Abstract
PCR detection of Toxoplasma gondii in blood has been suggested as a possibly efficient method for the diagnosis of ocular toxoplasmosis (OT) and furthermore for genotyping the strain involved in the disease. To assess this hypothesis, we performed PCR with 121 peripheral blood samples from 104 patients showing clinical and/or biological evidence of ocular toxoplasmosis and from 284 (258 patients) controls. We tested 2 different extraction protocols, using either 200 μl (small volume) or 2 ml (large volume) of whole blood. Sensitivity was poor, i.e., 4.1% and 25% for the small- and large-volume extractions, respectively. In comparison, PCR with ocular samples yielded 35.9% sensitivity, while immunoblotting and calculation of the Goldmann-Witmer coefficient yielded 47.6% and 72.3% sensitivities, respectively. Performing these three methods together provided 89.4% sensitivity. Whatever the origin of the sample (ocular or blood), PCR provided higher sensitivity for immunocompromised patients than for their immunocompetent counterparts. Consequently, PCR detection of Toxoplasma gondii in blood samples cannot currently be considered a sufficient tool for the diagnosis of OT, and ocular sampling remains necessary for the biological diagnosis of OT.
INTRODUCTION
Ocular toxoplasmosis (OT) is the leading cause of posterior uveitis worldwide (1–3). It is the consequence of a congenital or postnatal infection by the protozoan Toxoplasma gondii (4). Classically, the diagnosis is based on ophthalmologic examination and confirmed with a favorable outcome following appropriate therapy. In cases where the fundus is hidden by vitreous inflammation or clinical findings are atypical, physicians are obliged to draw ocular fluid samples to confirm the toxoplasmic origin of the lesions and discard other causes. In these cases, 3 biological methods can contribute to establishing a correct diagnosis: PCR, the calculation of the Goldmann-Witmer coefficient (GWC), and Western blot analysis (WB) (5). The last two methods are used to compare the specific antibody profiles in the ocular compartment and in the serum. Combining the 3 methods usually provides high sensitivity and specificity (6, 7). Ocular fluid extraction is an invasive procedure, however, and thus not without risk for the patient. Therefore, any method that may contribute to the establishment of a reliable diagnosis using simpler means would be a welcome addition. For example, detecting toxoplasmic DNA in the blood would be of great interest in clinical practice.
OT is generally the consequence of cyst reactivation in a setting of chronic infection. It has long been considered a local event, but publications have reported the detection of parasite DNA in blood in the course of OT, suggesting that this impression may be erroneous (8, 9). PCR using blood samples may therefore be useful for the diagnosis of OT. In order to assess this hypothesis, we performed real-time PCR to detect toxoplasmic DNA in blood samples of patients with OT versus a control group. We studied two protocols of DNA extraction, with either a small volume (200 μl) or a large volume (2 ml) of blood.
MATERIALS AND METHODS
Patients and methods.
The study was conducted at La Pitié-Salpêtrière Hospital, an 1,800-bed tertiary care medical center in Paris, France, over a 50-month period between January 2008 and February 2012. The study was approved by the local ethics committee (CPP Ile-de France IV). The study group comprised 405 patients (184 females and 221 males) for a total of 450 samples. Mean age was 43.4 years (range, 14 to 87). To establish an accurate diagnosis, aqueous humor (n = 368) or vitreous humor (n = 82 cases) was sampled. Blood was sampled concomitantly. The OT group consisted of patients with clinical findings suggestive of Toxoplasma gondii retinochoroiditis (i.e., focal retinal necrosis and choroidal edema with possible adjacent old pigmented scars) associated with successful outcomes of specific treatment and for whom other etiologies were discarded. Others patients were assigned to the control group.
Laboratory tests.
Aqueous humor samples were centrifuged at 1,500 × g for 10 min. Supernatants were used for antibody analysis (i.e., calculation of the GWC and immunoblotting) and pellets for real-time PCR detection of toxoplasmic DNA. When possible (according to the quantity of the ocular sample), biological diagnosis included PCR in the ocular sample, calculation of the GWC, and immunoblotting, as previously described (6). GWC was considered positive when greater than 2. Due to the particularities of sampling, vitreous humors are often diluted; therefore, only PCR and immunoblotting were performed with these types of samples.
DNA extraction.
