Abstract
The performance of a matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) workflow using an extensive reference database for dermatophyte identification was evaluated on 176 clinical strains. Using a direct-deposit procedure after 3 incubation days yielded 40% correct identification. Both increasing incubation time and using an extraction procedure resulted in 100% correct identification.
TEXT
Dermatophytes, which invade and infect keratinized tissues, affect a large proportion of the population, and over $500,000,000 per year is spent on antifungal drugs (1). This specific group of filamentous fungi can be separated in three anamorphic genera, Epidermophyton, Microsporum, and Trichophyton, and divided into geophilic, zoophilic, and anthropophilic species. Though resistance is uncommon (2), a proper identification of the infection agent is essential from an epidemiological point of view as well as for the choice of the antifungal treatment regimen and determining the source of infection (1).
Conventional identification of dermatophyte species relies mainly on the morphological characteristics of the strains, but it can take several weeks before the discriminative characteristics appear. In recent decades, however, molecular methods have been employed for dermatophyte species identification. Although DNA sequencing has become the gold standard for identifications, it is quite costly and time-consuming. Nowadays, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) represents a powerful tool for rapid and accurate identification of microorganisms (3–9) and has also proven to be promising for identification of filamentous fungi (10–17) and dermatophytes (18–24). The approach is based on the acquisition of a protein profile (between 2 and 20 kDa) and its comparison to a database with reference spectra (25). Since current databases for dermatophytes are quite limited and generally contain only the most common species, the robustness of a homemade, extensive database was evaluated using a MALDI-TOF MS-based workflow.
The database was constructed with a total of 195 reference strains and 58 species of Arthroderma, Epidermophyton, Microsporum, and Trichophyton, originating from the BCCM/IHEM fungal collection and the mycology laboratory of the CHU Timone in Marseille, France, and their identity was determined by DNA sequencing (see the full list in Table S1 in the supplemental material). Reference spectra included in the library were obtained after 3 days and for some slow-growing strains after 7 and/or 14 days of culture at 25°C on solid Sabouraud medium supplemented with chloramphenicol. In order to enhance the effectiveness and diversity of the library, 10 raw mass spectra from 4 different subcultures from each strain were generated (26). For the acquisition of the mass spectra, a formic acid/acetonitrile extraction protocol described for molds (27) was applied and main spectra (MSP) were created using the MSP creation function of the Maldi Biotyper software. Instrument calibration was performed with a bacterial test standard (BTS; Bruker Daltonics) on a MicroFlex (Bruker Daltonics) mass spectrometer. Spectra were recorded in the positive linear mode in a mass range from 2 to 20 kDa using the MALDI Biotyper Automation Control software.
All analyzed isolates were subcultured at 25°C on Sabouraud-chloramphenicol plates. Overall, 168 dermatophyte isolates originating from nail (66%), skin (29%), and hair (5%) specimens and collected between July 2012 and July 2013 in the microbiological laboratory from the University Hospital in Brussels, Belgium (UZ Brussels), were analyzed. Additionally, 8 isolates from two tinea capitis outbreaks in Belgian schools were investigated using both conventional methods and MALDI-TOF MS. After 72 h, fresh spores and filaments of each colony were scraped out using a wooden toothpick and smeared onto eight spots of a MALDI 96 polished steel target plate (Bruker Daltonics). After drying, four of the eight spots were covered with 1 μl 70% formic acid (Sigma-Aldrich) and again allowed to air dry. Thereafter, all spots were covered with 1 μl of an HCCA (α-cyano-4-hydroxycinnamic acid) matrix solution (in 50% acetonitrile–47.5% water–2.5% trifluoroacetic acid) and dried at room temperature. If no correct identification was obtained, the isolates were reanalyzed after 7 and 14 days following the formic acid-acetonitrile extraction protocol used to build the reference database (27). For each isolate, the spectrum of each spot was compared with the reference library spectra and analyzed with the MALDI BioTyper 3.0 software (Brüker Daltonics, Germany). Each isolate was analyzed in quadruplicate, and the MS-based identification was considered correct only if at least three of the four spots displayed the same identification, with the mean of their best-match log score (LS) being ≥1.70. Discrepancies from the classical identification methods were resolved by sequencing the internal transcribed spacer (ITS) ribosomal DNA (rDNA) regions with primers ITS 4 and 5 (28) and the beta-tubulin (BT) region with primers BT2A and BT2B (29) as the identification gold standard.
