Abstract
Increasing entomologic and epidemiologic evidence suggests that spotted fever group rickettsiae (SFGR) other than Rickettsia rickettsii are responsible for spotted fever rickettsioses in the United States. A retrospective seroepidemiologic study was conducted on stored acute- and convalescent-phase sera that had been submitted for Rocky Mountain spotted fever testing to the North Carolina State Laboratory of Public Health. We evaluated the serologic reactivity of the paired sera to R. rickettsii, Rickettsia parkeri, and Rickettsia amblyommii antigens. Of the 106 eligible pairs tested, 21 patients seroconverted to one or more antigens. Cross-reactivity to multiple antigens was observed in 10 patients, and seroconversions to single antigens occurred in 11 patients, including 1 against R. rickettsii, 4 against R. parkeri, and 6 against R. amblyommii. Cross-absorption of cross-reactive sera and/or Western blots identified two presumptive cases of infection with R. parkeri, two presumptive cases of infection with R. rickettsii, and one presumptive case of infection with R. amblyommii. These findings suggest that species of SFGR other than R. rickettsii are associated with illness among North Carolina residents and that serologic testing using R. rickettsii antigen may miss cases of spotted fever rickettsioses caused by other species of SFGR.
INTRODUCTION
Rocky Mountain spotted fever (RMSF), caused by the bacterium Rickettsia rickettsii, is the most commonly reported fatal tick-borne disease in the United States. The incidence of spotted fever rickettsioses in the United States (including RMSF) has been rising rapidly in recent years, from 2.5 cases per million in 2001 to 9.5 cases per million in 2011 (1). Mounting evidence suggests that infections with other species of spotted fever group rickettsiae (SFGR) may be at least partly responsible for the apparent increase in cases, which may reflect an increase in reporting or incidence or both. Seroepidemiologic surveys of adults and children in the United States show that seroprevalence to SFGR is between 6% and 12% (2, 3). Because subclinical infection or mild infections with R. rickettsii are considered to be rare or even nonexistent and there is cross-reactivity among SFGR in serologic tests, exposure to other species of SFGR could account for this relatively high seroprevalence (4). For many years, Rickettsia parkeri was considered to be a nonpathogenic SFGR, until it was isolated from a patient in Virginia in 2002 (5). R. parkeri is now recognized as a human pathogen, causing an illness characterized by formation of an eschar at the site of inoculation and generally milder symptoms than classic RMSF (6, 7). In California, Rickettsia 364D has been implicated in causing an eschar-associated illness decades after it was first identified in Dermacentor occidentalis ticks (8). Recent studies have suggested that Rickettsia amblyommii, which is present in a large percentage of Lone Star ticks (Amblyomma americanum), may cause a mild rickettsiosis in humans (9–15). In this study, we evaluated the reactivity of paired sera from North Carolina patients who had been tested for RMSF to a panel of SFGR, including R. rickettsii, R. amblyommii, and R. parkeri.
MATERIALS AND METHODS
Selection of case patients.
Case patients were identified from a database of patients who were tested for RMSF at the North Carolina State Laboratory of Public Health (NCSLPH) between 2008 and 2010. Samples were submitted from across the entire state, with the majority from patients in the Piedmont region (central North Carolina). Eligibility criteria included having paired sera available (from the acute and convalescent phases) and at least one sample with a titer of ≥1:64 against R. rickettsii in the original test. Sera from patients tested for SFGR that do not demonstrate reactivity are not routinely retained for long-term storage at the NCSLPH and were therefore unavailable for testing. Patients were excluded from the study if there was only a single serum specimen available, if there were not sufficient sera remaining for additional testing, or if the sera could not be located.
Antigens.
