious Diseases, Barcelona, Spain, May 2014.)
One C. difficile reference strain, ATCC 700057 (Cruinn Diagnostics, Ireland), and one uncharacterized clinical isolate (Diagnostic Laboratory, Beaumont Hospital, Dublin, Ireland) were included in the study. A modification of the C. difficile spore preparation protocol described by Chilton et al. was used (13). Briefly, C. difficile was inoculated into prereduced brain heart infusion supplemented with yeast extract and l-cysteine, incubated anaerobically at 37°C for 48 h, and spread onto 10 Columbia blood agar plates (Oxoid, United Kingdom), which were incubated anaerobically at 37°C for 10 days (14). Growth was collected from the plates using a cell scraper and suspended in 1 ml phosphate-buffered saline (PBS)–ethanol (50% [vol/vol]). The suspensions were incubated at room temperature for 1 h with periodic mixing. Spore suspensions were centrifuged for 10 min at 16,000 × g, and the pellets were resuspended in 1 ml of PBS. Spore numbers and purity were determined by microscopy using the Schaeffer and Fulton spore stain kit (Sigma-Aldrich, Ireland). Suspensions were adjusted to a concentration of approximately 105 CFU/ml (one colony is equivalent to one spore), and serial 1:10 dilutions were prepared in PBS.
C. difficile spore suspensions (50 μl), ranging from 105 to 10 CFU/ml, were added in duplicate to 25-cm2 sections of polyurethane mattress fabric (Meditec Medical, Ireland), polypropylene (GoodFellow Cambridge, Ltd., United Kingdom) and stainless steel, all decontaminated as described previously (15). Spore suspensions applied to the surfaces were air dried over 2 h. The surfaces were sampled using premoistened rayon swabs and nylon flocked swabs (Copan, Italy) and placed in 3 ml PBS. Serial dilutions of the swab suspensions were inoculated onto prepoured C. difficile selective plates—C. difficile agar base CM0601 plus C. difficile supplement SR0096 (250 mg/liter d-cycloserine, 8 mg/liter cefoxitin) and 7% (vol/vol) defibrinated horse blood (SR0050)—provided by Oxoid for enumeration of CFU/ml. Sterile contact plates (VWR) were poured in the laboratory with C. difficile selective agar and applied to each section for 20 s, ensuring firm contact with the surface. Subcultured plates from both swabs and contact plates were incubated overnight anaerobically at 37°C. Colony enumeration was performed the following day. The limit of detection (LOD) was defined as the lowest concentration of spores applied that was detected by a specific method. All methods were assessed independently targeting the entire section, and their abilities to detect and recover C. difficile spores were compared. Statistical analysis was carried out using GraphPad Prism 5.00 software. The means of three independent experiments of the percentage of recovery for the different methods were analyzed by one-way analysis of variance (ANOVA) and Tukey's multiple-comparison test.
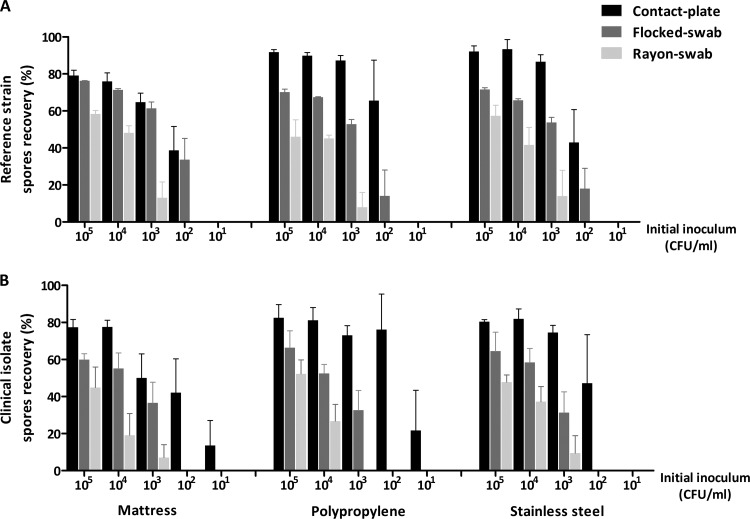
In 1983 one of the first reports comparing methods for the recovery of C. difficile spores from an environmental glass surface showed that of swabs, adhesive paddles, and contact plates, contact plates were by far the most efficient method, detecting spores at low levels, being simpler to use and relatively rapid (16). Since then, various methods, including swabs, contact plates, gloves, and sponges, have been used for research and outbreak investigations with variable results. Here, we demonstrate that contact plates achieved the highest recovery of C. difficile spores (14% to 92%) from various surfaces, including the mattress material, polypropylene, and stainless steel, confirming and extending the results from Buggy et al. (16) for recovery from glass. Recovery was also efficient for flocked swabs (14% to 76%), and the least efficient method was the rayon swab (7% to 58%) (Fig. 1A and B). The recovery percentage was directly proportional to the size of the initial inoculum—i.e., an inoculum of 105 CFU/ml allowed contact plates to recover as much as 92% and as little as 14% for a 10-CFU/ml inoculum. Contact plates were also most sensitive at detecting spores at a minimum inoculum of 10 CFU/ml, while the LOD was 102 CFU/ml for flocked swabs and 103 CFU/ml for rayon swabs, but this was only true for the clinical isolate. Statistically, contact plates were better than flocked swabs on polypropylene for the clinical isolate (P < 0.05) (Table 1) and persistently superior to rayon swabs (P < 0.05 to P < 0.001) (Table 1), while there was no significant difference between flocked and rayon swabs. Although statistical analysis did not reveal any significant difference in the recoveries between all surfaces studied, the recoveries from the mattress material were slightly reduced compared to those from the other two surfaces. Furthermore, no statistical difference was seen between the two isolates.
FIG 1.
Percentage recovery of Clostridium difficile spores of the reference strain (A) and clinical isolate (B) from three environmental surfaces using contact plates, flocked swabs, and rayon swabs. Error bars represent the standard errors of the means (SEM) from at least three independent experiments (n ≥ 3).
TABLE 1.
Summary of the results of statistical analysis carried out using one-way ANOVA and Tukey's multiple-comparison test
| Methods compared |
P value from statistical analysis ofa: |
||||
|---|---|---|---|---|---|
| Strain |
Surface |
||||
| Reference | Clinical | Mattress | Polypropylene | Stainless steel | |
| Contact plate vs flocked swab | NS | <0.05 | NS | <0.05 | NS |
| Contact plate vs rayon swab | <0.01 | <0.001 | <0.05 | <0.01 | <0.05 |
| Flocked swab vs rayon swab | NS | NS | NS | NS | NS |
The results were obtained by statistical analysis of the mean percentages of recovery from three different methods tested on three different surfaces with two isolates of C. difficile. NS, not significant.
C. difficile spores can be shed to the environment by both asymptomatic and symptomatic patients and may survive for up to 5 months on inanimate surfaces (17). They resist the bactericidal effects of most hospital disinfectants and most other decontamination techniques (18). Therefore, interventions should be monitored in rooms currently or previously occupied by C. difficile carriers. The reporting of improved methods in terms of speed and sensitivity, such as the contact plate and flocked swabs reported here, gives a more accurate estimate of the C. difficile burden compared to previously reported methods. Given that C. difficile is the most prominent endospore in today's health care setting and presents a significant infection risk to vulnerable patients, there is a growing need to accurately quantify C. difficile spore contamination in the health care environment. This would be for the purpose of assessing new methods of decontamination and correlating levels of environmental contamination with the risk of infection and the occurrence of outbreaks. Nevertheless, improving accepted recovery methods using materials that represent those found in the hospital setting are a necessary prerequisite.
ACKNOWLEDGMENTS
This research was supported by a Translational Research Award from Science Foundation Ireland and the Health Research Board (TRA/2010/10).
As potential conflicts of interest, H.H. has recent research collaborations with Steris Corporation, Inov8 Science, Pfizer, and Cepheid and has also received lecture and other fees from Novartis, AstraZeneca, and Astellas. All other authors declare no potential conflict of interest.