For ocular samples, we extracted toxoplasmic DNA from up to 10 μl of the ocular sample using the QIAmp DNA Blood Minikit (Qiagen). For blood, we used two different extraction protocols. The initial protocol was performed using 200 μl of whole blood and the QIAmp DNA Blood Minikit. We followed this up with a second protocol using 2 ml of whole blood and the QIAmp DNA Blood Midi kit (Qiagen). PCR performed with extract retrieved from 200 μl of whole blood was called SV-PCR (small volume), and that performed with extract retrieved from 2 ml of blood was named LV-PCR (large volume). DNA was eluted with 100 μl of elution solution for SV-PCR and 300 μl for LV-PCR. The albumin gene was amplified as a control for extraction. After DNA extraction, samples were kept at +4°C for up to 48 h or frozen at −35°C for use within the week.
DNA amplification.
For both blood and ocular samples, we used TaqMan technology and the 7500 fast real-time PCR system (Applied Biosystems). Cycling conditions were as follows: activation at 95°C for 30 s, 50 cycles of denaturation at 95°C for 3 s, and annealing/extension at 60°C for 30 s. The targets were the B1 gene (GenBank accession number AF179871) and the 529-bp repeat element sequence (RE) (GenBank accession number AF146527) (10). The primers were 5′-GAAAGCCATGAGGCACTCCA-3′ (sense) and 5′-TTCACCCGGACCGTTTAGC-3′ (antisense) for the B1 gene, 5′-AGAGACACCGGAATGCGATCT-3′ (sense) and 5′-TTCGTCCAAGCCTCCGACT-3′ (antisense) for the RE sequence, and 5′-TGA AAC ATA CGT TCC CAA AGA GTT T-3′ (sense) and 5′-CTC TCC TTC TCA GAA AGT GTG CAT AT-3′ (antisense) for the albumin gene. The probes were 5′(6FAM) CGGGCGAGTAGCACCTGAGGAGATACA (TAMRA)-3′ for the B1 gene, 5′(6FAM) TCGTGGTGATGGCGGAGAGAATTGA (TAMRA)-3′ for the RE sequence, and 5′(6FAM) TGC TGA AAC ATT CAC CTT CCA TGC AGA(TAMRA)-3′ for the albumin gene. Each target was amplified in duplicate (two wells). For both SV-PCR and LV-PCR, 5 μl of DNA extract was used. Final concentrations were 0.5 μM for primers and 0.2 μM for probes. Absence of inhibitors was checked for each well by using an internal positive control (TaqMan exogenous positive control; Applied Biosystems).
Statistical analysis.
Results were compared using the chi-square test.
RESULTS
A flow chart describing number of patients and samples enrolled in the study (control and OT groups) is presented in Fig. 1. The OT group included 114 patients (132 samples). In this group, PCR was performed with 121 blood samples from 104 patients. All but two of the OT group members had serological evidence of chronic toxoplasmic infection; the first of the two exceptions had evidence of recent infection, and the second had a serological profile compatible with toxoplasmic reactivation. The control group consisted of 291 patients, 39 of whom were immunocompromised, i.e., 19.9% of the 196 control patients for whom these data were available. Sixteen of these immunocompromised patients were infected with HIV, while 23 had other immunosuppressive factors. The control group comprised cases of nontoxoplasmic ocular infection (e.g., herpes simplex virus, varicella-zoster virus [VZV], and Toxocara) and noninfectious ocular disorders. In the control group, 69.1% of the patients had serologic evidence of chronic toxoplasmosis. In the OT group, among the 84 patients for whom immune status was available, 18 (21.4%) had immunosuppressive factors. Among them, 15 were infected with HIV and 3 had other causes of immunosuppression. No significant relation was found between the immune status and occurrence of OT (P = 0.78). However, there was statistically more HIV infection in the OT group than in the control group (P = 0.018), and HIV infection was a more common immunosuppressive factor in the OT group than in the control group (P < 0.005).
FIG 1.
