Abstract
Microsporidia are highly specialized obligate intracellular organisms that are closely related to fungi. Although traditionally associated with diarrheal illness in patients with AIDS, extraintestinal infections involving various organs have been reported with increasing frequency in the past decade, particularly in immunocompromised hosts. Diagnosis is usually accomplished by light microscopic identification of spores in body fluids and tissues, using a variety of stains. Transmission electron microscopy, immunofluorescence assays, or molecular methods are necessary for identification to the genus and species level. Early diagnosis is essential for preventing the significant associated morbidity and mortality of extraintestinal microsporidiosis.
INTRODUCTION
Microsporidia are primitive eukaryotic obligate intracellular organisms capable of infecting a large number of invertebrate and vertebrate hosts. They are closely related to fungi by phylogenetic molecular analysis but have an unusual genomic biology, mechanism of infection, and cell structure. Microsporidia were initially identified in 1857 as the causative agent of European silkworm disease and were known to infect fish and invertebrates long before human infection was described (1). Interest in microsporidia exploded in the medical community during the mid-1980s and 1990s due to their association with gastrointestinal and systemic disease in AIDS patients. Fortunately, the incidence of intestinal microsporidiosis in HIV-infected patients has since decreased in developed countries where the use of highly active antiretroviral therapy and resultant immune reconstitution are widespread. However, microsporidiosis is increasingly reported in non-HIV immunocompromised patients, such as transplant recipients, as well as in some immunocompetent contact lens wearers, travelers, children, and the elderly (1). There have been numerous reports of localized and disseminated extraintestinal microsporidiosis affecting almost any organ system in the past decade, predominantly in immunocompromised patients. This minireview will discuss the taxonomy, epidemiology, clinical features, diagnosis, and management of extraintestinal microsporidiosis.
TAXONOMY AND LIFE CYCLE
The term microsporidia encompasses over 1,300 described species belonging to 170 named genera in the phylum Microsporidia (formerly Microspora) (2). Among these are at least 15 species known to be human pathogens (3). The genus designation of many human microsporidia has changed over the years as new ultrastructural and molecular data have become available. For example, Nosema algerae was renamed Brachiola algerae and later Anncaliia algerae, while Septata intestinalis was renamed Encephalitozoon intestinalis (2).
Microsporidia possess some of the smallest autonomous nuclear genomes known today. This is thought to be due to reductive evolution, during which their reduced biosynthetic pathways have made them highly dependent on the host cell to complete their life cycle; hence, their obligate intracellular (“parasitic”) nature (4). Microsporidia can infect almost any phylum of animals, including mammals, insects, and fish.
Microsporidia derive their name from their characteristically small spore stage. Mature spores of human-infective species range from 0.8 to 5 μm in length and are comparable in size to many bacteria and small yeasts. Spores are the infective stage of the organism and are able to withstand adverse environmental conditions, often surviving outside the host for prolonged periods of time. They contain a nucleus, posterior vacuole, anterior anchoring disc, and Golgi-like apparatus within the sporoplasm. There is also a polar filament wrapped tightly around the periphery of the sporoplasm. The polar filament is unique to the microsporidia and plays an important role in host cell invasion. In the appropriate host environment, changes in pH and osmotic pressure cause rapid eversion of the polar filament, with sufficient force to penetrate a host cell (Fig. 1). The filament then becomes a long polar tubule through which infective sporoplasm is transferred to the cell, much like injection with a syringe. After entering the host cell, the sporoplasm undergoes two stages of development: an asexual proliferative phase and a later sexual sporogonic phase. The host cell eventually ruptures due to the high intracellular parasite burden, and new infective spores are released into the local environment (4).
FIG 1.
