Abstract
Patients with primary immunodeficiencies are usually susceptible to enterovirus infections and have higher risks to develop severe clinical forms. We report a unique description of a boy with major histocompatibility complex class II (MHC-II) deficiency infected by 9 different enterovirus serotypes during a 2-year period, with very mild clinical symptoms, probably due to the immunoglobulin therapy he was receiving.
CASE REPORT
The case patient is the older child of Tunisian consanguineous healthy parents. He was born in 2002 with, apparently, no neonatal pathology. Breastfed for 20 months, he had a history of multiple episodes of upper respiratory infections and diarrhea with failure to thrive (−2 standard deviations in weight and size). At the age of 3 months, he developed a chronic diarrhea (6 to 7 stools per day) which required hospitalization. He was hospitalized again at 4 years of age for diarrhea and dehydration; the diagnoses of mucovicidosis and of celiac disease were ruled out based on negative sweat testing and the results of duodenal fibroscopy and biopsy, respectively. At 5 years of age, because of multiple respiratory infections, a computed tomography thoracic scan was performed and found a diffuse bronchiectasis. His vaccination history against poliomyelitis included four doses of trivalent oral poliovirus vaccine (OPV) administered at 3, 4, 5, and 18 months of age; he received another dose of OPV at 4 years of age during a subnational polio mass vaccination campaign. In December 2007, major histocompatibility (MHC) class II deficiency was diagnosed based on the defect of expression of MHC class II molecules on the surface of resting peripheral blood mononuclear cells, with confirmation by expression study on phytohemagglutinin-activated blast cells, as assessed by flow cytometry. A severe CD4 lymphopenia (6%) was found, along with abnormal immunoglobulin titers of 644 mg/dl for IgG (normal, 929 ± 228 mg/dl), 74 mg/dl for IgA (normal, 56 ± 18 mg/dl), and 132 mg/dl for IgM (normal, 93 ± 27 mg/dl) (1). Subsequently, he was treated on a monthly basis with substitutive intravenous immunoglobulins and was regularly followed by the expert physicians and nurses of the Bone Marrow Transplantation Center in Tunisia, specialized in managing and providing care for these patients. Bone marrow transplantation could not be performed due to the unavailability of a compatible donor. The molecular analysis did not show the presence of the recurrent 752delG26/RFXANK gene mutation that is observed in most MHC class II-deficient Maghrebian patients (2). At each visit and prior to immunoglobulin transfusion, a blood sample was collected for residual immunoglobulin titration. A complete medical examination was performed; all clinical symptoms present at the day of the visit and those that occurred during the previous month were recorded and appropriate treatment prescriptions were provided.
In July 2009, when the patient was 7 years old, a WHO collaborative study searching for chronic poliovirus excretors among patients with immunodeficiencies was initiated, and he was enrolled in the study in October 2009. The study protocol was approved by the Ethical Review Board of the Pasteur Institute of Tunis and the Ethical Review Board of WHO, Geneva. Written informed consent was obtained from the patient's parents. Part of the clinical history and the virological findings for the patient (data up to day 454 [D454], approximately 15 months of follow-up) were previously published in a paper summarizing the study results for the whole cohort (4). This patient was specifically selected for attention because we continued to identify new episodes of enterovirus (EV) infections with additional serotypes. Herein, we report detailed clinical data and the results of virological investigations up to day 1,270, i.e., approximately 42 months of follow-up. The initial stool samples were collected at the patient's home residence, and subsequent samples were obtained during the monthly follow-up medical visits that the patient had for his substitutive immunoglobulin therapy. A total of 30 samples were collected. Stool extracts were inoculated onto cells of three cell lines: RD (human rhabdomyosarcoma cell lines), L20B (transgenic mouse cell line expressing the gene of human cellular receptor for poliovirus), and HEp-2C (human larynx epidermoid carcinoma cell lines). The inoculated cells were assessed daily for 10 days for cytopathic effect (CPE). Polioviruses were identified by the presence of a CPE on L20B cells and then serotyped and intratyped by real-time PCR using poliovirus serotype-specific and vaccine-specific primers. Nonpoliovirus enteroviruses were identified by the presence of a CPE on RD and/or HEp2-C cells and the absence of CPE on L20B cells. The EV types were determined by partial sequencing of a 356-nucleotide fragment in the VP1 region of the enterovirus genome. Briefly, RNA extraction and PCR amplifications were carried out as described previously (5). The PCR products were sequenced in both directions, the sequence of each isolate was deduced by aligning the respective forward and reverse sequences, and the serotype was determined by comparison to the sequences of prototype strains of the different EV serotypes as described previously (6).