Indirect immunofluorescence assay (IFA) testing completed at the North Carolina State Laboratory of Public Health (NCSLPH) utilized rickettsiae grown in chicken egg yolk (R. rickettsii Sheila Smith) or Vero cells (R. parkeri Portsmouth and R. amblyommii Darkwater) provided by William Nicholson, Rickettsial Zoonoses Branch, CDC (16). Antigen preparations of infected Vero cells (R. parkeri and R. amblyommii) or cell-free bacteria in egg yolk suspension (R. rickettsii) were spotted onto the wells of glass templated slides using capillary tubes. Antigen spots were allowed to air dry and then fixed in acetone for 10 to 15 min at room temperature. Slides were then stored in sealed boxes at −70°C until they were ready to be used. IFA testing conducted at the Tennessee Vector-Borne Disease Laboratory (TNVBDL) utilized slides prespotted with rickettsiae grown in Vero cells (R. rickettsii Sheila Smith, R. parkeri Portsmouth, and R. amblyommii WB-82-like North Texas) and corresponding Renografin-purified rickettsial lysates for cross-absorption and Western blotting, prepared by Nicole Mendell, Department of Pathology, University of Texas Medical Branch at Galveston (17). Rickettsiae were cultivated in confluent Vero cells and monitored until the cells were determined to be approximately 70 to 80% infected. The infected monolayer was scraped, harvested, washed with 0.1 M phosphate-buffered saline (PBS) by centrifugation at 500 × g for 5 min, and resuspended in PBS with 1% bovine calf serum (BCS). The cell suspension was pipetted onto poly-l-lysine-coated 12-well Teflon masked IFA slides at 10 μl per well, allowed to dry, and fixed for 10 min with acetone. The rickettsial antigen lysate was generated by scraping heavily infected (80 to 100%) Vero cell monolayers and centrifugation at 17,000 × g for 15 min to pellet the infected Vero cells. The infected Vero cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 2 mM sucrose and lysed by sonication on ice for four 15-s pulses at 60% amplitude to release the rickettsiae. Host cell debris was removed by centrifugation at 1,000 × g for 5 min. The supernatant was passed through a 0.22-μm-pore syringe-driven filter, and cell-free rickettsiae were pelleted 17,000 × g for 15 min and resuspended in PBS with 0.05% sodium azide. Rickettsiae were sonicated on ice for eight 15-s pulses at 60% amplitude to lyse the bacteria.
IFA.
Assays were conducted using previously published methods for rickettsial diagnosis using the IFA (18). Briefly, frozen slides were placed at room temperature and allowed to warm while serial dilutions were prepared. The primary dilution of 1:64 was prepared in 3% egg yolk for the R. rickettsii antigen and 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for R. parkeri and R. amblyommii. The remaining serial 2-fold dilutions were made in PBS with 1% BSA for all antigens. Sera were diluted to a final dilution of 1:2,048, except for positive-control sera, which were diluted to 1:16,384. Positive-control sera from a patient with a known titer against R. rickettsii antigen were included in each run (including dilutions from 1:64 to 1:16,384). Negative controls, including PBS only, PBS with BSA, and a negative-control serum that is nonreactive to R. rickettsii (primary dilution only) were also included in each run. Dilutions were applied to the slides and incubated in a humidity chamber at 37°C for 30 min. Slides were rinsed in PBS, washed in PBS for 10 min, and then washed in distilled water for 10 min and air dried. Fluorescein isothiocyanate (FITC)-labeled goat anti-human IgG conjugate (Scimedx, Danville, NJ) with Eriochrome black counterstain was applied to each well, and the wells were incubated in a humid chamber at 37°C for 30 min. Slides were washed as described above, and once dried, a drop of buffered glycerol was added to each well and overlaid with a coverslip. Slides were stored in the dark until they were read (within 24 h).
Reading of slides and interpretation of results.
Paired sera were always tested and read together by the same technician. Slides were read on a UV epifluorescence microscope, and wells were initially examined at low power (100×) and then high power (400×). After the negative-control wells were read, the positive-control and test samples were read from the highest dilution to the lowest dilution. Staining for negative controls was confirmed by the absence of specific fluorescence. Runs for which the positive control was within one dilution of the known titer were considered acceptable. Fluorescence was scored according to brightness and consistency of staining throughout the well on a scale of 4+, 3+, 2+, 1+, +/−, and −, with 4+ being the most intense fluorescence. Endpoint titers were recorded as the reciprocal of the dilution with 1+ fluorescence, unless the fluorescence at the 1:2,048 dilution was 1+ or greater, in which case the endpoint titer was recorded as ≥1:2,048. If staining intensity did not increase as expected from higher dilutions to lower dilutions or if nonspecific staining patterns were observed, then this was noted, and no endpoint titer could be determined. If a sample had a questionable result or if problems with the staining were noted, the senior technician reevaluated the slide and either made the final call on the endpoint titer or suggested that the test be repeated. If a test needed to be repeated, both samples (acute and convalescent phases) were repeated together. For samples that did not yield a titer against R. rickettsii that was within one dilution of the original titer, the assay was repeated, and the titer that was found in the majority of the assays was accepted as the correct titer.
A seroconversion was defined as a 4-fold or greater change in IgG titer against an antigen between acute- and convalescent-phase samples. For each antigen, the patient was classified as either a seroconversion, stationary titer (lack of 4-fold or greater change in titer), or unknown (if one or more of the samples had nonspecific fluorescence or were unreadable).
Cross-absorption and IFA.