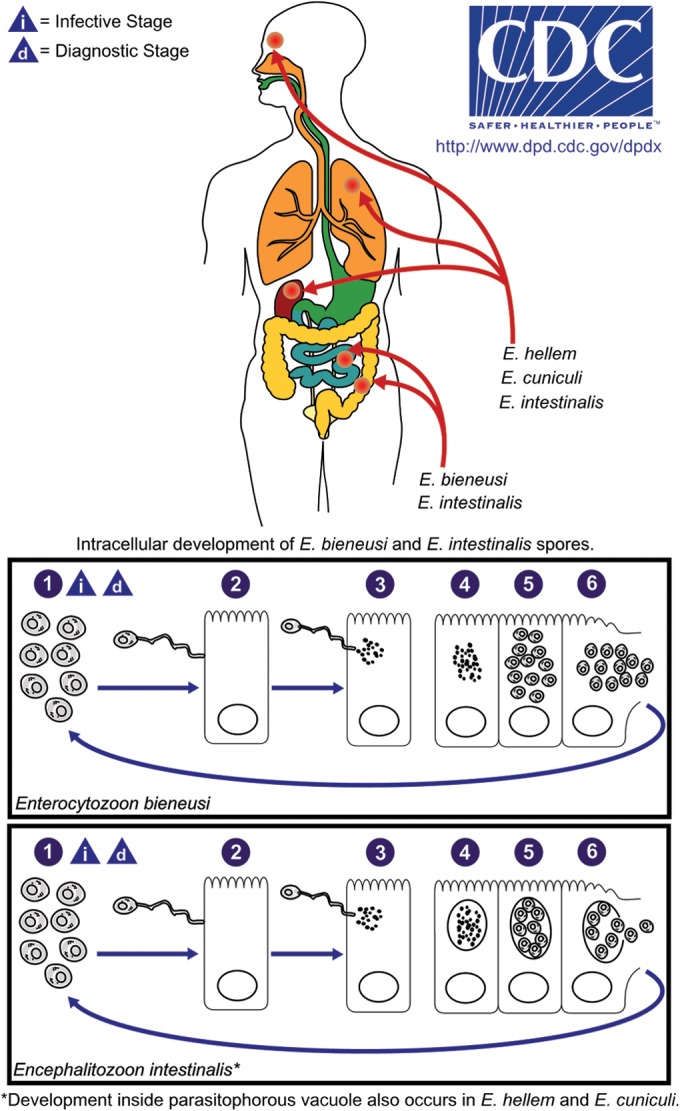
Microsporidial life cycle as exemplified by E. bieneusi and E. intestinalis. Spores are the infectious form of the organism (1). Spores infect cells when the polar filament protrudes from the spore and forms the polar tubule, which inserts into the eukaryotic host cell (2). The infective sporoplasm is injected through the polar tubule into the host cell, where it replicates asexually by binary fission (merogony) or multiple fission (schizogony) (3 to 5). Some species divide directly in the host cell cytoplasm (e.g., E. bieneusi), while others are found within a parasitophorous vacuole (e.g., Encephalitozoon spp.) or sporophorous vesicle (e.g., Pleistophora spp.). Mature spores are produced by a form of sexual reproduction called sporogony (5) and eventually, following cell disruption, are released into the surrounding environment (6) to infect new cells. (Courtesy of the CDC DPDx Service.)
EPIDEMIOLOGY AND MODE OF TRANSMISSION
Human microsporidiosis has been described worldwide. The species known to infect humans have been isolated from a large variety of birds, fish, insects, and other animals, as well as from food and water sources (3, 5, 6). Modes of transmission are thought to include ingestion, inhalation, direct trauma to skin or mucous membrane, sexual contact, and transplacental (mother to child) (2).
CLINICAL MANIFESTATIONS
Microsporidia can cause localized or disseminated infection. The clinical features depend on the mode of transmission, the type of infecting species, and the underlying host immune status and response. Enterocytozoon bieneusi and Encephalitozoon intestinalis are the most common species associated with infection in humans, historically being associated with chronic diarrheal illness, weight loss, and abdominal pain in patients with AIDS. They also may cause an acute, self-limiting diarrheal illness in immunocompetent people. While Enterocytozoon bieneusi infection is mostly confined to the intestinal and biliary tract, Encephalitozoon species are known to cause disseminated infection involving the kidneys, lungs, eyes, and other organs. Other microsporidia can also involve the eye, causing localized ocular infection (keratitis), particularly in contact lens wearers. Disseminated infections usually occur in immunocompromised hosts (mostly reported in solid organ and bone marrow transplant recipients) and can affect any organ system. Most cases of pulmonary microsporidiosis have been reported in patients with hematological malignancies and bone marrow transplantation. In addition, cases of brain abscess, sinusitis, endocarditis, myocarditis, osteomyelitis, cutaneous, and genitourinary infections have been described. Most extraintestinal infections occur as part of disseminated disease and carry a poorer prognosis than gastrointestinal infections due to underlying host comorbidities and to difficulty in diagnosis and resultant delays in treatment. Table 1 lists the various microsporidia species that have been associated with extragastrointestinal infections.