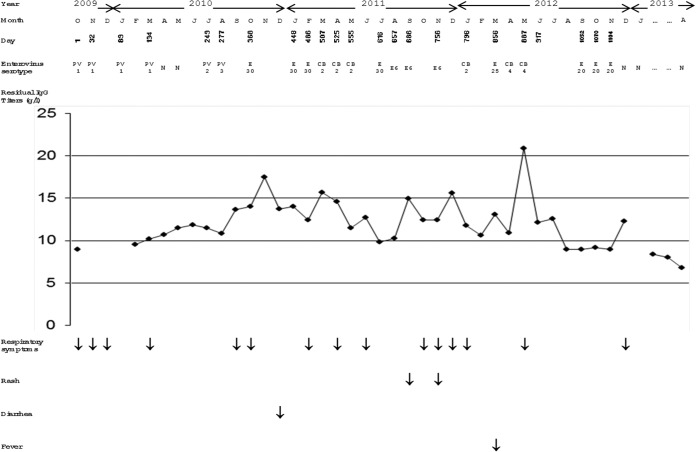
The enterovirus serotypes detected in the immunodeficient patient during the whole study period are indicated in Fig. 1. A vaccine-related poliovirus type 1 was isolated in the initial sample (D1), and the patient excreted the same serotype during more than 4 months, up to D132. Vaccine-related polioviruses of types 2 and 3 were then detected in the specimens collected at D249 and D277, respectively. The patient was then reinfected successively by enteroviruses of 6 different serotypes, as follows: echovirus type 30 during a 118-day period (D368 to D486), coxsackievirus group B2 during a 48-day period (D507 to D555), echovirus type 30 detected at day 616, echovirus type 6 during a 99-day period (D657 to D756), coxsackievirus group B2 at day 796, echovirus type 25 at day 856, coxsackievirus group B4 during a 30-day period (D887 to D917), and echovirus type 20 during a 53-day period (D1052 to D1104). Comparison of the partial VP1 sequences of isolates of the same serotypes revealed identical sequences for echovirus type 6 and coxsackie B4 isolates and very few mutations for isolates of the other serotypes, including a maximum of two nucleotide differences for echovirus type 20 and coxsackievirus group B2 isolates and three nucleotide differences for echovirus type 30 isolates.
FIG 1.
Enterovirus excretion and clinical features during the study period. PV1, poliovirus type 1; PV2, poliovirus type 2; PV3, poliovirus type 3; E30, echovirus type 30; CB2, coxsackievirus group B type 2; E6, echovirus type 6; E25, echovirus type 25; CB4, coxsackievirus group B type 4; E20, echovirus type 20; N, negative; IgG, immunoglobulin G.
During the whole study period, the patient's residual serum IgG titers were persistently satisfactory, with a minimal value of 681 mg/dl. The clinical features were marked by multiple episodes of upper respiratory infections of bacterial origin, including Haemophilus influenzae and Streptococcus pneumoniae, isolated repetitively in the respiratory tract specimens collected at D448, D486, D756, and D1104 (Fig. 1). A few episodes of rash, diarrhea, and fever were also noted during the study period but with no serious disease that could be related to enteroviral infections like meningitis, encephalitis, or paralysis.