A subset of samples for which patient clinical data and sufficient sera were available were selected for additional analyses at the TNVBDL, in an attempt to identify the presumptive causative agent. Additional testing included Western blots and cross-absorption followed by IFA. To cross-absorb sera, equal volumes of diluted sera (1:32) and antigen lysate (2 mg/ml) were incubated for 20 h at room temperature on a rotator. Sera were then centrifuged at 10,000 × g for 11 min, and the supernatant was collected for use in IFAs (19). IFAs were completed as described above with the following modifications (according to the protocol used at the TNVBDL): serum dilutions were prepared in PBS with 1% BSA, 0.01% sodium azide, and 0.1% Tween 20, the wash buffer was PBS with 0.1% Tween 20, FITC-labeled goat anti-human IgG conjugate was purchased from KPL, and slides were counterstained with 1% Evans blue. A presumptive agent was identified when cross-absorption resulted in the removal (or at least 4-fold decrease) of both homologous and heterologous antibodies, while cross-absorption with the cross-reactive species removed homologous antibodies only.
Western blotting.
Purified antigen (30 μg) was denatured with RunBlue dithiothreitol (DTT) reducer (Expedeon, San, Diego, CA) at 70°C for 10 min and separated by SDS-PAGE with a 4-to-12% gradient gel (Expedeon) using a Dual Cool electrophoresis system (CBS Scientific, San Diego, CA) at 180 V for 1 h. Resolved antigens were transferred to nitrocellulose membranes (Expedeon) using a semidry blotter (Amersham Biosciences) at 22 V for 1 h. Membranes were blocked in Tris-buffered saline (TBS; 10 mM Tris-HCl [pH 7.5], 150 mM NaCl) with 5% nonfat dry milk for 1 h at room temperature. Sera, diluted 1:125 in blocking buffer, were incubated with membranes overnight at room temperature. Membranes were washed once in TBS with 0.05% Tween 20 for 10 min and once in TBS for 10 min, followed by incubation with phosphatase-labeled goat anti-human IgG purified antibody (KPL, Gaithersburg, MD) diluted 1:20,000 in blocking buffer for 1 h at room temperature. Membranes were washed twice in TBS for 10 min, and bands were visualized using the BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium) phosphatase substrate (KPL) and washed in distilled water to stop the reaction. Membranes were examined for the presence of the typical rickettsial lipopolysaccharide (LPS) antigen washboard pattern at 20 to 60 kDa and specific protein antigens (SPAs) in the 110- to 140-kDa region (20, 21).
Surveillance reports.
Surveillance reports from the North Carolina Division of Public Health (NCDPH) were requested for all patients included in this study. Sample numbers from the NCSLPH were cross-referenced with event numbers in the North Carolina Electronic Disease Surveillance System (NCEDSS), and all available reports were provided in paper format (deidentified) by the North Carolina Public Health Data Group. A form was created in Qualtrics, and relevant fields were entered into the form for each patient.
Data analysis.
Standard descriptive statistics were calculated for demographic and clinical variables. Positive percentage of agreement was calculated for each antigen using seroconversion against R. rickettsii as the referent comparison. All analyses were performed using SAS (version 9.2, SAS Institute, Inc., Cary, NC).
Human subjects.
This research was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. The use of the data was approved by the North Carolina Division of Public Health through a Data Use Agreement. Since all data were deidentified, informed consent was not required as the risk to subjects was minimal.
RESULTS
Case patients.
IFA test results from patients in the NCSLPH database with paired sera that had been tested for RMSF between 2008 and 2010 were reviewed (n = 311). Of these patients, 126 (40.5%) had at least one sample with a titer of ≥1:64 against R. rickettsii. Results for testing against Rickettsia typhi were also reviewed for these patients, all of which were negative. Samples from 8 patients were no longer available. Twelve patients were excluded from the analysis due to nonspecific fluorescence or an unreadable result, and assays for 22 subjects were repeated for which endpoint titers were all resolved. Endpoints were not reached for 11 samples, and these titers were recorded as ≥1:2,048. Of the 106 patients included in the study, surveillance reports were available for 53. The majority of these patients were white males, with a median age of 50 (range, 1 to 80) (see Table S1 in the supplemental material). The demographic profile was similar for patients who seroconverted to at least one of the SFGR antigens and patients with stationary titers.
Seroconversions.
Of the 106 eligible pairs tested, 10 patients seroconverted to R. rickettsii antigen in the original testing. In our subsequent testing, only 8 of the 10 seroconverted to R. rickettsii antigen. The two patients that seroconverted to R. rickettsii antigen in the original testing but did not meet the criteria for seroconversion to R. rickettsii in subsequent testing both produced titers that were within the one-dilution allowance for intra-assay variability of the IFA. In our subsequent testing, one of these patients seroconverted to R. amblyommii antigen and the other did not seroconvert to any of the SFGR antigens.