TABLE 1.
List of microsporidia that cause extraintestinal human infectiona
| Organ system involved (disorder caused) | Species of Microsporidia (site of infection) |
|---|---|
| Cardiovascular system (endocarditis, myocarditis) | Encephalitozoon cuniculi |
| Anncaliia connori | |
| Trachipleistophora anthropopthera | |
| Central nervous system | Encephalitozoon cuniculi (brain abscess) |
| Trachipleistophora anthropopthera | |
| Disseminated | Encephalitozoon spp.: E. intestinalis, E. hellem, E. cuniculi |
| Tubulinosema acridophagus | |
| Anncaliia connori | |
| Trachipleistophora anthropopthera | |
| Eye (keratitis, keratoconjunctivitis, blindness) | Encephalitozoon hellem |
| Encephalitozoon intestinalis (conjunctiva) | |
| Vittaforma corneae; formerly Nosema corneum | |
| Trachipleistophora hominis | |
| Nosema ocularum | |
| Microsporidium spp.: M. africanum, M. ceylonensis | |
| Anncaliia algerae | |
| Endocrine system | Trachipleistophora anthropopthera |
| Thyroid, parathyroid, adrenal cortex | Anncaliia connori |
| Genital tract: endometrium, fallopian tubes, urethritis | Encephalitozoon spp. |
| Liver, pancreas, peritoneum | Encephalitozoon spp. |
| Enterocytozoon bieneusi (biliary tract) | |
| Trachipleistophora anthropopthera | |
| Hematologic system: spleen, bone marrow, lymph nodes | Trachipleistophora anthropopthera |
| Musculoskeletal system (myositis) | Anncaliia algerae |
| Braciola vesicularum | |
| Microsporidium spp. | |
| Novel species closely related to Endoreticularis spp. | |
| Pleistophora spp. | |
| Trachipleistophora hominis | |
| Tubulinosema | |
| Upper respiratory tract: nasal cavity, sinuses, pharynx, larynx, vocal cords | Anncaliia algerae |
| Encephalitozoon spp. | |
| Enterocytozoon bieneusi | |
| Trachipleistophora hominis | |
| Lower respiratory tract: trachea, bronchi, bronchioles, lungs | Encephalitozoon spp. |
| Enterocytozoon bieneusi | |
| Renal system | Anncaliia connori |
| Encephalitozoon spp. (especially in kidney allografts) | |
| Trachipleistophora anthropopthera | |
| Skin | Anncaliia algerae |
| Encephalitozoon intestinalis |
LABORATORY DIAGNOSIS
A number of laboratory techniques have been described for the identification of microsporidia in human specimens, including light microscopy, transmission electron microscopy (TEM), immunofluorescence assays (IFAs) using polyclonal or monoclonal antibodies, cell culture, and molecular methods (2). Many of these methods require significant expertise or specialized equipment and are therefore limited to reference, research, and public health laboratories, such as the Centers for Disease Control and Prevention (CDC).
