Abstract
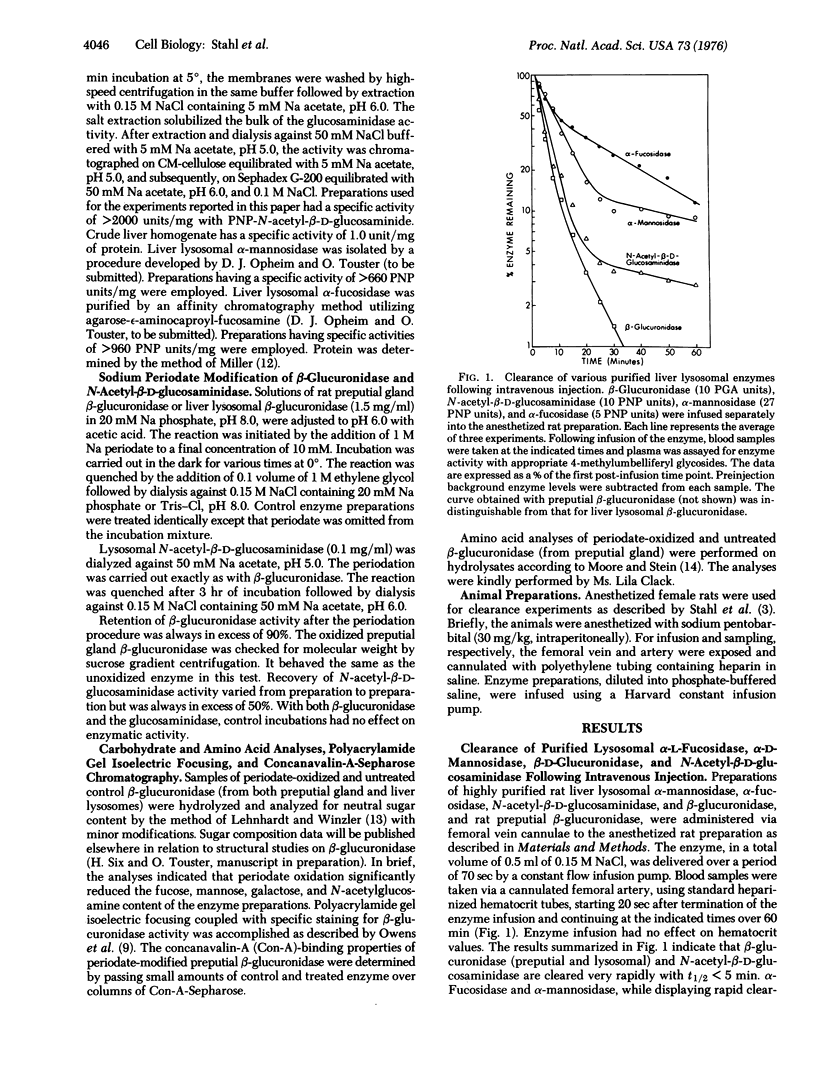
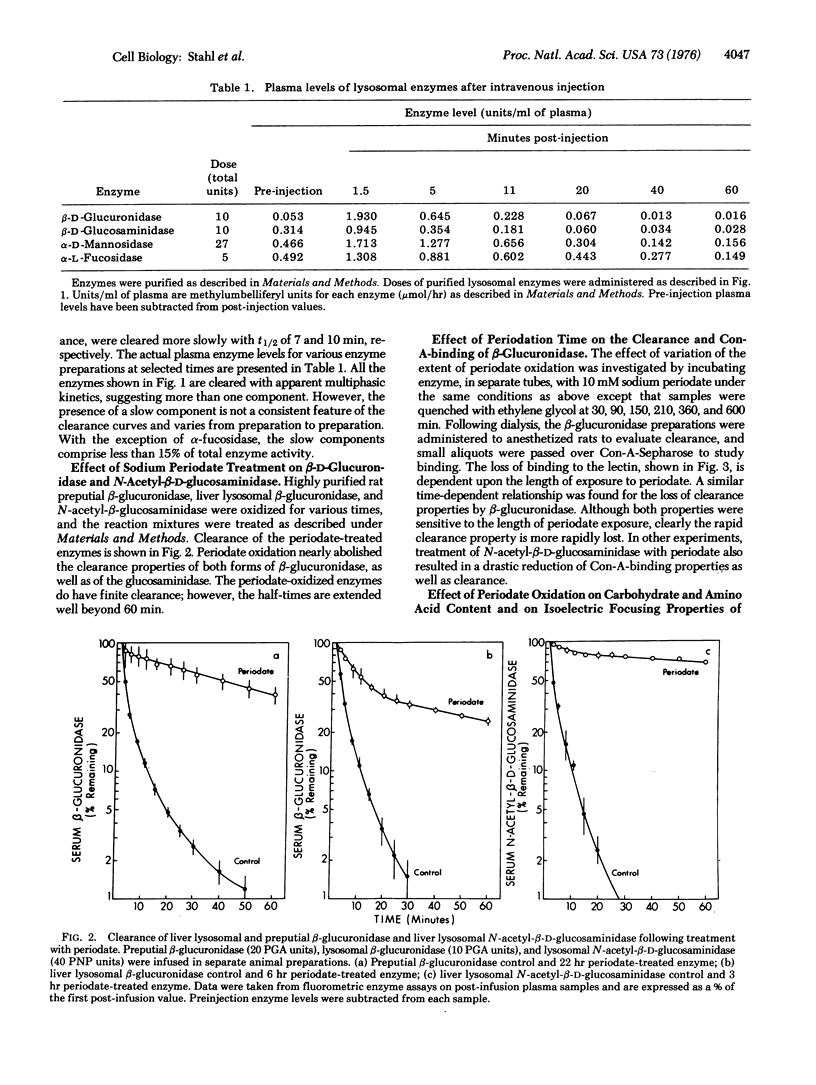
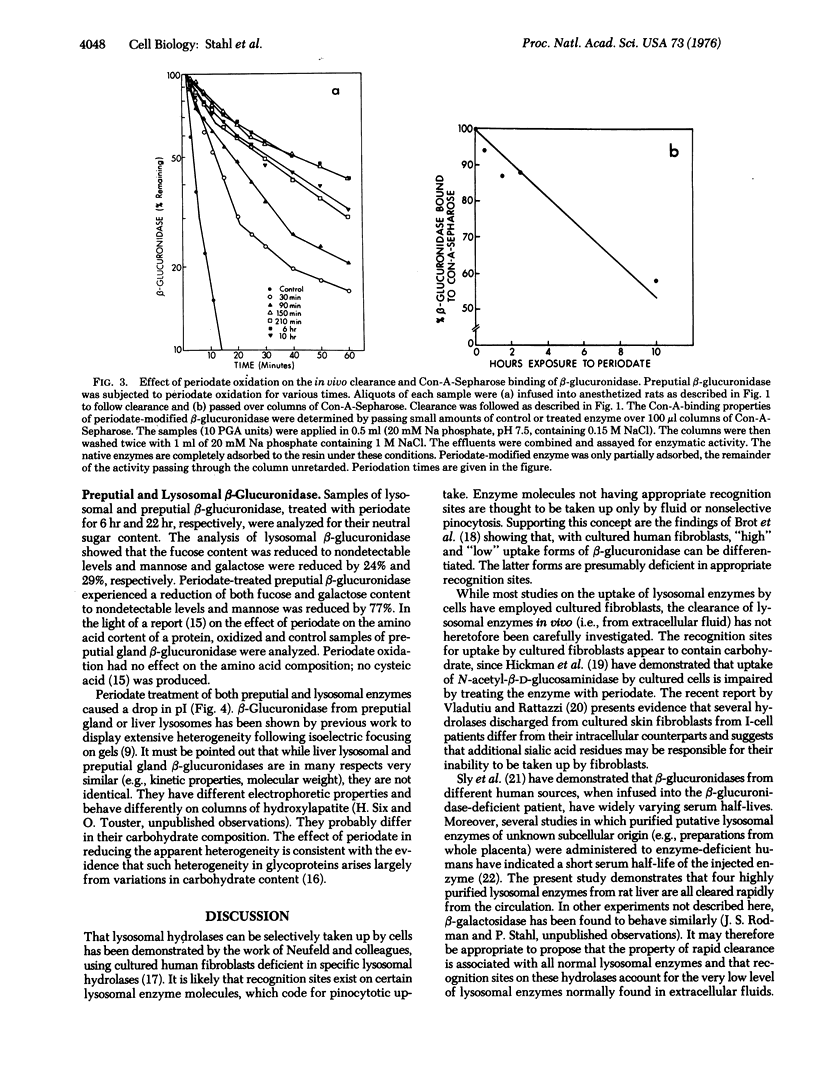
A study of the clearance of liver lysosomal enzymes was carried out in the rat. Purified rat liver lysosomal beta-D-glucuronidase (EC 3.2.1.31), N-acetyl-beta-D-glucosaminidase (EC 3.2.1.30), alpha-L-fucosidase (EC 3.2.1.51), and alpha-D-mannosidase (EC 3.2.1.24), as well as rat preputial gland beta-glucuronidase, were infused intravenously into anesthetized rats. All of the enzymes were rapidly cleared from the circulation. Sodium periodate oxidation of lysosomal beta-glucuronidase resulted in a near abolition of rapid clearance, a reduction in concanavilin-A-Sepharose binding, and a reduction in neutral sugar content, accompanied by alteration in isoelectric focusing properties. Similarly, periodate oxidation of lysosomal N-acetyl-beta-D-glucosaminidase resulted in a loss of the rapid clearance property. These results suggest that specific recognition sites occur on lysosomal hydrolases which mediate clearance following intravenous injection, and that these sites involve the carbohydrate portions of the enzymes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Baccino F. M., Zuretti M. F. On the structure-linked sedimentability of rat liver beta-N-acetylglucosaminidase. Biochim Biophys Acta. 1971 May 12;235(2):353–357. doi: 10.1016/0005-2744(71)90214-2. [DOI] [PubMed] [Google Scholar]
- Brady R. O., Pentchev P. G., Gal A. G. Investigations in enzyme replacement therapy in lipid storage diseases. Fed Proc. 1975 Apr;34(5):1310–1315. [PubMed] [Google Scholar]
