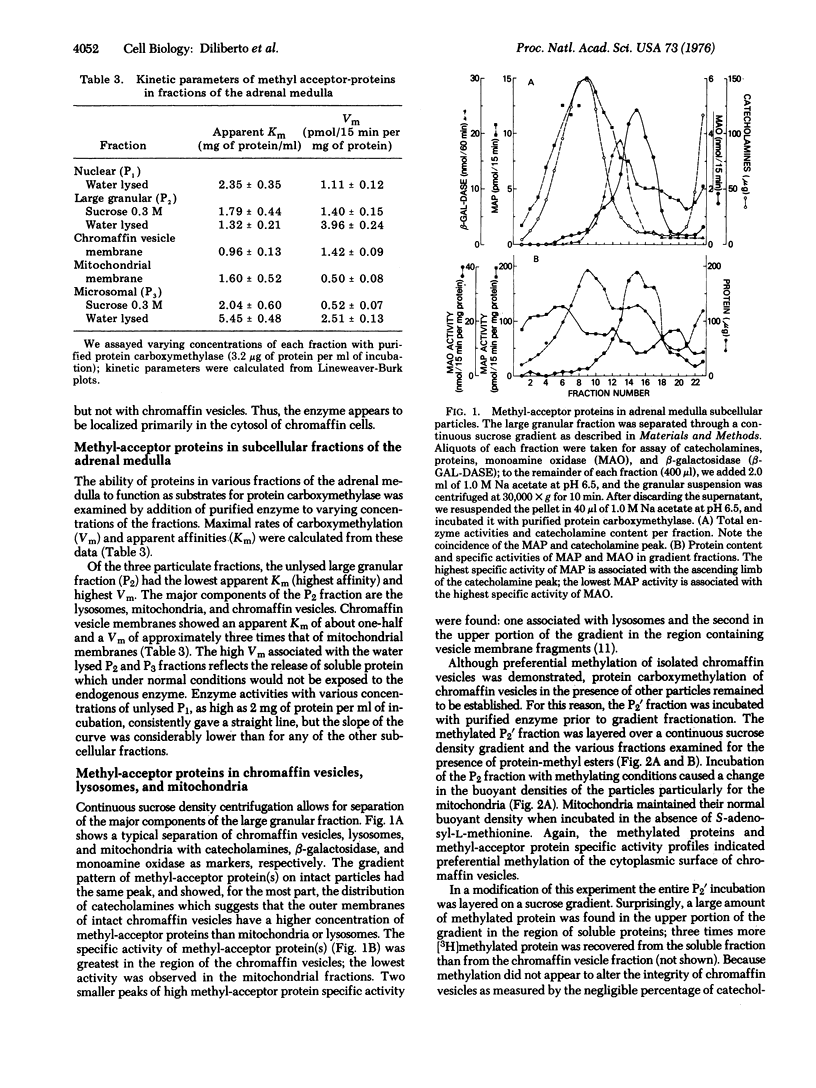
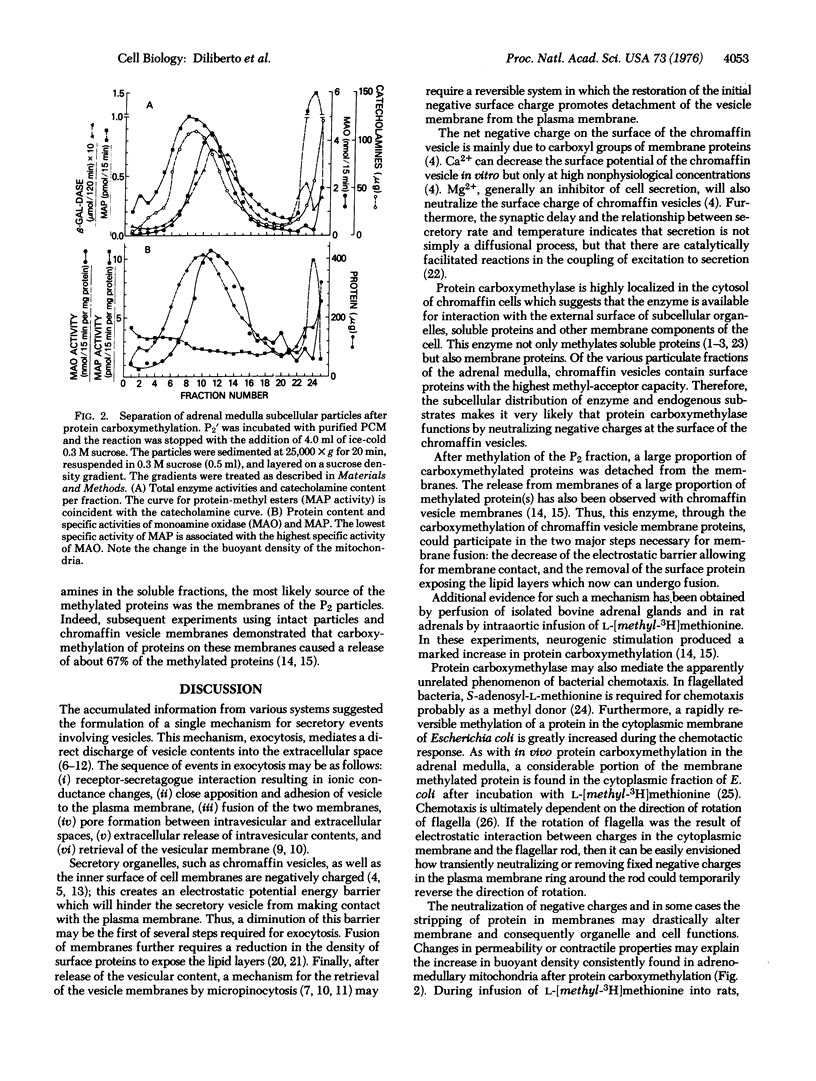
Abstract
Protein carboxymethylase (S-adenosyl-L-methionine:protein O-methyltransferase, EC 2.1.1.24) transfers a methyl group from S-adenoxyl-L-methionine to carboxyl side chains of proteins to form labile protein-methyl esters which, thus, neutralize negative charges. This enzyme was examined for its possible participation in excitation-secretion coupling in the adrenal medulla. Protein carboxymethylase has a specific activity several times higher in the adrenal medulla than in the adrenal cortex; also, the medulla has a higher concentration of methyl-acceptor proteins. In the adrenal medulla, 97% of the enzyme was localized in the cytosol. Of the various subcellular fractions of the medulla, the catecholamine-containing chromaffin vesicles had the highest concentrations of substrat(s) for protein carboxymethylase. Carboxymethylation of proteins in intact chromaffin vesicles results in stripping of methylated protein(s) from the membranes. Thus, protein carboxymethylase appears to be involved in the neutralization of charges on the surface of chromaffin vesicles and in the release of surface proteins; both phenomena are likely to be required for exocytosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. Mechanisms of cell fusion. Nature. 1975 Jan 17;253(5488):194–195. doi: 10.1038/253194a0. [DOI] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Role of methionine in bacterial chemotaxis. J Bacteriol. 1974 May;118(2):640–645. doi: 10.1128/jb.118.2.640-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE ROBERTIS E., VAZ FERREIRA A. Electron microscope study of the excretion of cathecol-containing droplets in the adrenal medulla. Exp Cell Res. 1957 Jun;12(3):568–574. doi: 10.1016/0014-4827(57)90172-6. [DOI] [PubMed] [Google Scholar]
- Dean P. M. Exocytosis modelling: an electrostatic function for calcium in stimulus-secretion coupling. J Theor Biol. 1975 Oct;54(2):289–308. doi: 10.1016/s0022-5193(75)80132-9. [DOI] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Axelrod J. Characterization and substrate specificity of a protein carboxymethylase in the pituitary gland. Proc Natl Acad Sci U S A. 1974 May;71(5):1701–1704. doi: 10.1073/pnas.71.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Axelrod J. Regional and subcellular distribution of protein carboxymethylase in brain and other tissues. J Neurochem. 1976 Jun;26(6):1159–1165. doi: 10.1111/j.1471-4159.1976.