Diagnosis is most widely performed using light microscopic identification of spores in stool and body fluid samples and tissue touch preps and smears (2). The most commonly used staining methods for these specimens are the chromotrope 2R technique and modifications of it (e.g., Ryan blue modified trichrome) (Fig. 2). With this method, microsporidial spores stain a deep red-pink, often with an accentuated central beltlike stripe running perpendicular to the long axis of the spore. Optical fluorescent brighteners, such as calcofluor white and Uvitex 2B, will also highlight microsporidial spores and are useful for rapid screening; however, these stains are nonspecific and will also highlight yeasts of similar appearance (2). Spores (and yeasts) will also stain positively with a routine Gram stain. A rapid Gram-chromotrope combined method has been described that provides a result in 10 min or less and highlights the beltlike stripe within spores, thus providing a more specific method for detecting microsporidial spores than Gram alone (11). Of note, there is some variation in the sizes of spores between the different microsporidia; the spores most commonly seen in stool (E. bieneusi and Encephalitozoon spp.) are very small, measuring 0.8 to 1.5 μm in length by approximately 1 μm in width, while spores of other microsporidia (e.g., Nosema, Brachiola, and Anncaliia species) can measure up to 5 μm in the greatest dimension and overlap with the sizes of small yeasts, such as Histoplasma capsulatum and Candida glabrata (2). It is important for the clinical microbiology laboratory to be aware of these size differences and keep microsporidia in their differential diagnosis when examining specimens, particularly from extraintestinal sites. In general, light microscopic preparations can be challenging to interpret, given the relatively small size of the spores and their morphological similarities to yeasts.
FIG 2.
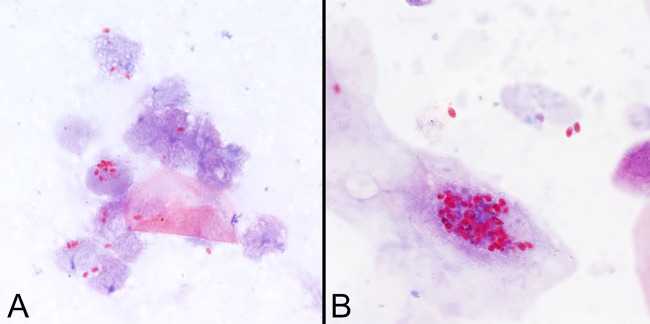
Spores of Encephalitozoon cuniculi in urine (A) and Anncaliia algerae in sputum (B) stained with the modified trichrome (Ryan blue) stain. Note that the spores of A. algerae are significantly larger than spores of Encephalitozoon spp. and may therefore be confused with yeasts. All images are shown at ×1,000 magnification.
In histologic sections, microsporidial spores stain pink using hematoxylin and eosin (H&E) and stain positively with Periodic acid-Schiff (PAS) stain, tissue Gram stain (e.g., Brown and Brenn's or Brown and Hopps' method), and Warthin-Starry stain. They may also be focally positive with the Gomori methenamine silver (GMS) fungal stain and Ziehl-Neelsen (Z-N) acid-fast stain (Fig. 3) (2). The PAS stain produces a characteristic polar dotlike staining pattern which is particularly evident in larger spores; this appearance is unlike the staining pattern of yeasts, thus aiding in their differentiation. Microsporidial spores also polarize using birefringent light (Fig. 4) (2). These microscopic features allow the identification of microsporidia but do not provide definitive genus or species information.
FIG 3.
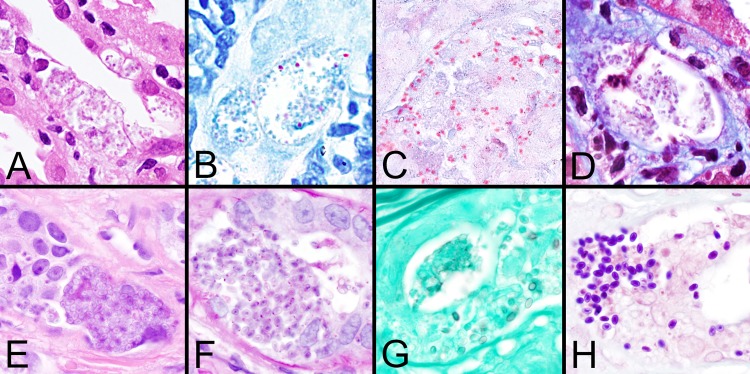
Histologic staining patterns for microsporidial spores in tissue. (A to D) Images demonstrate spores of Encephalitozoon cuniculi in sections of kidney stained with H&E, Z-N, modified trichrome (Ryan blue), and Weber's modified trichome stain, respectively. Note that the spores are only focally positive with the Z-N acid-fast stain. (E to H) Images demonstrate the larger spores of Anncaliia algerae in eccrine skin glands stained with H&E, PAS, GMS, and Brown and Brenn (tissue Gram stain) stain, respectively. Note the dot-like PAS positivity and the focal staining with GMS. All images are shown at ×1,000 magnification.