In this study, 21 patients seroconverted to one or more of the SFGR antigens. The frequency of seroconversions to each antigen and cross-reactivity between antigens is depicted in Fig. 1. Of the eight patients that seroconverted to R. rickettsii, seven also seroconverted to both R. parkeri and R. amblyommii. Eleven patients had seroconversions against a single antigen: 1 against R. rickettsii, 4 against R. parkeri, and 6 against R. amblyommii. Three patients had seroconversions against both R. parkeri and R. amblyommii antigens.
FIG 1.
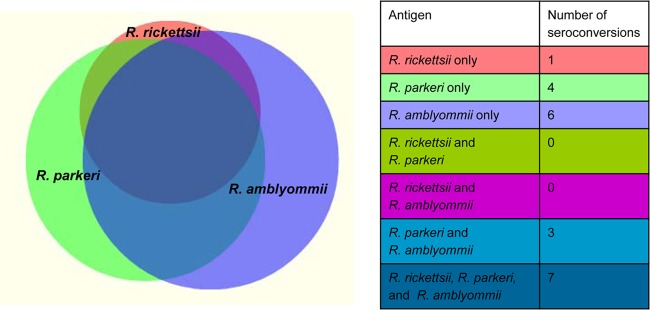
Proportional Venn diagram showing seroconversions to R. rickettsii, R. parkeri, and R. amblyommii antigens and degree of cross-reactivity by indirect immunofluorescent assay. The diagram was generated by a program available at http://www.cmbi.ru.nl/∼timhulse/venn/.
The ability to detect seroconversions to R. parkeri and R. amblyommii using R. rickettsii antigen was poor, as measured by positive percentages of agreement of 50% (95% confidence interval [CI], 24.0 to 76.0%) and 43.8% (95% CI, 20.8 to 69.4%), respectively. The majority of positive agreement between seroconversion classifications was due to broad cross-reactivity to all three antigens.
Cross-absorption and Western blot assays.
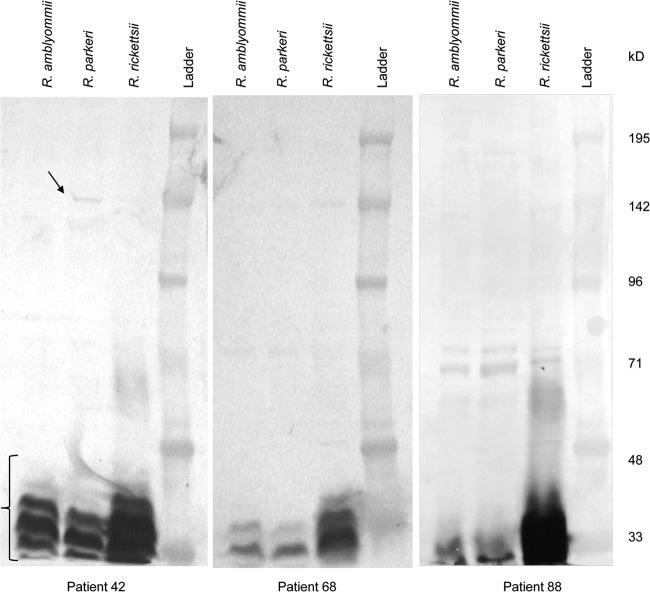
Convalescent-phase sera from seven patients were cross-absorbed against all three antigens followed by IFA. Patterns in IFA titers consistent with R. parkeri infection were identified in patients 3 and 42, R. rickettsii infection in patients 56 and 58, and R. amblyommii infection in patient 88 (Table 1). IFA patterns were indeterminate in cross-absorbed sera from patients 68 and 70. Western blots of all seven patient sera showed reactivity to LPS antigen from all three species, but in many cases, specific protein antigens were not visible or were extremely faint (e.g., patients 68 and 88) (Fig. 2). In contrast, the convalescent-phase serum from patient 42 was clearly reactive with an ∼150-kDa protein of R. parkeri (Fig. 2), supporting the results of the cross-absorption and IFA testing.
TABLE 1.
