Abstract
Neurobiological studies in higher primates indicate that the processing of stereoscopic information takes place at early levels in the visual cortex. To map the anatomical structures in the human brain participating in pure stereopsis based upon binocular disparity, we measured with positron emission tomography the changes in regional cerebral blood flow as an indicator of metabolic activity in 10 healthy young men during visual discrimination of binocular disparity. The data demonstrate that the discrimination of pure stereo-optic disparity information takes place in the polar striate cortex and the neighboring peri-striate cortices, as well as in the parietal lobe, the prefrontal cortex, and the cerebellum. The discrimination of stereoscopic depth is dependent on a network composed of multiple functional fields localized in occipital- and parietal-lobe visual areas as well as in the dorsolateral and mesial prefrontal cortex. The findings support the importance of coactivated occipitoparietal visual areas in the processing and analysis of binocular depth information in humans.
Full text
PDF

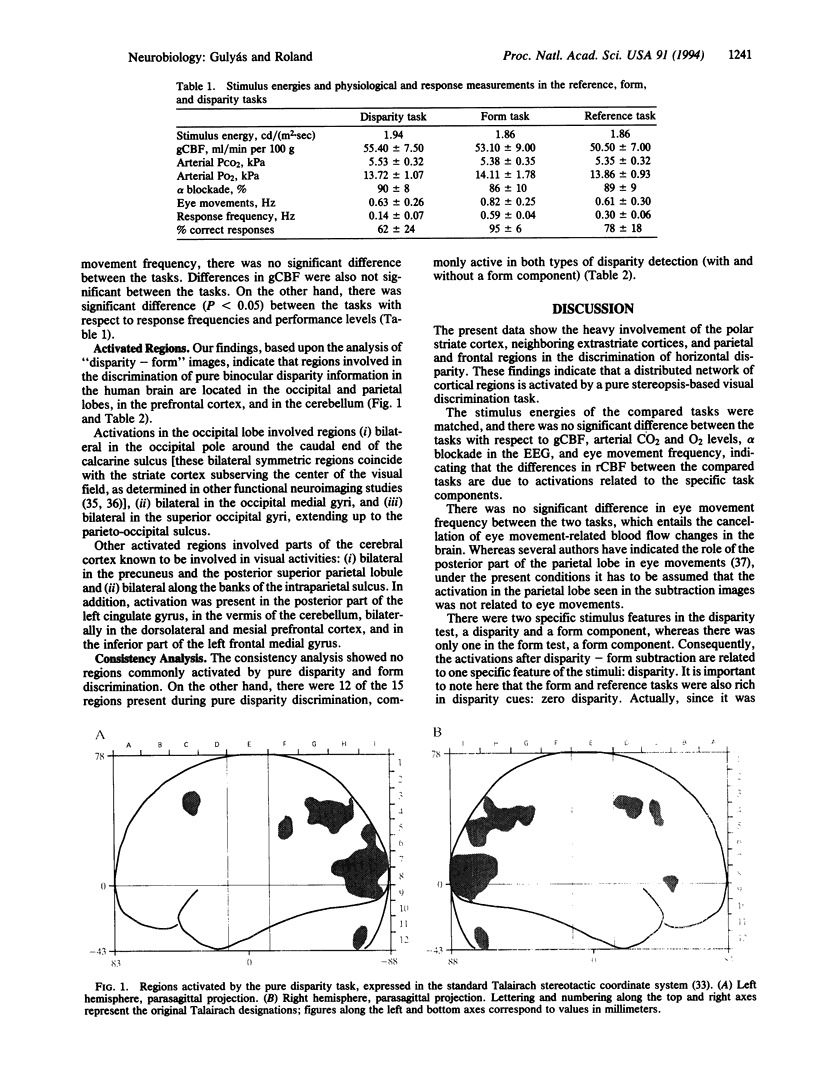

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. A., Brotchie P. R., Mazzoni P. Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol. 1992 Dec;2(6):840–846. doi: 10.1016/0959-4388(92)90143-9. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Berridge M. S., Adler L. P., Nelson A. D., Cassidy E. H., Muzic R. F., Bednarczyk E. M., Miraldi F. Measurement of human cerebral blood flow with [15O]butanol and positron emission tomography. J Cereb Blood Flow Metab. 1991 Sep;11(5):707–715. doi: 10.1038/jcbfm.1991.127. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Pettigrew J. D. Neural mechanisms of binocular vision. Vision Res. 1986;26(9):1587–1600. doi: 10.1016/0042-6989(86)90177-x. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Hague B. Evidence for disparity detecting neurones in the human visual system. J Physiol. 1972 Sep;225(2):437–455. doi: 10.1113/jphysiol.1972.sp009948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm C., Greitz T., Blomqvist G., Farde L., Forsgren P. O., Kingsley D., Sjögren I., Wiesel F., Wik G. Applications of a computerized adjustable brain atlas in positron emission tomography. Acta Radiol Suppl. 1986;369:449–452. [PubMed] [Google Scholar]
- Bohm C., Greitz T., Kingsley D., Berggren B. M., Olsson L. Adjustable computerized stereotaxic brain atlas for transmission and emission tomography. AJNR Am J Neuroradiol. 1983 May-Jun;4(3):731–733. [PMC free article] [PubMed] [Google Scholar]
- Carney T., Shadlen M., Switkes E. Parallel processing of motion and colour information. Nature. 1987 Aug 13;328(6131):647–649. doi: 10.1038/328647a0. [DOI] [PubMed] [Google Scholar]