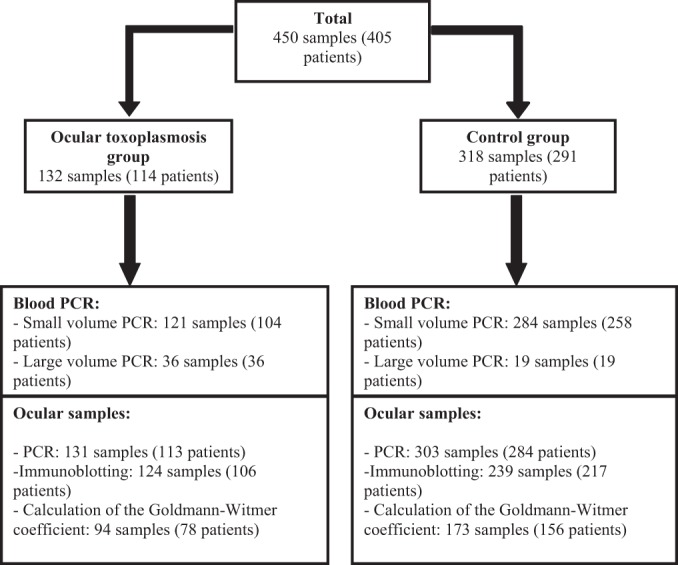
Flow chart describing the number of patients (patients with ocular toxoplasmosis and control patients) and samples enrolled in the study.
SV-PCR.
SV-PCR was performed for 362 patients (405 samples). As previously reported (10), we considered that a single positive amplification in a duplicate assay was sufficient for positive diagnosis. Results are presented in Table 1. Characteristics of the patients (both OT and control groups) who were positive for DNA amplification (both SV-PCR and LV-PCR) are summarized in Table 2. In the OT group, parasitic DNA was detected using SV-PCR in samples from only 5 patients, 4 of whom were immunocompromised. Of note, for these 4 patients, ocular samples had a positive PCR result. A false-positive result occurred for an immunocompetent patient for whom the final diagnosis was retinal syphilis (OT was excluded). This patient had evidence of chronic toxoplasmic infection but no sign of disseminated or localized toxoplasmosis at the time of sampling and during a 6-month follow-up period. The use of SV-PCR on peripheral blood as a diagnostic tool for OT yielded extremely poor sensitivity, i.e., 4.1%. However, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 99.6%, 83.3%, and 70.9%, respectively. SV-PCR was more often positive for immunocompromised patients (16.7%; 4/24) than for immunocompetent patients (1.4%; 1/70) (P < 0.005).
TABLE 1.
Performance of PCR detection of Toxoplasma gondii in blood and ocular samples, calculation of Goldmann-Witmer coefficient, and immunoblotting for diagnosis of ocular toxoplasmosisa
| Sample | Method (nb) | Immune statusc | Group | No. of samples with result |
% sensitivity | % specificity | Positive predictive value (%) | Negative predictive value (%) | P valued | |
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||||
| Blood samples | SV-PCR (405) | All patients | OT | 5 | 116 | 4.1 | 99.6 | 83.3 | 70.9 | |
| Control | 1 | 283 | ||||||||
| Immunocompetent | OT | 1 | 69 | 1.4 | 99.4 | 50 | 70.5 | |||
| Control | 1 | 165 | ||||||||
| Immunocompromised | OT | 4 | 20 | 16.7 | 100 | 100 | 67.7 | |||
| Control | 0 | 42 | <0.005 | |||||||
| LV-PCR (55) | All patients | OT | 9 | 27 | 25 | 89.5 | Not applicable | Not applicable | ||
| Control | 2 | 17 | ||||||||
| Immunocompetent | OT | 4 | 21 | 16 | 100 | Not applicable | Not applicable | |||
| Control | 0 | 12 | ||||||||
| Immunocompromised | OT | 5 | 6 | 45.4 | 71.4 | Not applicable | Not applicable | |||
| Control | 2 | 5 | 0.06 | |||||||
| Ocular samples | PCR (434) | All patients | OT | 47 | 84 | 35.9 | 100 | 100 | 78.3 | |
| Control | 0 | 303 | ||||||||
| Immunocompetent | OT | 18 | 56 | 24.3 | 100 | 100 | 74.3 | |||
| Control | 0 | 162 | ||||||||
| Immunocompromised | OT | 16 | 10 | 61.5 | 100 | 100 | 81.5 | |||
| Control | 0 | 44 | <0.001 | |||||||
| Immunoblotting (363) | All patients | OT | 59 | 65 | 47.6 | 100 | 100 | 78.6 | ||
| Control | 0 | 239 | ||||||||
| Immunocompetent | OT | 33 | 39 | 45.8 | 100 | 100 | 76.9 | |||
| Control | 0 | 130 | ||||||||
| Immunocompromised | OT | 9 | 12 | 42.9 | 100 | 100 | 74.5 | |||
| Control | 0 | 35 | 0.88 | |||||||
| GWC (267) | All patients | OT | 68 | 26 | 72.3 | 98.6 | 98.6 | 73.5 | ||
| Control | 1 | 172 | ||||||||
| Immunocompetent | OT | 35 | 14 | 71.4 | 99 | 97.2 | 87.6 | |||
| Control | 1 | 99 | ||||||||
| Immunocompromised | OT | 12 | 5 | 70.6 | 100 | 100 | 82.8 | |||
| Control | 0 | 24 | 0.88 | |||||||
SV-PCR, small-volume PCR; LV-PCR, large-volume PCR; OT, ocular toxoplasmosis; GWC, calculation of the Goldmann-Witmer coefficient.
