Abstract
Anaerobic bacteria are often difficult to detect, especially after the initiation of antibiotics. We describe the application of PCR-electrospray ionization mass spectrometry (PCR/ESI-MS) using a sample of cerebrospinal fluid to identify an anaerobic Gram-negative bacillus, Fusobacterium nucleatum, in a patient with “culture-negative” meningitis and cerebral abscesses.
CASE REPORT
A 33-year-old healthy man presented with a 1-month history of fevers, cough, and headaches. On examination, he appeared ill and was tachycardic and febrile, with a maximum temperature of 38.3°C. He demonstrated poor dentition, and rales were appreciated on auscultation of the right middle and lower lung fields. Neurological examination was unremarkable. Chest X ray revealed a right middle lobe cavitary lesion, and magnetic resonance imaging (MRI) of the brain showed multiple abscesses (Fig. 1). Blood cultures drawn on admission remained sterile, and serologic testing for human immunodeficiency virus (HIV) was negative.
FIG 1.
Magnetic resonance imaging of brain with contrast showing multiple rim-enhancing foci in the bilateral cerebral hemispheres, right greater than left.
A lumbar puncture (LP) yielded cerebrospinal fluid (CSF) consistent with a bacterial infection: neutrophilic pleocytosis (white blood cell [WBC] count of 2,827 cells/mm3:77% neutrophils, 20% lymphocytes, 3% monocytes), glucose level of <10 mg/dl, and total protein level of 198 mg/dl. Gram stain revealed 3+ granulocytes, and no organisms were seen. Acute inflammation was noted on the pathology slides of the CSF. CSF culture was negative. Bacterial, fungal, and mycobacterial cultures of a transbronchial biopsy specimen of the pulmonary abscess were negative, as were all direct smears and cultures, including anaerobic culture from stereotactic aspiration of one of the brain lesions performed on day 5 of hospitalization. The interval time from collection of abscess material to the lab was 2 h, and samples were submitted to the lab in appropriate anaerobic containers. Histopathology of the abscess revealed acute inflammation with liquefactive necrosis, consistent with abscess.
Before LP and abscess evacuation, this patient was prescribed broad-spectrum antimicrobials, including vancomycin and meropenem on day 1 of hospitalization. Trimethoprim-sulfamethoxazole was added on day 13 for presumptive Nocardia infection. Fever resolved, but on day 17 the patient developed left-sided weakness, and repeat MRI showed enlargement and coalescence of cerebral abscesses, ultimately requiring urgent hemicraniectomy and decompression on days 18 and 19.
Tissue and CSF specimens collected from evacuation of the abscess on day 18 were submitted for culture, including anaerobic cultures, which were negative. PCR followed by electrospray ionization mass spectrometry (PCR/ESI-MS) was performed on CSF. The primers used in the sterile fluid bacteria and Candida assay (BAC detection assay) consisted of three ribosomal primers, P 348, P 361, and P 349, all targeting bacterial 16S rRNA genes as per the PCR/ESI-MS protocol previously described (1).
In addition, formalin-fixed paraffin-embedded tissue specimens from the brain biopsy and brain abscess were also submitted to the Infectious Diseases Pathology Branch (IDPB), Centers for Disease Control and Prevention, for histopathological, molecular, and immunohistochemical evaluation. Paraffin-embedded tissues were deparaffinized and digested with proteinase K, and DNA was extracted by using the QIAamp UCP pathogen minikit (Qiagen). Paneubacterial PCR and sequencing analysis were performed targeting 16S rRNA genes (2). The investigational immunohistochemical assay used an in-house polyclonal rabbit antibody against Fusobacterium necrophorum known to cross-react with a wide variety of other bacteria.
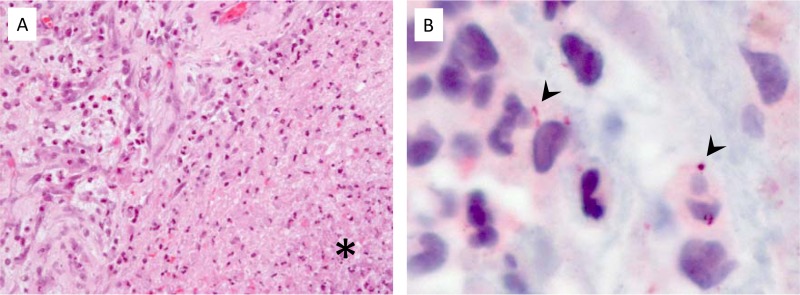
PCR/ESI-MS detected Fusobacterium nucleatum in CSF. This was confirmed by an independent analysis of brain tissue and brain abscess wall biopsy specimens by using eubacterial 16S rRNA gene sequencing, which detected a 382-bp fragment of DNA with 100% homology to F. nucleatum. In addition, immunohistochemical staining using an investigational Fusobacterium species assay showed granular staining associated with inflammatory infiltrates. Lillie-Twort Gram and Warthin-Starry silver stains performed on the tissue were nondiagnostic for bacteria (Fig. 2). Hematoxylin and eosin-stained sections of the brain abscess wall biopsy specimen demonstrated areas of neutrophilic infiltrates, necrotic debris, granulation tissue, and fibrosis. Gram stain, Grocott's methenamine silver stain, acid-fast bacillus smear, and Fite stains were reviewed and were negative. Retrospective analysis of the bronchial biopsy specimen by PCR and sequencing detected the presence of F. nucleatum DNA.
FIG 2.