- Brot F. E., Glaser J. H., Roozen K. J., Sly W. S., Stahl P. D. In vitro correction of deficient human fibroblasts by beta-glucuronidase from different human sources. Biochem Biophys Res Commun. 1974 Mar 15;57(1):1–8. doi: 10.1016/s0006-291x(74)80349-9. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Tandt W. R., Lassila E., Philippart M. Leroy's l-cell disease: markedly increased activity of plasma acid hydrolases. J Lab Clin Med. 1974 Mar;83(3):403–408. [PubMed] [Google Scholar]
- Gan J., Papkoff H., Li C. H. The reaction of pituitary interstitial cell-stimulating hormone with periodate. Biochim Biophys Acta. 1968 Nov 12;170(1):189–197. doi: 10.1016/0304-4165(68)90172-4. [DOI] [PubMed] [Google Scholar]
- Hickman S., Neufeld E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Hickman S., Shapiro L. J., Neufeld E. F. A recognition marker required for uptake of a lysosomal enzyme by cultured fibroblasts. Biochem Biophys Res Commun. 1974 Mar 15;57(1):55–61. doi: 10.1016/s0006-291x(74)80356-6. [DOI] [PubMed] [Google Scholar]
- Lehnhardt W. F., Winzler R. J. Determination of neutral sugars in glycoproteins by gas-liquid chromatography. J Chromatogr. 1968 May 7;34(4):471–479. doi: 10.1016/0021-9673(68)80091-3. [DOI] [PubMed] [Google Scholar]
- Otsuka K., Wakabayashi M. Purification and characterization of the preputial gland beta-glucuronidase. Enzymologia. 1970 Aug 31;39(2):109–124. [PubMed] [Google Scholar]