IFA titers and clinical characteristics for patients who seroconverted to R. rickettsii, R. parkeri, and/or R. amblyommii antigensa
| Patient no. (n = 21) | IFA titer (acute, convalescent phase) |
Presumptive agent by: |
Symptom(s) |
Hospitalized | Discharge diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R. rickettsii | R. parkeri | R. amblyommii | Cross-absorption with IFA | Western blot | Fever | Rash | Headache | Myalgia | Other | |||
| Patients who seroconverted to all 3 antigens | ||||||||||||
| 3 | <1:64, 1:256 | <1:64, 1:1,024 | <1:64, 1:512 | R. parkeri | IND | + | + | − | − | − | − | Presumed RMSF |
| 42 | 1:128, 1:1,024 | 1:512, >1:2,048 | 1:128, >1:2048 | R. parkeri | R. parkeri | + | − | − | + | Leukopenia | − | NA |
| 56 | <1:64, 1:512 | <1:64, ≥1:2,048 | <1:64, ≥1:2,048 | R. rickettsii | IND | + | + | + | + | Lethargy, thrombocytopenia | − | RMSF |
| 58 | <1:64, 1:1,024 | <1:64, 1:512 | 1:64, 1:512 | R. rickettsii | IND | + | − | + | + | Nausea, vomiting | Febrile illness, possible RMSF | |
| 65 | <1:64, 1:512 | <1:64, 1:256 | <1:64, 1:256 | NA | NA | NA | NA | NA | NA | NA | ||
| 68 | <1:64, 1:2,048 | <1:64, ≥1:2,048 | <1:64, 1:2,048 | IND | IND | − | + | − | − | Cough | − | Rash, unspecified |
| 85 | <1:64, 1:2,048 | 1:64, 1:1,024 | 1:64, 1:1,024 | NA | NA | NA | NA | NA | NA | NA | ||
| Patients who seroconverted to 2 antigens | ||||||||||||
| 55 | 1:64, 1:64 | 1:256, 1:1,024 | 1:256, 1:2,048 | NA | NA | NA | NA | NA | NA | NA | ||
| 88 | 1:512, 1:256 | 1:1,024, 1:256 | 1:1,024, 1:256 | R. amblyommii | IND | + | − | + | + | Multipleb | − | NA |
| 109 | 1:256, 1:128 | 1:256, 1:1,024 | 1:256, 1:1,024 | + | − | NA | NA | NA | − | NA | ||
| Patients who seroconverted to 1 antigen | ||||||||||||
| 5 | 1:128, 1:64 | 1:64, 1:64 | 1:64, 1:256 | NA | NA | NA | NA | NA | NA | NA | ||
| 7 | 1:256, 1:256 | 1:256, <1:64 | 1:128, <1:64 | NA | NA | NA | NA | NA | NA | NA | ||
| 19 | 1:512, 1:512 | ≥1:2,048, 1:1,024 | 1:2,048, 1:512 | NA | NA | NA | NA | NA | NA | NA | ||
| 25 | 1:128, 1:1,024 | 1:256, 1:256 | 1:1,024, ≥1:2,048 | NA | NA | NA | NA | NA | NA | NA | ||
| 29 | 1:64, 1:128 | 1:128, 1:512 | 1:128, 1:256 | NA | NA | NA | NA | NA | NA | NA | ||
| 31c | 1:128, 1:256 | 1:256, 1:256 | 1:256, 1:1,024 | + | − | − | − | Multipled | + | Tick-borne illness | ||
| 44 | 1:128, 1:256 | 1:512, 1:1,024 | 1:64, 1:512 | NA | NA | NA | NA | NA | − | NA | ||
| 70 | 1:256, 1:256 | 1:256, 1:1,024 | 1:256, 1:256 | IND | IND | + | − | + | + | Multiplee | + | NA |
| 76 | 1:512, 1:256 | 1:1,024, 1:2,048 | 1:512, 1:2,048 | − | − | − | + | Fatigue | − | NA | ||
| 93 | 1:512, 1:512 | 1:2,048, 1:512 | 1:1,024, 1:1,024 | − | − | − | − | − | − | NA | ||
| 116 | 1:128, 1:128 | 1:128, 1:64 | 1:512, 1:128 | NA | NA | NA | NA | NA | NA | NA | ||
Seroconversions are highlighted in bold. For subjects with sufficient remaining sera, the presumptive agent was determined from the results of cross-absorption of convalescent-phase sera followed by IFA and/or Western blotting. IND, indeterminate; NA, not available; +, positive; −, negative.
Nausea, diarrhea, weakness, cough, and sweats.
In the original testing, this patient seroconverted to E. chaffeensis with acute- and convalescent-phase titers of 1:128 and 1:8,192.
Elevated liver enzymes, acute renal failure, thrombocytopenia, leukopenia, and anemia.
Elevated liver enzymes, thrombocytopenia, leukopenia, and anemia.
FIG 2.
Western blots of rickettsial antigens reacted with convalescent-phase patient sera (1:125 dilution). The bracket in the lower left indicates reactivity to nonspecific LPS antigen, and the arrow indicates reactivity with a specific protein antigen.
Clinical characteristics of seroconverters.
Surveillance reports were available for half of the patients (n = 53). Of these, 12 reports were available from patients who seroconverted to at least one of the SFGR antigens. Fever, thrombocytopenia, leukopenia, elevated liver enzymes, and acute renal failure occurred more frequently among seroconverters, while skin rash was more common among patients who did not seroconvert (see Table S1 in the supplemental material). None of the patients who seroconverted exclusively to R. parkeri and/or R. amblyommii antigens reported a rash, compared to three of five patients with seroconversions to all three antigens. Hospitalization occurred in two seroconverters: in a patient who seroconverted to R. parkeri (patient 70) and in another patient who seroconverted to R. amblyommii (patient 31, who also had a confirmed case of ehrlichiosis). Both Ehrlichia chaffeensis and R. amblyommii share a tick vector (A. americanum), thus this case was a potential coexposure.