(A) Brain biopsy specimen showing cerebral abscess with necrosis and neutrophilic infiltrates (asterisk) (hematoxylin and eosin stain). (B) Granular antigens are seen within infiltrates by Fusobacterium immunohistochemistry (arrowheads) (immunoalkaline phosphatase-fast red chromogen).
The antimicrobial regimen was then narrowed on day 56 to meropenem only, and the patient ultimately completed a 4-month course of intravenous meropenem followed by a 9-month course of oral metronidazole, after which all antibiotics were discontinued. Improvement in neurological deficits was noted on follow-up outpatient physical exam, and repeat brain MRI at the completion of intravenous antimicrobial treatment was notable for decrease in size and number of nodules. Associated diffusion restriction to suggest residual abscess at 12-month follow-up was not found (Fig. 3).
FIG 3.
MRI after completion of 12 months of antimicrobial therapy. Right temporal surgical defect with surrounding gliosis involving the right temporal and, to a lesser extent, parietal and occipital lobes. Scattered small nodular foci of enhancement in these regions are stable to minimally smaller in size. No new enhancing lesions were identified.
F. nucleatum is a fastidious, strictly anaerobic, non-spore-forming Gram-negative bacillus. It is a rare isolate in routine laboratory practice. Nevertheless, F. nucleatum is associated with important infectious disease syndromes. The true spectrum of disease is felt to be underappreciated by clinicians due to inability to identify Fusobacterium by traditional culture techniques (3).
PCR/ESI-MS is a novel diagnostic technique that has been used to identify environmental and human pathogens using selected gene targets amplified by PCR and detected by ESI-MS (4–6). PCR/ESI-MS is most promising when cultures are negative (7), particularly in the setting of suspected anaerobic infection and when cultures are obtained following the initiation of antimicrobial treatment. In a prospective comparison of conventional microbiological testing and PCR/ESI-MS, 72% of cultures obtained following initiation of antimicrobial treatment were nondiagnostic (8). PCR/ESI-MS detected pathogenic bacteria in 60% of the cases in which cultures were nondiagnostic.
Our case illustrates the ability of molecular techniques such as eubacterial 16S rRNA gene sequencing and PCR/ESI-MS to identify difficult-to-detect pathogens such as F. nucleatum. The application of nucleic acid-based detection methods with greatly increased sensitivity compared to that of culture can have an important impact on directing antimicrobial therapy and limiting antimicrobial resistance.
ACKNOWLEDGMENTS
Funds and facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program award number 1I01BX001974, and the Geriatric Research Education and Clinical Center VISN 10 to R.A.B. supported this work. This work was also supported by funds from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R01AI063517 and R01AI100560 to R.A.B. and through the Antibiotic Resistance Leadership Group under National Institutes of Health award number UM1AI104681.
Funding organizations were not involved in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation, review, or approval of the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the Centers for Disease Control and Prevention.
R.A.B. has received grant funding from STERIS Corporation, AstraZeneca, Merck, Pfizer, and Rib-X Pharmaceuticals. R.S. is a salaried employee of Ibis Biosciences, a division of Abbott.
Footnotes
Published ahead of print 25 June 2014
REFERENCES
- 1.Kaleta EJ, Clark AE, Johnson DR, Gamage DC, Wysocki VH, Cherkaoui A, Schrenzel J, Wolk DM. 2011. Use of PCR coupled with electrospray ionization mass spectrometry for rapid identification of bacterial and yeast bloodstream pathogens from blood culture bottles. J. Clin. Microbiol. 49:345–353. 10.1128/JCM.00936-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kommedal O, Lekang K, Langeland N, Wiker HG. 2011. Characterization of polybacterial clinical samples using a set of group-specific broad-range primers targeting the 16S rRNA gene followed by DNA sequencing and RipSeq analysis. J. Med. Microbiol. 60:927–936. 10.1099/jmm.0.028373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huggan PJ, Murdoch DR. 2008. Fusobacterial infections: clinical spectrum and incidence of invasive disease. J. Infect. 57:283–289. 10.1016/j.jinf.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia NS, Farrell JJ, Sampath R, Ranken R, Rounds MA, Ecker DJ, Bonomo RA. 2012. Identification of Streptococcus intermedius central nervous system infection by use of PCR and electrospray ionization mass spectrometry. J. Clin. Microbiol. 50:4160–4162. 10.1128/JCM.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caliendo AM. 2011. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin. Infect. Dis. 52(Suppl 4):S326–S330. 10.1093/cid/cir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ecker DJ, Sampath R, Li H, Massire C, Matthews HE, Toleno D, Hall TA, Blyn LB, Eshoo MW, Ranken R, Hofstadler SA, Tang YW. 2010. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev. Mol. Diagn. 10:399–415. 10.1586/erm.10.24. [DOI] [PubMed] [Google Scholar]
- 7.Sampath R, Hall TA, Massire C, Li F, Blyn LB, Eshoo MW, Hofstadler SA, Ecker DJ. 2007. Rapid identification of emerging infectious agents using PCR and electrospray ionization mass spectrometry. Ann. N. Y. Acad. Sci. 1102:109–120. 10.1196/annals.1408.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell JJ, Sampath R, Ecker DJ, Bonomo RA. 2013. “Salvage microbiology”: detection of bacteria directly from clinical specimens following initiation of antimicrobial treatment. PLoS One 8:e66349. 10.1371/journal.pone.0066349. [DOI] [PMC free article] [PubMed] [Google Scholar]