- Cavada C., Goldman-Rakic P. S. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989 Sep 22;287(4):422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Miezin F. M., Dobmeyer S., Shulman G. L., Petersen S. E. Attentional modulation of neural processing of shape, color, and velocity in humans. Science. 1990 Jun 22;248(4962):1556–1559. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Miezin F. M., Dobmeyer S., Shulman G. L., Petersen S. E. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991 Aug;11(8):2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoe E. A., Van Essen D. C. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 1988 May;11(5):219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- Felleman D. J., Van Essen D. C. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991 Jan-Feb;1(1):1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Felleman D. J., Van Essen D. C. Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex. J Neurophysiol. 1987 Apr;57(4):889–920. doi: 10.1152/jn.1987.57.4.889. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Miezin F. M., Allman J. M., Van Essen D. C., Raichle M. E. Retinotopic organization of human visual cortex mapped with positron-emission tomography. J Neurosci. 1987 Mar;7(3):913–922. doi: 10.1523/JNEUROSCI.07-03-00913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greitz T., Bergström M., Boëthius J., Kingsley D., Ribbe T. Head fixation system for integration of radiodiagnostic and therapeutic procedures. Neuroradiology. 1980;19(1):1–6. doi: 10.1007/BF00369080. [DOI] [PubMed] [Google Scholar]
- Greitz T., Bohm C., Holte S., Eriksson L. A computerized brain atlas: construction, anatomical content, and some applications. J Comput Assist Tomogr. 1991 Jan-Feb;15(1):26–38. [PubMed] [Google Scholar]
- Gulyás B., Roland P. E. Cortical fields participating in form and colour discrimination in the human brain. Neuroreport. 1991 Oct;2(10):585–588. doi: 10.1097/00001756-199110000-00008. [DOI] [PubMed] [Google Scholar]
- Hamsher K. D. Stereopsis and unilateral brain disease. Invest Ophthalmol Vis Sci. 1978 Apr;17(4):336–343. [PubMed] [Google Scholar]
- Haxby J. V., Grady C. L., Horwitz B., Ungerleider L. G., Mishkin M., Carson R. E., Herscovitch P., Schapiro M. B., Rapoport S. I. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1621–1625. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987 Nov;7(11):3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M., Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988 May 6;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lueck C. J., Zeki S., Friston K. J., Deiber M. P., Cope P., Cunningham V. J., Lammertsma A. A., Kennard C., Frackowiak R. S. The colour centre in the cerebral cortex of man. Nature. 1989 Aug 3;340(6232):386–389. doi: 10.1038/340386a0. [DOI] [PubMed] [Google Scholar]
- Maunsell J. H., Van Essen D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J Neurophysiol. 1983 May;49(5):1148–1167. doi: 10.1152/jn.1983.49.5.1148. [DOI] [PubMed] [Google Scholar]