In the literature, the implementation and identification of different MALDI-TOF MS systems have already been extensively discussed for dermatophytes (18–24). However, no standardized workflow where both the extraction procedure and the incubation time were assessed has been described so far. Based on the MALDI-TOF MS identification of yeasts, all clinical strains were tested after 3 days of incubation using a direct-deposit methodology with or without addition of 1 μl of formic acid (30, 31).
Trichophyton rubrum was the most prevalent species (73%) of all clinical isolates, followed by T. interdigitale (17%), Microsporum audouinii (3.6%), Arthroderma benhamiae (3%), T. tonsurans and T. violaceum (1.2%), and M. canis and M. gypseum (0.6%). After 3 days of incubation at 25°C and using the direct-deposit methodology, 33% of the isolates were already correctly identified. The use of 1 μl 70% formic acid increased this percentage to 40%, with exact identification for all strains belonging to A. benhamiae and T. interdigitale. For all the other species, waiting 7 days and using the extraction protocol increased the identification to 80% and after 14 days 100% of the isolates were correctly identified (Fig. 1). The classical (morphological) identifications, performed at the University Hospital, were shown to be erroneous in 11 cases (7%) (Table 1). Additionally, the proposed MALDI-TOF MS flowchart (Fig. 2) was used to identify 8 dermatophytes involved in two tinea capitis outbreaks. After 3 incubation days using the direct-deposit methodology, with and without the addition of 1 μl 70% formic acid on the smear, the responsible dermatophytes was correctly identified by MALDI-TOF MS as T. tonsurans and M. audouinii, both with a mean LS of ≥1.7. Compared with the conventional methodology, which took 7 to 14 days, depending on the appearance of the morphological characteristics of the strains, the correct identification by MALDI-TOF MS was significantly faster.
FIG 1.
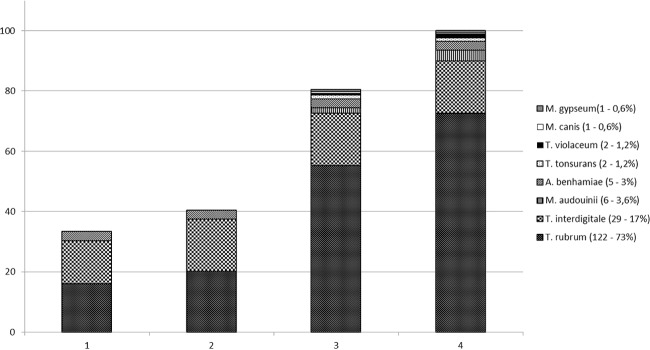
Percentages of correct identification of the clinical dermatophytes (number of tested isolates per species, percentage of total) using the direct-deposit methodology after 3 incubation days without (column 1) and with (column 2) addition of 1 μl 70% formic acid and the extraction protocol after 7 incubation days (column 3) and after 14 days (column 4).
TABLE 1.