DISCUSSION
The purpose of this study was to evaluate reactivity of paired sera from suspected RMSF patients against R. rickettsii, R. parkeri, and R. amblyommii antigens concurrently. These antigens are likely to represent the species of SFGR that occur in tick vectors most frequently in North Carolina, based on current knowledge. Although the seroconversions observed cannot be used to infer etiology, the greater number of unique seroconversions to R. parkeri and R. amblyommii than to R. rickettsii indicates that species of SFGR other than R. rickettsii may be causing infections among North Carolina residents. This theory is supported by identification of R. parkeri as the presumptive agent in two patients and R. amblyommii in one patient using cross-absorption and Western blot assays. The low positive percentage of agreement for R. parkeri and R. amblyommii seroconversion classification also suggests that serologic testing using R. rickettsii antigen may result in missed cases of spotted fever rickettsioses caused by other species of SFGR. Thus, even the current “gold standard” for serologic diagnosis of SFGR has serious limitations.
The large relative frequency of seroconversions to R. amblyommii was not unexpected in the context of previous work by Apperson et al. (12). Active surveillance for tick-borne diseases in a central North Carolina county resulted in the identification of several patients with mild illness in which initial testing failed to confirm RMSF. Upon further testing with both R. rickettsii and R. amblyommii antigens, three patients seroconverted against R. amblyommii in that study. At this time, R. amblyommii has not been recognized as a human pathogen, but the high infection prevalence in A. americanum, the most ubiquitous and aggressive human-biting tick in this region, creates ample opportunity for potential infection of human and animal hosts (10, 13, 15). In Oklahoma, canine infection with R. amblyommii was demonstrated among dogs naturally exposed to ticks (22). In a longitudinal study of outdoor workers in North Carolina, more than 90% of ticks removed from subjects were A. americanum (23), while less than 5% of ticks were D. variabilis and less than 1% of ticks were Amblyomma maculatum (Charles Apperson, unpublished data). The predominant rickettsial species found in ticks removed from subjects in that study was R. amblyommii (51.6%), while R. rickettsii was found in only one tick, an A. americanum nymph (Apperson, unpublished).
We also anticipated that we would detect seroconversions to R. parkeri, a known human pathogen that was found in 29% of gulf coast ticks (Amblyomma maculatum) in a recent entomologic survey in North Carolina (24). A recent study identified R. parkeri in Lone Star ticks from Tennessee and Georgia, suggesting that A. americanum may serve as a vector for R. parkeri (25). Seroconversions to R. parkeri and R. amblyommii among ill patients in this study provide further evidence that infections caused by these species of SFGR are likely to account for some of the increase in spotted fever rickettsioses reported in this region.
It is well known that there is serologic cross-reactivity between species of SFGR, and as a result, serologic tests cannot be used to infer etiology (26). Serologic cross-reactivity between R. rickettsii and R. parkeri antigens has been described previously. Raoult and Paddock retested sera from 15 patients diagnosed with RMSF using class-specific IFA with R. rickettsii and R. parkeri antigens (27). Equal titers were observed for six patients, while 4 patients had higher titers to R. rickettsii and 5 patients had higher titers to R. parkeri. Western blot analysis of 4 patients with higher titers against R. parkeri provided additional evidence of infection with R. parkeri. Paddock et al. conducted comparative class-specific IFA with samples from 6 confirmed and 6 probable cases of R. parkeri rickettsiosis using R. parkeri, R. rickettsii, R. amblyommii, and Rickettsia akari antigens (28). IgG geometric mean titers were higher against R. rickettsii than for any other antigen. These studies illustrate that comparison of single titers is unreliable for distinguishing cases of RMSF and R. parkeri rickettsiosis. By evaluating reactivity of acute- and convalescent-phase paired sera, we found that some patients showed rising or falling titers to multiple rickettsiae, which is consistent with the cross-reactivity observed in previous studies. It is also possible that patients could have been coinfected with multiple species of rickettsiae or with species not included in the testing for this study. Notably, we found that some patients seroconverted to only a single antigen, indicating that extensive cross-reactivity between SFGR antigens may not be present in all cases.