- Olesen J., Paulson O. B., Lassen N. A. Regional cerebral blood flow in man determined by the initial slope of the clearance of intra-arterially injected 133Xe. Stroke. 1971 Nov-Dec;2(6):519–540. doi: 10.1161/01.str.2.6.519. [DOI] [PubMed] [Google Scholar]
- Petrides M., Pandya D. N. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984 Sep 1;228(1):105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Poggio G. F., Fischer B. Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving rhesus monkey. J Neurophysiol. 1977 Nov;40(6):1392–1405. doi: 10.1152/jn.1977.40.6.1392. [DOI] [PubMed] [Google Scholar]
- Poggio G. F., Poggio T. The analysis of stereopsis. Annu Rev Neurosci. 1984;7:379–412. doi: 10.1146/annurev.ne.07.030184.002115. [DOI] [PubMed] [Google Scholar]
- Poggio G. F., Talbot W. H. Mechanisms of static and dynamic stereopsis in foveal cortex of the rhesus monkey. J Physiol. 1981 Jun;315:469–492. doi: 10.1113/jphysiol.1981.sp013759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptito A., Zatorre R. J. Impaired stereoscopic detection thresholds after left or right temporal lobectomy. Neuropsychologia. 1988;26(4):547–554. doi: 10.1016/0028-3932(88)90111-x. [DOI] [PubMed] [Google Scholar]
- Ptito A., Zatorre R. J., Larson W. L., Tosoni C. Stereopsis after unilateral anterior temporal lobectomy. Dissociation between local and global measures. Brain. 1991 Jun;114(Pt 3):1323–1333. doi: 10.1093/brain/114.3.1323. [DOI] [PubMed] [Google Scholar]
- Ramachandran V. S., Gregory R. L. Does colour provide an input to human motion perception? Nature. 1978 Sep 7;275(5675):55–56. doi: 10.1038/275055a0. [DOI] [PubMed] [Google Scholar]
- Roland P. E., Eriksson L., Stone-Elander S., Widen L. Does mental activity change the oxidative metabolism of the brain? J Neurosci. 1987 Aug;7(8):2373–2389. [PMC free article] [PubMed] [Google Scholar]
- Roland P. E., Gulyás B., Seitz R. J., Bohm C., Stone-Elander S. Functional anatomy of storage, recall, and recognition of a visual pattern in man. Neuroreport. 1990 Sep;1(1):53–56. doi: 10.1097/00001756-199009000-00015. [DOI] [PubMed] [Google Scholar]
- Roland P. E., Mortensen E. Somatosensory detection of microgeometry, macrogeometry and kinesthesia in man. Brain Res. 1987 Mar;434(1):1–42. doi: 10.1016/0165-0173(87)90017-8. [DOI] [PubMed] [Google Scholar]
- Rothstein T. B., Sacks J. G. Defective stereopsis in lesions of the parietal lobe. Am J Ophthalmol. 1972 Feb;73(2):281–284. doi: 10.1016/0002-9394(72)90146-8. [DOI] [PubMed] [Google Scholar]
- Seitz R. J., Bohm C., Greitz T., Roland P. E., Eriksson L., Blomqvist G., Rosenqvist G., Nordell B. Accuracy and precision of the computerized brain atlas programme for localization and quantification in positron emission tomography. J Cereb Blood Flow Metab. 1990 Jul;10(4):443–457. doi: 10.1038/jcbfm.1990.87. [DOI] [PubMed] [Google Scholar]
- Zeki S., Watson J. D., Lueck C. J., Friston K. J., Kennard C., Frackowiak R. S. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991 Mar;11(3):641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]