Identification of the 168 clinical strains using either MALDI-TOF MS or microscopya
| Correct species identification (multilocus sequencing) | Identification (n) by: |
|
|---|---|---|
| Microscopy | MALDI-TOF MS | |
| Arthroderma benhamiae (5) | T. mentagrophytes (5) | A. benhamiae (5) |
| M. audouinii (6) | M. audouinii (6) | |
| M. canis (1) | M. canis (1) | |
| M. gypseum (1) | M. gypseum (1) | |
| T. interdigitale (29) | T. interdigitale (29) | |
| Trichophyton rubrum (122) | T. rubrum (117) | T. rubrum (122) |
| T. interdigitale (4) | ||
| T. tonsurans (1) | ||
| Trichophyton tonsurans (2) | T. tonsurans (1) | T. tonsurans (2) |
| T. rubrum (1) | ||
| T. violaceum (2) | T. violaceum (2) | |
| Total | 93% (157) | 100% (168) |
If different identification results were obtained, multilocus sequencing was used as the gold standard.
FIG 2.
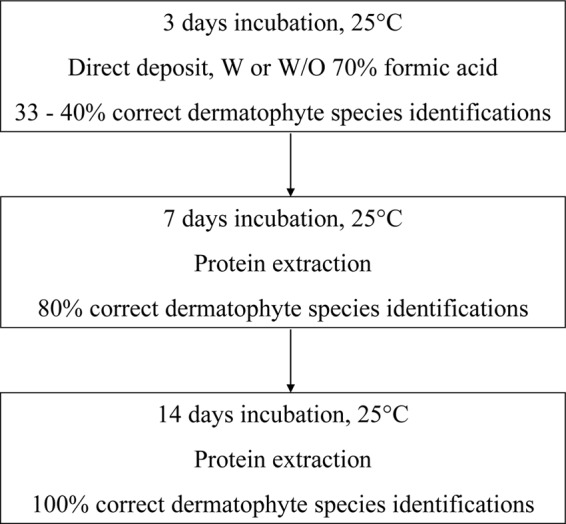
Proposed flowchart for the routine identification of dermatophytes in a clinical laboratory using MALDI-TOF MS. W, with; W/O, without.
In the present study, a comprehensive and diverse dermatophyte library was evaluated and validated using a workflow obtaining 100% correct species identification after a maximum incubation period of 14 days and using an extended extraction protocol if necessary. The efficacy of this protocol was highlighted by the fast identification of dermatophytes species involved in two tinea capitis outbreaks.
Supplementary Material
ACKNOWLEDGMENTS
We thank Karin Goens, Sam Roesems, Johan Breynaert, Luc Van Dijck, Danny Cnudde, and Iris Ottoy for skilled technical assistance.
Molecular identification was performed at the Platform Biotechnology and Molecular Biology unit at the Scientific Institute of Public Health.
Footnotes
Published ahead of print 16 July 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01428-14.
REFERENCES
- 1.Gräser Y, Scott J, Summerbell R. 2008. The new species concept in dermatophytes—a polyphasic approach. Mycopathologia 166:239–256. 10.1007/s11046-008-9099-y. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee PK, Leidich SD, Isham N, Leitner I, Ryder NS, Ghannoum MA. 2003. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob. Agents Chemother. 47:82–86. 10.1128/AAC.47.1.82-86.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader O, Weig M, Taverne-Ghadwal L, Lugert R, Gross U, Kuhns M. 2011. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 17:1359–1365. 10.1111/j.1469-0691.2010.03398.x. [DOI] [PubMed] [Google Scholar]
- 4.Dhiman N, Hall L, Wohlfiel SL, Buckwalter SP, Wengenack NL. 2011. Performance and cost analysis of matrix-assisted laser desorption ionization–time of flight mass spectrometry for routine identification of yeast. J. Clin. Microbiol. 49:1614–1616. 10.1128/JCM.02381-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marklein G, Josten M, Klanke U, Muller E, Horre R, Maier T, Wenzel T, Kostrzewa M, Bierbaum G, Hoerauf A, Sahl HG. 2009. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J. Clin. Microbiol. 47:2912–2917. 10.1128/JCM.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martiny D, Busson L, Wybo I, El Haj RA, Dediste A, Vandenberg O. 2012. Comparison of the Microflex LT and Vitek MS systems for routine identification of bacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 50:1313–1325. 10.1128/JCM.05971-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto A, Halliday C, Zahra M, van Hal S, Olma T, Maszewska K, Iredell JR, Meyer W, Chen SC. 2011. Matrix-assisted laser desorption ionization-time of flight mass spectrometry identification of yeasts is contingent on robust reference spectra. PLoS One 6:e25712. 