Limitations of this study include the lack of clinical data for all patients and the variability in the level of completeness of those for which data are available. The small number of patients with surveillance reports available prevents us from making conclusions on the association between seroconversion to specific antigens and clinical signs and symptoms. Due to the eligibility requirement of having paired sera, which represents a small minority of patients, the patients in this study are likely to have suffered from more severe illnesses, which led the medical provider to order repeated testing for tick-borne pathogens. By limiting our study to these patients, it is possible that we excluded many cases of mild disease caused by SFGR. Curiously, there were some patients with paired sera who had no reported signs or symptoms. In several surveillance reports, it was noted that testing was done as a precaution after multiple tick bites, although none of those patients seroconverted in this study. The scarcity of appropriately timed paired samples with accompanying clinical information further compounds the diagnostic confusion for spotted fever rickettsioses, indicating a need for acceptability criteria for serologic testing of patient samples (29).
It is clear that while R. rickettsii is still circulating and causing disease among people living in the southern and southeastern United States, residents are also being infected with other SFGR carried by ticks in these regions. Some of these SFGR, such as R. parkeri, have been shown to cause human disease, while the pathogenic potential of others, such as R. amblyommii, R. montanensis, and R. rhipicephali, has yet to be determined. Until more specific serologic diagnostic methods are developed that can distinguish between species of SFGR or the use of molecular detection techniques becomes routine, the relative contributions of different species of SFGR to human morbidity will remain unclear. Active surveillance for cases of suspected tick-borne illness, which include paired serology and molecular detection, are needed to determine the etiologies of SFGR infections in this region. If current serological methods continue to be the standard for diagnosis and surveillance of spotted fever group rickettsioses, inclusion of antigens for all species known to cause human disease in the relevant geographic region should be considered to prevent missed cases.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kalpana Patel, Erin Umberger, and Brenda Cummins (North Carolina State Laboratory of Public Health) for assistance with the serological testing.
This work was supported by a grant from the Centers for Disease Control and Prevention and the National Institute for Occupational Safety and Health (grant no. 5R01-OH009874).
The North Carolina Public Health Data Group do not take responsibility for the scientific validity or accuracy of methodology, results, statistical analyses, or conclusions presented. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 3 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01733-14.
REFERENCES
- 1.Adams DA, Gallagher KM, Jajosky RA, Kriseman J, Sharp P, Anderson WJ, Aranas AE, Mayes M, Wodajo MS, Onweh DH, Abellera JP, Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC 2013. Summary of Notifiable Diseases—United States, 2011. MMWR Morb. Mortal. Wkly. Rep. 60:1–117. [PubMed] [Google Scholar]
- 2.Marshall GS, Stout GG, Jacobs RF, Schutze GE, Paxton H, Buckingham SC, DeVincenzo JP, Jackson MA, San Joaquin VH, Standaert SM, Woods CR. 2003. Antibodies reactive to Rickettsia rickettsii among children living in the southeast and south central regions of the United States. Arch. Pediatr. Adolesc Med. 157:443–448. 10.1001/archpedi.157.5.443. [DOI] [PubMed] [Google Scholar]
- 3.Graf PCF, Chretien JP, Ung L, Gaydos JC, Richards AL. 2008. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse US sample. Clin. Infect. Dis. 46:70–77. 10.1086/524018. [DOI] [PubMed] [Google Scholar]
- 4.Paddock CD. 2005. Rickettsia parkeri as a paradigm for multiple causes of tick-borne spotted fever in the Western Hemisphere. Ann. N. Y. Acad. Sci. 1063:315–326. 10.1196/annals.1355.051. [DOI] [PubMed] [Google Scholar]
- 5.Whitman TJ, Richards AL, Paddock CD, Tamminga CL, Sniezek PJ, Jiang J, Byers DK, Sanders JW. 2007. Rickettsia parkeri infection after tick bite, Virginia. Emerg. Infect. Dis. 13:334–336. 10.3201/eid1302.061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker RR, Kohls GM, Cox GW, Davis GE. 1939. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 54:1482–1484. 10.2307/4582985. [DOI] [Google Scholar]
- 7.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA. 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 38:805–811. 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro MR, Fritz CL, Tait K, Paddock CD, Nicholson WL, Abramowicz KF, Karpathy SE, Dasch GA, Sumner JW, Adem PV, Scott JJ, Padgett KA, Zaki SR, Eremeeva ME. 2010. Rickettsia 364D: a newly recognized cause of eschar-associated illness in California. Clin. Infect. Dis. 50:541–548. 10.1086/649926. [DOI] [PubMed] [Google Scholar]