FIG 4.
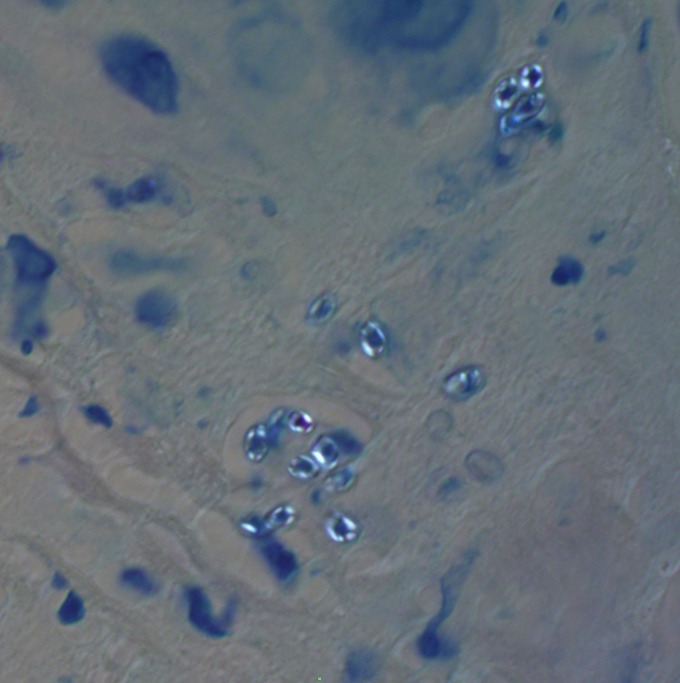
Microsporidial spores are birefringent with many commonly used histopathology stains using polarized light. The image was obtained using Z-N stain and ×2,000 magnification.
Historically, TEM has been used for confirmation of diagnosis and microsporidial species determination (Fig. 5) (2). It has also been important in the taxonomic classification of new species. Ultrastructural characteristics that are used to differentiate the microsporidia include the number of polar filament cross sections, arrangement of polar filaments (e.g., number of rows), and characteristics of the parasite-host interface (i.e., whether the spores are directly within the host cell cytoplasm or are within a parasitophorous vacuole or sporophorous vesicle). Unfortunately, electron microscopy is not widely utilized due to the cost of the necessary equipment and the level of technical expertise required to perform the test and interpret the results.
FIG 5.
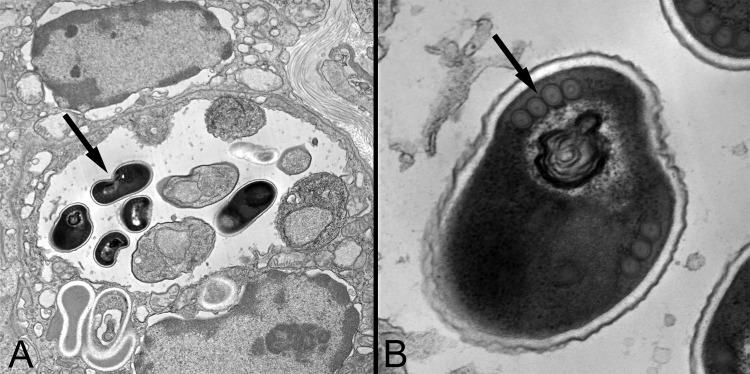
Transmission electron micrographs of Encephalitozoon cuniculi organisms within a renal tubular cell. (A) Note that the dark spores (arrow) are within a parasitophorous vacuole, as is characteristic for Encephalitozoon spp. (×11,000 magnification). (B) On higher magnification (×68,000 magnification), a single row of polar filament cross sections is seen (arrow), which is also consistent with the diagnosis of Encephalitozoon spp. The final species determination was made by PCR.