- Owens J. W., Gammon K. L., Stahl P. D. Multiple forms of beta-glucuronidase in rat liver lysosomes and microsomes. Arch Biochem Biophys. 1975 Jan;166(1):258–272. doi: 10.1016/0003-9861(75)90387-2. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Touster O. Beta-glucuronidase of rat liver lysosomes. Purification, properties, subunits. J Biol Chem. 1971 Sep 10;246(17):5398–5406. [PubMed] [Google Scholar]
- Stahl P., Mandell B., Rodman J. S., Schlesinger P., Lang S. Different forms of rat beta-glucuronidase with rapid and slow clearance following intravenous injection: selective serum enhancement of slow clearance forms by organophosphate compounds. Arch Biochem Biophys. 1975 Oct;170(2):536–540. doi: 10.1016/0003-9861(75)90149-6. [DOI] [PubMed] [Google Scholar]
- Thorpe S. R., Fiddler M. B., Desnick R. J. Enzyme therapy IV. A method for determining the in vivo fate of bovine beta-glucuronidase in beta-glucuronidase deficient mice. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1464–1470. doi: 10.1016/s0006-291x(74)80448-1. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Keller R. K., Touster O. The preparation and chemical composition of the multiple forms of beta-glucuronidase from the female rat preputial gland. J Biol Chem. 1975 Jun 25;250(12):4770–4776. [PubMed] [Google Scholar]
- Vladutiu G. D., Rattazzi M. C. Abnormal lysosomal hydrolases excreted by cultured fibroblasts in I-cell disease (mucolipidosis II). Biochem Biophys Res Commun. 1975 Dec 1;67(3):956–964. doi: 10.1016/0006-291x(75)90768-8. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- Wiesmann U., Vassella F., Herschkowitz N. "I-cell" disease: leakage of lysosomal enzymes into extracellular fluids. N Engl J Med. 1971 Nov 4;285(19):1090–1091. doi: 10.1056/NEJM197111042851922. [DOI] [PubMed] [Google Scholar]