Footnotes
Published ahead of print 9 July 2014
REFERENCES
- 1.Crobach MJ, Dekkers OM, Wilcox MH, Kuijper EJ. 2009. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin. Microbiol. Infect. 15:1053–1066. 10.1111/j.1469-0691.2009.03098.x. [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536. 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 3.Kyne L, Hamel MB, Polavaram R, Kelly CP. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin. Infect. Dis. 34:346–353. 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 4.Dubberke ER, Reske KA, Noble-Wang J, Thompson A, Killgore G, Mayfield J, Camins B, Woeltje K, McDonald JR, McDonald LC, Fraser VJ. 2007. Prevalence of Clostridium difficile environmental contamination and strain variability in multiple health care facilities. Am. J. Infect. Control 35:315–318. 10.1016/j.ajic.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Otter JA, French GL. 2009. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J. Clin. Microbiol. 47:205–207. 10.1128/JCM.02004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce JM, Havill NL, Otter JA, McDonald LC, Adams NM, Cooper T, Thompson A, Wiggs L, Killgore G, Tauman A, Noble-Wang J. 2008. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect. Control Hosp. Epidemiol. 29:723–729. 10.1086/589906. [DOI] [PubMed] [Google Scholar]
- 7.Otter JA, Yezli S, French GL. 2011. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect. Control Hosp. Epidemiol. 32:687–699. 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- 8.Otter JA, Yezli S, Salkeld JA, French GL. 2013. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 41:S6–S11. 10.1016/j.ajic.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Galvin S, Cahill O, O'Connor N, Cafolla AA, Daniels S, Humphreys H. 2013. The antimicrobial effects of helium and helium-air plasma on Staphylococcus aureus and Clostridium difficile. Lett. Appl. Microbiol. 57:83–90. 10.1111/lam.12091. [DOI] [PubMed] [Google Scholar]
- 10.Zhang A, Nerandzic MM, Kundrapu S, Donskey CJ. 2013. Does organic material on hospital surfaces reduce the effectiveness of hypochlorite and UV radiation for disinfection of Clostridium difficile? Infect. Control Hosp. Epidemiol. 34:1106–1108. 10.1086/673148. [DOI] [PubMed] [Google Scholar]
- 11.Malik DJ, Patel KV, Clokie MR, Shama G. 2013. On the difficulties of isolating Clostridium difficile from hospital environments. J. Hosp. Infect. 84:181–183. 10.1016/j.jhin.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox MH, Fawley WN, Parnell P. 2000. Value of lysozyme agar incorporation and alkaline thioglycollate exposure for the environmental recovery of Clostridium difficile. J. Hosp. Infect. 44:65–69. 10.1053/jhin.1999.0253. [DOI] [PubMed] [Google Scholar]
- 13.Chilton CH, Freeman J, Baines SD, Crowther GS, Nicholson S, Wilcox MH. 2013. Evaluation of the effect of oritavancin on Clostridium difficile spore germination, outgrowth and recovery. J. Antimicrob. Chemother. 68:2078–2082. 10.1093/jac/dkt160. [DOI] [PubMed] [Google Scholar]
- 14.Sorg JA, Dineen SS. 2009. Laboratory maintenance of Clostridium difficile. Curr. Protoc. Microbiol. Chapter 9:Unit9A.1. 10.1002/9780471729259.mc09a01s12. [DOI] [PubMed] [Google Scholar]
- 15.Claro T, Galvin S, Cahill OJ, Fitzgerald-Hughes D, Daniels S, Humphreys H. 2014. What is the best method? Recovery of methicillin-resistant Staphylococcus aureus and extended-spectrum β-lactamase producing Escherichia coli from inanimate hospital surfaces. Infect. Control Hosp. Epidemiol. 35:869–871. 10.1086/676858. [DOI] [PubMed] [Google Scholar]
- 16.Buggy BP, Wilson KH, Fekety R. 1983. Comparison of methods for recovery of Clostridium difficile from an environmental surface. J. Clin. Microbiol. 18:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fekety R, Kim KH, Brown D, Batts DH, Cudmore M, Silva J., Jr 1981. Epidemiology of antibiotic-associated colitis; isolation of Clostridium difficile from the hospital environment. Am. J. Med. 70:906–908. 10.1016/0002-9343(81)90553-2. [DOI] [PubMed] [Google Scholar]
- 18.Dubberke ER, Gerding DN, Classen D, Arias KM, Podgorny K, Anderson DJ, Burstin H, Calfee DP, Coffin SE, Fraser V, Griffin FA, Gross P, Kaye KS, Klompas M, Lo E, Marschall J, Mermel LA, Nicolle L, Pegues DA, Perl TM, Saint S, Salgado CD, Weinstein RA, Wise R, Yokoe DS. 2008. Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect. Control Hosp. Epidemiol. 29(Suppl 1):S81–S92. 10.1086/591065. [DOI] [PubMed] [Google Scholar]