10.1371/journal.pone.0025712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sendid B, Ducoroy P, Francois N, Lucchi G, Spinali S, Vagner O, Damiens S, Bonnin A, Poulain D, Dalle F. 2013. Evaluation of MALDI-TOF mass spectrometry for the identification of medically-important yeasts in the clinical laboratories of Dijon and Lille hospitals. Med. Mycol. 51:25–32. 10.3109/13693786.2012.693631. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson LG, Drake SK, Shea YR, Zelazny AM, Murray PR. 2010. Evaluation of matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of clinically important yeast species. J. Clin. Microbiol. 48:3482–3486. 10.1128/JCM.00687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Carolis E, Posteraro B, Lass-Florl C, Vella A, Florio AR, Torelli R, Girmenia C, Colozza C, Tortorano AM, Sanguinetti M, Fadda G. 2012. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 18:475–484. 10.1111/j.1469-0691.2011.03599.x. [DOI] [PubMed] [Google Scholar]
- 11.De Respinis S, Vogel G, Benagli C, Tonolla M, Petrini O, Samuels G. 2010. MALDI-TOF MS of Trichoderma: a model system for the identification of microfungi. Mycol. Prog. 9:79–100. 10.1007/s11557-009-0621-5. [DOI] [Google Scholar]
- 12.Del Chierico F, Masotti A, Onori M, Fiscarelli E, Mancinelli L, Ricciotti G, Alghisi F, Dimiziani L, Manetti C, Urbani A, Muraca M, Putignani L. 2012. MALDI-TOF MS proteomic phenotyping of filamentous and other fungi from clinical origin. J. Proteomics 75:3314–3330. 10.1016/j.jprot.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 13.Hettick JM, Green BJ, Buskirk AD, Kashon ML, Slaven JE, Janotka E, Blachere FM, Schmechel D, Beezhold DH. 2008. Discrimination of Aspergillus isolates at the species and strain level by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Anal. Biochem. 380:276–281. 10.1016/j.ab.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 14.Hettick JM, Green BJ, Buskirk AD, Kashon ML, Slaven JE, Janotka E, Blachere FM, Schmechel D, Beezhold DH. 2008. Discrimination of Penicillium isolates by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Rapid Commun. Mass Spectrom. 22:2555–2560. 10.1002/rcm.3649. [DOI] [PubMed] [Google Scholar]
- 15.Iriart X, Lavergne RA, Fillaux J, Valentin A, Magnaval JF, Berry A, Cassaing S. 2012. Routine identification of medical fungi by the new Vitek MS matrix-assisted laser desorption ionization–time of flight system with a new time-effective strategy. J. Clin. Microbiol. 50:2107–2110. 10.1128/JCM.06713-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marinach-Patrice C, Lethuillier A, Marly A, Brossas JY, Gen Jé Symoens F, Datry A, Guarro J, Mazier D, Hennequin C. 2009. Use of mass spectrometry to identify clinical Fusarium isolates. Clin. Microbiol. Infect. 15:634–642. 10.1111/j.1469-0691.2009.02758.x. [DOI] [PubMed] [Google Scholar]
- 17.Tao J, Zhang G, Jiang Z, Cheng Y, Feng J, Chen Z. 2009. Detection of pathogenic Verticillium spp. using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 23:3647–3654. 10.1002/rcm.4296. [DOI] [PubMed] [Google Scholar]
- 18.Alshawa K, Beretti JL, Lacroix C, Feuilhade M, Dauphin B, Quesne G, Hassouni N, Nassif X, Bougnoux ME. 2012. Successful identification of clinical dermatophyte and Neoscytalidium species by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 50:2277–2281. 10.1128/JCM.06634-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Respinis S, Tonolla M, Pranghofer S, Petrini L, Petrini O, Bosshard PP. 2013. Identification of dermatophytes by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Med. Mycol. 51:514–521. 10.3109/13693786.2012.746476. [DOI] [PubMed] [Google Scholar]