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman E., Teichberg S., Abrahams S. J., Citkowitz E., Crain S. M., Kawai N., Peterson E. R. Notes on synaptic vesicles and related structures, endoplasmic reticulum, lysosomes and peroxisomes in nervous tissue and the adrenal medulla. J Histochem Cytochem. 1973 Apr;21(4):349–385. doi: 10.1177/21.4.349. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I. Mechanism of transmitter release. Prog Biophys Mol Biol. 1970;21:33–124. [PubMed] [Google Scholar]
- Kim S., Pail W. K. Studies on the structural requirements of substrate protein for protein methylase II. Biochemistry. 1971 Aug 3;10(16):3141–3145. doi: 10.1021/bi00792a024. [DOI] [PubMed] [Google Scholar]
- Kim S., Wasserman L., Lew B., Paik W. K. Studies on the natural substrate for protein methylase II in mammalian brain and blood. J Neurochem. 1975 Apr;24(4):625–629. [PubMed] [Google Scholar]
- Kirshner N., Viveros O. H. The secretory cycle in the adrenal medulla. Pharmacol Rev. 1972 Jun;24(2):385–398. [PubMed] [Google Scholar]
- Kort E. N., Goy M. F., Larsen S. H., Adler J. Methylation of a membrane protein involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3939–3943. doi: 10.1073/pnas.72.10.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Evans R. J., Dean P. M. The ionogenic nature of the secretory-granule membrane. Electrokinetic properties of isolated chromaffin granules. Biochem J. 1972 Dec;130(3):825–832. doi: 10.1042/bj1300825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Experiments on internally perfused squid giant axons. Ann N Y Acad Sci. 1966 Jul 14;137(2):807–817. doi: 10.1111/j.1749-6632.1966.tb50201.x. [DOI] [PubMed] [Google Scholar]
- Satir B., Schooley C., Satir P. Membrane fusion in a model system. Mucocyst secretion in Tetrahymena. J Cell Biol. 1973 Jan;56(1):153–176. doi: 10.1083/jcb.56.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman J. F., Jr, Brady R. O., Suzuki K. Enzymic activities associated with membranous cytoplasmic bodies and isolated brain lysosomes. J Neurochem. 1971 Sep;18(9):1775–1777. doi: 10.1111/j.1471-4159.1971.tb03754.x. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- Viveros O. H., Arqueros L., Kirshner N. Mechanism of secretion from the adrenal medulla. VII. Effect of insulin administration on the buoyant density, dopamine -hydroxylase, and catecholamine content of adrenal storage vesicles. Mol Pharmacol. 1971 Jul;7(4):444–454. [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]