Culture is another classic technique for studying microsporidia and has been successfully performed for many of the human-infecting species, using a number of different cell culture lines, including human diploid fibroblasts (e.g., MRC-5) (12). Culture is especially useful for amplifying the number of spores from a specimen so that subsequent molecular analysis or TEM can be performed for definitive species identification.
Finally, molecular amplification, primarily using PCR, has been employed to detect microsporidia and identify the infecting species (2). There are no assays approved by the Federal Drug Administration (FDA), but they may be available through some reference, research, and public health laboratories. Assays have been described for a number of microsporidia, including those that detect and/or discriminate between the most common human pathogens, E. bieneusi, Encephalitozoon cuniculi, Encephalitozoon hellem, and Encephalitozoon intestinalis, from common specimen types, including stool and urine (13–17). Commonly used targets include the intergenic spacer region gene and small-subunit ribosomal DNA (16, 17). In most cases, PCR assays are significantly more sensitive than microscopy, and they may allow semiquantitative or quantitative analysis and species differentiation, thus having an impact on treatment decisions. Given the challenges associated with subjective microscopic examination, PCR may someday be the test of choice for microsporidia.
PATIENT MANAGEMENT
Reduction of immunosuppression, if possible, is often essential for clearing the infection. This has been demonstrated in patients with AIDS who were started on antiretroviral therapy which resulted in immune reconstitution; however, there are fewer data for other immunocompromised hosts. Albendazole is the primary drug of choice for most species that infect humans, especially the Encephalitozoon species, but it has limited efficacy against Enterocytozoon bieneusi and Vittaforma species. Oral fumagillin is active against E. bieneusi; however, its use is limited due to significant bone marrow toxicity. Topical fumagillin is routinely used for the management of ocular infections (18), but systemic formulations are not readily available in the United States.
CONCLUSION
Microsporidia are a diverse group of highly specialized fungi that have established themselves as human pathogens since the AIDS epidemic. In recent years, there has been a change in this trend that is specifically related to the epidemiology and clinical features of microsporidiosis. Extraintestinal infections affecting various organs and caused by new species have been reported with increasing frequency in the past decade. Therefore, microsporidiosis should be considered in the differential diagnosis of life-threatening infections involving almost any organ system in immunocompromised hosts. Early diagnosis will help decrease the mortality of these infections. There is an urgent need to develop more effective antimicrobial agents with acceptable toxicity profiles for use against E. bieneusi and in complicated or refractory cases.
Biography

Bobbi Pritt, MD, is an Associate Professor of Pathology at Mayo Clinic, MN, where she serves as the Director of the Clinical Parasitology Laboratory, Program Director of the Clinical Microbiology MD fellowship program, and the Vice Chair of Education in the Department of Laboratory Medicine and Pathology. Dr. Pritt completed her medical degree and pathology residency at the University of Vermont, College of Medicine, followed by a fellowship in Clinical Microbiology at Mayo Clinic. She then received a Master's degree in Medical Parasitology from the London School of Hygiene and Tropical Medicine and was awarded the Patrick Buxton memorial medal for best student in her class. Dr. Pritt has received numerous other awards for education, including the Mayo Clinic Innovation and Leadership Award in Education. She has published over 70 manuscripts, 10 book chapters, and a Parasitology Reference Guide. Her interests include clinical parasitology, infectious disease pathology, and molecular diagnostics.