This study reports unusual sequential and very frequent enterovirus infections in a patient with MHC class II deficiency. Interestingly, no severe clinical symptoms could be clearly related to the enterovirus infections. MHC class II deficiency is a relatively common primary immunodeficiency (PID) which affects infants and young children. The affected T-cell and B-cell responses expose the patient to repeated microbial infections, including enteroviral infections, and severe forms, especially with neurological presentations (encephalitis, meningitis, and paralysis) have frequently been reported in patients with immune deficiencies (7–9). Our patient had received substitutive immunoglobulin therapy regularly since he was 5 years of age, and during the study period, he had adequate residual IgG serum levels. This may suggest the possible role of this treatment in preventing severe forms of enterovirus infections, although it seems that this treatment does not protect against the infection itself. In fact, the very high susceptibility of our patient to enteroviral infections is remarkable, especially if we consider the relatively low endemicity of enterovirus infection in Tunisia; according to the national enterovirus surveillance data, regularly conducted since 1991, enterovirus shedding was found in only 4 to 5% of acute flaccid paralysis cases and their healthy contacts during the last decade (4, 6). We have previously noted high susceptibility to enterovirus infections in a cohort of patients with various types of PIDs: 13.4% (11 of 82) of all patients and 20.7% (11 of 53) of those with humoral or combined immunodeficiency had enterovirus-positive specimens (4). However, the case patient was particularly prone to such infections, as he was sequentially infected by 9 different enterovirus serotypes during a period of 25 months. In contrast, no enterovirus was detected in his stool specimens starting from December 2012 and continuing up to April 2013. We could not identify, at the clinical level, any change or particular event that may explain this unusual high susceptibility to infection during the 2-year period and the absence of infection subsequently.
Regardless of the clinical presentation, enterovirus infection in patients with primary immunodeficiencies was frequently reported as chronic infection (4, 8, 10). In our case patient, the first virus detected was a Sabin-like poliovirus type 1; it was excreted during a period exceeding 4 months, from D1 to D134. It is well established that immunodeficient patients exposed to OPV may excrete poliovirus strains for many months or years (11–14), and these patients may constitute a reservoir for potentially neurovirulent polioviruses after the eradication of wild polioviruses. These patients may receive the live-attenuated vaccine before their immune deficiency is diagnosed; they may also be infected with vaccine strains excreted by OPV-vaccinated contacts. Screening for poliovirus excretors among patients with PIDs is now highly recommended. The identified poliovirus excretors should then be followed until poliovirus excretion stops; antiviral therapy may even be proposed to accelerate the clearance of the excreted viruses. Our case patient received OPV during the two first years of life, before his immune deficiency was diagnosed, but he received it again at school entry, a few months before sampling, despite the fact that his PID was already known and he should not have been given any live vaccine. Fortunately, he did not become a chronic poliovirus excretor, although the mechanism by which the excretion stopped at D134 remains unclear. Polioviruses type 2 and type 3 were then detected in his stool specimens at D249 and D277, respectively. Family data indicated that his newborn brother was given the first and second dose of OPV in the same period, which also did not have to happen, as no live vaccine should be given to the household contacts of immunodeficient patients. After the three poliovirus serotypes, an echovirus type 30 was detected over 4 months; it was replaced by a coxsackievirus group B2 and then reappeared. The same is true for the coxsackievirus group B2, which was replaced by echovirus types 30 and 6 for a few months and then reappeared. A reinfection with the same serotype is possible, but a latent carrier state during which the virus is not detected cannot be excluded. Only part of the VP1 genomic region was sequenced for typing purposes, and the identified sequences of isolates from the same serotype were very close to each other; thus, only more advanced molecular investigations may help to differentiate between the two hypotheses. For each of the remaining enterovirus serotypes, the excretion period was relatively time limited and did not exceed 3 to 4 months. In fact, it was previously established that enterovirus excretion may last up to 3 months or slightly more in apparently immunocompetent individuals (15).
Although it is well established that patients with PIDs are highly susceptible to viral infections, the novel finding from this case report is the occurrence of such diversified and numerous infections in a single patient and in a short period of time. It provides further evidence of the extreme susceptibility of patients with primary immunodeficiency to enterovirus infections. Since these patients can become chronically infected by enteroviruses, and in particular, polioviruses, they can be seen as potential reservoirs for pathogenic enteroviruses. They are also at risk for severe forms of enterovirus infection, but interestingly, no severe clinical consequence was associated with the sequential presence of several enterovirus serotypes in this patient receiving substitutive immunoglobulins regularly. Adequate surveillance, clinical management, and follow-up for these patients is essential to prevent the occurrence of severe forms for the patient and the propagation of virus strains that may be of concern for the global polio eradication initiative.
ACKNOWLEDGMENT
This study was partially supported by the Tunisian Ministry for Scientific Research and Technology (LR11-IPT09 and LR11-IPT-02).