n, no. of samples.
Patients for whom immune status was available were categorized as “immunocompetent” or “immunocompromised.” Global analysis independent of the immune status is indicated on the “All patients” line.
As calculated by the chi-square test between immunocompetent and immunocompromised groups.
TABLE 2.
Characteristics of patients with positive blood PCR resulta
| Patient no. | Sex | Age (yrs) | Group | Immune status/medical history | Onset of symptoms (days) | Result |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SV-PCR |
LV-PCR |
PCR, ocular sample |
GWC | WB | |||||||||
| RE | B1 | RE | B1 | RE | B1 | ||||||||
| 1 | F | 70 | OT | Horton's disease (corticoid + immunosuppressive regimen) | 30 | + | − | + | − | + | + | ND | − |
| 2 | M | 45 | OT | HIV infected | 3 | − | − | − | + | + | + | 1 | − |
| 3 | F | 66 | OT | CREST syndrome | 90 | − | − | − | + | + | + | 1.1 | − |
| 4 | M | 35 | CTRL | HIV infected | 10 | − | − | + | − | − | − | 0.8 | − |
| 5 | M | 48 | CTRL | HIV infected (300 CD4+/mm3) | NA | − | − | + | − | − | − | NA | − |
| 6 | M | 48 | CTRL | Immunocompetent, retinal syphilis | 180 | + | − | ND | ND | − | − | NA | − |
| 7 | F | 56 | OT | HIV infected (21 CD4+/mm3) | 15 | + | − | − | − | + | − | 0.5 | − |
| 8 | F | 22 | OT | Immunocompetent | 14 | − | − | + | − | − | − | 333.4 | + |
| 9 | F | 51 | OT | HIV infected | 14 | − | − | + | + | + | − | 0.4 | − |
| 10 | F | 52 | OT | HIV infected (110 CD4+/mm3) | 10 | + | − | ND | ND | + | + | ||
| 11 | F | 76 | OT | Lupus (corticoid and immunosuppressive regimen) | 15 | + | − | + | − | + | + | 5.7 | − |
| 12 | M | 28 | OT | Immunocompetent | NA | − | − | − | + | − | − | 5.5 | + |
| 13 | F | 22 | OT | Immunocompetent | 7 | − | − | − | + | − | − | 1.2 | − |
| 14 | M | 61 | OT | Immunocompetent | 90 | − | − | − | + | + | + | 4.1 | + |
| 15 | M | 35 | OT | Immunocompetent | 6 | + | + | ND | ND | − | − | ||
F, female; M, male; OT, ocular toxoplasmosis; CTRL, control; SV-PCR, small-volume PCR; LV-PCR, large-volume PCR; +, positive test; −, negative test; GWC, Goldmann-Witmer coefficient; WB, Western blot; ND, not done; NA, not available.
LV PCR.