Footnotes
Published ahead of print 14 May 2014
REFERENCES
- 1.Didier ES. 2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 94:61–76. 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Cali A, Takvorian NRPM. 2011. Microsporidiosis, p 61–76 In Meyers WM, Firpo A, Wear DJ. (ed), Topics on the pathology of protozoan and invasive arthropod diseases. Armed Forces Institute of Pathology (AFIP), Washington, DC: http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA545141 Accessed 3 April 2014. [Google Scholar]
- 3.Mathis A, Weber R, Deplazes P. 2005. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 18:423–445. 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams BAP. 2009. Unique physiology of host-parasite interactions in microsporidia infections. Cell. Microbiol. 11:1551–1560. 10.1111/j.1462-5822.2009.01362.x. [DOI] [PubMed] [Google Scholar]
- 5.Decraene V, Lebbad M, Botero-Kleiven S, Gustavsson AM, Lofdahl M. 2012. First reported foodborne outbreak associated with microsporidia, Sweden, October 2009. Epidemiol. Infect. 140:519–527. 10.1017/S095026881100077X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvan AL, Sanchez AMM, Valentin MAP, Henriques-Gil N, Izquierdo F, Fenoy S, del Aguila C. 2011. First cases of microsporidiosis in transplant recipients in Spain and review of the literature. J. Clin. Microbiol. 49:1301–1306. 10.1128/JCM.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhary MM, Metcalfe MG, Arrambide K, Bern C, Visvesvara GS, Pieniazek NJ, Bandea RD, Deleon-Carnes M, Adem P, Choudhary MM, Zaki SR, Saeed MU. 2011. Tubulinosema sp. microsporidian myositis in immunosuppressed patient. Emerg. Infect. Dis. 17:1727–1730. 10.3201/eid1709.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kester KE, Visvesara GS, McEvoy P. 2000. Organism responsible for nodular cutaneous microsporidiosis in a patient with AIDS. Ann. Intern. Med. 133:925. 10.7326/0003-4819-133-11-200012050-00028. [DOI] [PubMed] [Google Scholar]
- 9.Meissner EG, Bennett JE, Qvarnstrom Y, da Silva A, Chu EY, Tsokos M, Gea-Banacloche J. 2012. Disseminated microsporidiosis in an immunosuppressed patient. Emerg. Infect. Dis. 18:1155–1158. 10.3201/eid1807.120047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suankratay C, Thiansukhon E, Nilaratanakul V, Putaporntip C, Jongwutiwes S. 2012. Disseminated infection caused by novel species of Microsporidium, Thailand. Emerg. Infect. Dis. 18:302–304. 10.3201/eid1802.111319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moura H, Schwartz DA, Bornay-Llinares F, Sodre FC, Wallace S, Visvesvara GS. 1997. A new and improved “quick-hot Gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch. Pathol. Lab. Med. 121:888–893. [PubMed] [Google Scholar]
- 12.Visvesvara GS. 2002. In vitro cultivation of microsporidia of clinical importance. Clin. Microbiol. Rev. 15:401–413. 10.1128/CMR.15.3.401-413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh K, Weiss LM. 2009. Molecular diagnostic tests for microsporidia. Interdiscip. Perspect. Infect. Dis. 2009:926521. 10.1155/2009/926521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Notermans DW, Peek R, de Jong MD, Wentink-Bonnema EM, Boom R, van Gool T. 2005. Detection and identification of Enterocytozoon bieneusi and Encephalitozoon species in stool and urine specimens by PCR and differential hybridization. J. Clin. Microbiol. 43:610–614. 10.1128/JCM.43.2.610-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subrungruang I, Mungthin M, Chavalitshewinkoon-Petmitr P, Rangsin R, Naaglor T, Leelayoova S. 2004. Evaluation of DNA extraction and PCR methods for detection of Enterocytozoon bienuesi in stool specimens. J. Clin. Microbiol. 42:3490–3494. 10.1128/JCM.42.8.3490-3494.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verweij JJ, Ten Hove R, Brienen EA, van Lieshout L. 2007. Multiplex detection of Enterocytozoon bieneusi and Encephalitozoon spp. in fecal samples using real-time PCR. Diagn. Microbiol. Infect. Dis. 57:163–167. 10.1016/j.diagmicrobio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Orlandi PA, Stenger DA. 2005. Simultaneous detection of four human pathogenic microsporidian species from clinical samples by oligonucleotide microarray. J. Clin. Microbiol. 43:4121–4128. 10.1128/JCM.43.8.4121-4128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagpal A, Pritt BS, Lorenz EC, Amer H, Nasr SH, Cornell LD, Iqbal S, Wilhelm MP. 2013. Disseminated microsporidiosis in a renal transplant recipient: Case report and review of the literature. Transpl. Infect. Dis. 5:526–532. 10.1111/tid.12119. [DOI] [PubMed] [Google Scholar]