- 20.Erhard M, Hipler UC, Burmester A, Brakhage AA, Wostemeyer J. 2008. Identification of dermatophyte species causing onychomycosis and tinea pedis by MALDI-TOF mass spectrometry. Exp. Dermatol. 17:356–361. 10.1111/j.1600-0625.2007.00649.x. [DOI] [PubMed] [Google Scholar]
- 21.L'Ollivier C, Cassagne C, Normand AC, Bouchara JP, Contet-Audonneau N, Hendrickx M, Fourquet P, Coulibaly O, Piarroux R, Ranque S. 2013. A MALDI-TOF MS procedure for clinical dermatophyte species identification in the routine laboratory. Med. Mycol. 51:713–720. 10.3109/13693786.2013.781691. [DOI] [PubMed] [Google Scholar]
- 22.Nenoff P, Erhard M, Simon JC, Muylowa GK, Herrmann J, Rataj W, Graser Y. 2013. MALDI-TOF mass spectrometry - a rapid method for the identification of dermatophyte species. Med. Mycol. 51:17–24. 10.3109/13693786.2012.685186. [DOI] [PubMed] [Google Scholar]
- 23.Packeu A, Hendrickx M, Beguin H, Martiny D, Vandenberg O, Detandt M. 2013. Identification of the Trichophyton mentagrophytes complex species using MALDI-TOF mass spectrometry. Med. Mycol. 51:580–585. 10.3109/13693786.2013.770605. [DOI] [PubMed] [Google Scholar]
- 24.Theel ES, Hall L, Mandrekar J, Wengenack NL. 2011. Dermatophyte identification using matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 49:4067–4071. 10.1128/JCM.01280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marvin LF, Roberts MA, Fay LB. 2003. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in clinical chemistry. Clin. Chim. Acta 337:11–21. 10.1016/j.cccn.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Normand AC, Cassagne C, Ranque S, L'ollivier C, Fourquet P, Roesems S, Hendrickx M, Piarroux R. 2013. Assessment of various parameters to improve MALDI-TOF MS reference spectra libraries constructed for the routine identification of filamentous fungi. BMC Microbiol. 13:76. 10.1186/1471-2180-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassagne C, Ranque S, Normand AC, Fourquet P, Thiebault S, Planard C, Hendrickx M, Piarroux R. 2011. Mould routine identification in the clinical laboratory by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLoS One 6:e28425. 10.1371/journal.pone.0028425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White TJ, Bruns T, Lee SS, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322 In Innis MA, Gelfand DH, Sninsky JJ, White TJ. (ed), PCR protocols, a guide to methods and applications. Academic Press, Inc., New York, NY. [Google Scholar]
- 29.Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bille E, Dauphin B, Leto J, Bougnoux ME, Beretti JL, Lotz A, Suarez S, Meyer J, Join-Lambert O, Descamps P, Grall N, Mory F, Dubreuil L, Berche P, Nassif X, Ferroni A. 2012. MALDI-TOF MS Andromas strategy for the routine identification of bacteria, mycobacteria, yeasts, Aspergillus spp. and positive blood cultures. Clin. Microbiol. Infect. 18:1117–1125. 10.1111/j.1469-0691.2011.03688.x. [DOI] [PubMed] [Google Scholar]
- 31.Cassagne C, Cella AL, Suchon P, Normand AC, Ranque S, Piarroux R. 2013. Evaluation of four pretreatment procedures for MALDI-TOF MS yeast identification in the routine clinical laboratory. Med. Mycol. 51:371–377. 10.3109/13693786.2012.720720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