Footnotes
Published ahead of print 16 July 2014
REFERENCES
- 1.Stiehm ER, Ochs HD, Winkelstein JA. 2004. Immunologic disorders in infants & children, 5th ed. W.B. Saunders, Philadelphia, PA. [Google Scholar]
- 2.Ben-Mustapha I, Ben-Farhat K, Guirat-Dhouib N, Dhemaied E, Larguèche B, Ben-Ali M, Chemli J, Bouguila J, Ben-Mansour L, Mellouli F, Khemiri M, Béjaoui M, Barbouche MR. 2013. Clinical, immunological and genetic findings of a large Tunisian series of major histocompatibility complex class II deficiency patients. J. Clin. Immunol. 33:865–870. 10.1007/s10875-013-9863-8. [DOI] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Driss N, Ben-Mustapha I, Mellouli F, Ben Yahia A, Touzi H, Bejaoui M, Ben Ghorbel M, Triki H, Barbouche MR. 2012. High susceptibility for enterovirus infection and virus excretion features in Tunisian patients with primary immunodeficiencies. Clin. Vaccine Immunol. 19:1684–1689. 10.1128/CVI.00293-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezig D, Ben Yahia A, Ben Abdallah H, Bahri O, Triki H. 2004. Molecular characterization of coxsackievirus B5 isolates. J. Med. Virol. 72:268–274. 10.1002/jmv.10579. [DOI] [PubMed] [Google Scholar]
- 6.Bahri O, Rezig D, Nejma-Oueslati BB, Yahia AB, Sassi JB, Hogga N, Sadraoui A, Triki H. 2005. Enteroviruses in Tunisia: virological surveillance over 12 years (1992–2003). J. Med. Microbiol. 54(Pt 1):63–69. 10.1099/jmm.0.45695-0. [DOI] [PubMed] [Google Scholar]
- 7.Hertel NT, Pedersen FK, Heilmann C. 1989. Coxsackie B3 virus encephalitis in a patient with agammaglobulinaemia. Eur. J. Pediatr. 148:642–643. 10.1007/BF00441520. [DOI] [PubMed] [Google Scholar]
- 8.McKinney RE, Jr, Katz SL, Wilfert CM. 1987. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev. Infect. Dis. 9:334–356. 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- 9.Cheng FW, Chan PK, Ho WC, Wong FY, Leung TF. 2009. Recurrent enterovirus encephalitis: chance or something else? BMJ Case Rep. 2009:bcr12.2008.1358. 10.1136/bcr.12.2008.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliday E, Winkelstein J, Webster AD. 2003. Enteroviral infections in primary immunodeficiency (PID): a survey of morbidity and mortality. J. Infect. 46:1–8. 10.1053/jinf.2002.1066. [DOI] [PubMed] [Google Scholar]
- 11.Bellmunt A, May G, Zell R, Pring-Akerblom P, Verhagen W, Heim A. 1999. Evolution of poliovirus type I during 5.5 years of prolonged enteral replication in an immunodeficient patient. Virology 265:178–184. 10.1006/viro.1999.0003. [DOI] [PubMed] [Google Scholar]
- 12.Kew OM, Sutter RW, Nottay BK, McDonough MJ, Prevots DR, Quick L, Pallansch MA. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 36:2893–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kew OM, Wright PF, Agol VI, Delpeyroux F, Shimizu H, Nathanson N, Pallansch MA. 2004. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. World Health Organ. 82:16–23. [PMC free article] [PubMed] [Google Scholar]
- 14.Martín J, Dunn G, Hull R, Patel V, Minor PD. 2000. Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J. Virol. 74:3001–3010. 10.1128/JVI.74.7.3001-3010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halsey NA, Pinto J, Espinosa-Rosales F, Faure-Fontenla MA, da Silva E, Khan AJ, Webster AD, Minor P, Dunn G, Asturias E, Hussain H, Pallansch MA, Kew OM, Winkelstein J, Sutter R, Polio Project Team 2004. Search for poliovirus carriers among people with primary immune deficiency diseases in the United States, Mexico, Brazil, and the United Kingdom. Bull. World Health Organ. 82:3–8. [PMC free article] [PubMed] [Google Scholar]