- 9.Dasch GA, Kelly DJ, Richards AL, Sanchez JL, Rives CC. 1993. Western blotting analysis of sera from military personnel exhibiting serological reactivity to spotted fever group rickettsiae, abstr 242. Program Abstr. Joint Annu. Meet. Am. Soc. Trop. Med. Hyg.-Am. Soc. Parasitologists, Atlanta, GA, 31 October to 4 November 1993. [Google Scholar]
- 10.Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA. 2006. Prevalence of Ehrlichia, Borrelia, and rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J. Med. Entomol. 43:1261–1268. 10.1603/0022-2585(2006)43[1261:POEBAR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Billeter SA, Blanton HL, Little SE, Levy MG, Breitschwerdt EB. 2007. Detection of Rickettsia amblyommii in association with a tick bite rash. Vector Borne Zoonotic Dis. 7:607–610. 10.1089/vbz.2007.0121. [DOI] [PubMed] [Google Scholar]
- 12.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. 2008. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 8:597–606. 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 13.Moncayo AC, Cohen SB, Fritzen CM, Huang E, Yabsley MJ, Freye JD, Dunlap BG, Huang J, Mead DG, Jones TF, Dunn JR. 2010. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am. J. Trop. Med. Hygiene 83:653–657. 10.4269/ajtmh.2010.09-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. 2010. Bacterial pathogens in Ixodid ticks from a Piedmont county in North Carolina: prevalence of rickettsial organisms. Vector Borne Zoonotic Dis. 10:939–952. 10.1089/vbz.2009.0178. [DOI] [PubMed] [Google Scholar]
- 15.Berrada ZL, Goethert HK, Cunningham J, Telford SR., III 2011. Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from Kansas. J. Med. Entomol 48:461–467. 10.1603/ME10130. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson WL, Masters E, Wormser GP. 2009. Preliminary serologic investigation of ‘Rickettsia amblyommii' in the aetiology of Southern tick associated rash illness (STARI). Clin. Microbiol. Infect. 15(Suppl 2):S235–S236. 10.1111/j.1469-0691.2008.02155.x. [DOI] [PubMed] [Google Scholar]
- 17.Ammerman NC, Beier-Sexton M, Azad AF. 2008. Laboratory maintenance of Rickettsia rickettsii. Curr. Protoc. Microbiol. Chapter 3:Unit 3A.5. 10.1002/9780471729259.mc03a05s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumler JS. 2004. Serodiagnosis of rickettsial infections: indirect immunofluorescent-antibody test, p 11.7.2.1–11.7.2.7 In Isenberg HD. (ed), Clinical microbiology procedures handbook, 2nd ed, 3 ASM Press, Washington, DC. [Google Scholar]
- 19.Hechemy KE, Raoult D, Fox J, Han Y, Elliott LB, Rawlings J. 1989. Cross-reaction of immune sera from patients with rickettsial diseases. J. Med. Microbiol. 29:199–202. 10.1099/00222615-29-3-199. [DOI] [PubMed] [Google Scholar]
- 20.Jensenius M, Fournier PE, Vene S, Ringertz SH, Myrvang B, Raoult D. 2004. Comparison of immunofluorescence, Western blotting, and cross-adsorption assays for diagnosis of African tick bite fever. Clin. Diagn. Lab. Immunol. 11:786–788. 10.1128/CDLI.11.4.786-788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anacker RL, Mann RE, Gonzales C. 1987. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J. Clin. Microbiol. 25:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrett A, Little SE, Shaw E. 2014. “Rickettsia amblyommii” and R. montanensis infection in dogs following natural exposure to ticks. Vector Borne Zoonotic Dis. 14:20–25. 10.1089/vbz.2013.1325. [DOI] [PubMed] [Google Scholar]
- 23.Vaughn MF, Funkhouser SW, Lin FC, Fine J, Juliano JJ, Apperson CS, Meshnick SR. 2014. Long-lasting permethrin impregnated uniforms: a randomized-controlled trial for tick bite prevention. Am. J. Prev. Med. 46:473–480. 10.1016/j.amepre.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Varela-Stokes AS, Paddock CD, Engber B, Toliver M. 2011. Rickettsia parkeri in Amblyomma maculatum ticks, North Carolina, USA, 2009–2010. Emerg. Infect. Dis. 17:2350–2353. 10.3201/eid1712.110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen SB, Yabsley MJ, Garrison LE, Freye JD, Dunlap BG, Dunn JR, Mead DG, Jones TF, Moncayo AC. 2009. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerg. Infect. Dis. 15:1471–1473. 10.3201/eid1509.090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Scola B, Raoult D. 1997. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J. Clin. Microbiol. 35:2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raoult D, Paddock CD. 2005. Rickettsia parkeri infection and other spotted fevers in the United States. N. Engl. J. Med. 353:626–627. 10.1056/NEJM200508113530617. [DOI] [PubMed] [Google Scholar]
- 28.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. 2008. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin. Infect. Dis. 47:1188–1196. 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 29.Crump JA, Corder JR, Henshaw NG, Reller LB. 2004. Development, implementation, and impact of acceptability criteria for serologic tests for infectious diseases. J. Clin. Microbiol. 42:881–883. 10.1128/JCM.42.2.881-883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.