Following up on the SV-PCR results discussed above, we decided to performed DNA extraction from a larger volume (2 ml), hoping to improve the performance of the technique. LV-PCR was performed for 36 blood samples of the OT group and for 19 blood samples from the control group (6 immunocompetent patients with no serological evidence of toxoplasmic infection, 6 immunocompetent patients with serological evidence of chronic toxoplasmic infection, and 7 immunocompromised patients with serological evidence of chronic toxoplasmic infection). Results are summarized in Table 1. In the OT group, 9 patients, of whom 5 were immunocompromised, had positive results. Of note, 6 of the 9 had positive PCR results for the ocular sample; for the 3 others, the OT diagnosis was assessed 2 times by GWC and/or immunoblotting. In one case, the LV-PCR was the only positive biological test. In the control group, we found 2 immunocompromised patients with positive results for whom ocular or localized or disseminated toxoplasmosis was excluded at the time of sampling and during a 6-month follow-up period. Of note, they showed serological evidence of chronic toxoplasmic infection. LV-PCR yielded 25% sensitivity and 89.5% specificity. Since LV-PCR was not performed prospectively, PPV and NPV were not calculated. LV-PCR appeared to show better sensitivity for immunocompromised patients than for immunocompetent patients, but the difference was not statistically significant (P = 0.06).
PCR with ocular samples.
PCR was carried out with 434 ocular samples. In the OT group, PCR was positive for 47 samples and negative for 84 samples (Table 1). In the control group, PCR results were all negative. Therefore, for PCR resultsfor ocular samples, sensitivity was 35.9%, specificity was 100%, PPV was 100%, and NPV was 78.3%. When focusing on patients for whom immune status was available, PCR was more often positive for immunocompromised patients (61.5%; 16/26) than for immunocompetent patients (24.3%; 18/74). This statistical difference was highly significant (P < 0.001).
Immunoblotting and calculation of Goldmann-Witmer coefficient.
Immunoblotting showed 47.6% sensitivity and 100% specificity, while the calculation of the GWC showed 72.3% sensitivity and 98.6% specificity. Contrary to the case with PCR methods, immunoblotting and GWC performed similarly for immunocompromised and immunocompetent patients. Performing the three methods that involved ocular samples (i.e., PCR, immunoblotting, and calculation of the GWC) together provided 89.4% sensitivity and 98.9% specificity.
DISCUSSION
Historically, the diagnostic gold standard for OT has been based on clinical examination (fundus) and the absence of evidence for other etiologies. The contribution of laboratory methods to improving diagnosis is now recognized. However, these methods require an invasive procedure to sample ocular fluid (aqueous or vitreous humor). PCR in ocular fluid provides intermediate sensitivity, ranging from 36% to 55% (6, 7, 11, 12).
PCR in peripheral blood is currently used with immunocompromised patients for the diagnosis of cerebral (especially in HIV-positive patients) or disseminated toxoplasmosis. It yields good sensitivity (between 89.3% and 95.5% in a study by Mesquita et al. [13]), although this is dependent on the marker that is used. The volume of the sample used for DNA extraction is thought to be of great importance also (see below). PCR performed in amniotic fluid for the diagnosis of congenital toxoplasmosis also provides good sensitivity (14), benefiting of course from sample extraction where the infection is localized.
In our study, SV-PCR in the OT group gave five positive results. Four patients were immunocompromised and also had positive PCR results for the ocular sample. As for LV-PCR, among the 9 patients in the OT group who had positive results, PCR in the ocular sample was also positive for 6. Among these 6 patients, 5 were immunocompromised and 1 was immunocompetent. The 3 other patients, with positive LV-PCR and negative ocular PCR, were immunocompetent. A high cycle threshold (CT) was observed for the majority of the blood samples positive by PCR, but we feel that this had no impact on the relevancy of the specificity of the signal, since no positive results were seen for patients without evidence of chronic/acute toxoplasmic infection. Whatever the volume used, the sensitivity was very low, as also reported by other authors (15). This may be explained in part by our use of whole blood in the study instead of peripheral blood mononuclear cells. These latter might be more relevant but would necessitate an additional step before processing the diagnosis. The potential interest of this should be tested in another study. Furthermore, the maximum volume used for extraction in our study was 2 ml; Contini et al. used larger volumes, up to 10 ml, to retrieve the peripheral blood mononuclear cells, with a subsequent sensitivity of up to 100%, although their study included only five patients, all immunocompetent with congenital toxoplasmosis (9). Indeed, in Toxoplasma encephalitis, it has been hypothesized that the volume of blood used for DNA extraction may lead to major differences in the sensitivity of PCR (16–18).
Bou et al. suggested 2 hypotheses for positive blood PCR in the setting of OT: (i) ocular lesions may be the source of parasitemia, or (ii) OT may be associated with reactivation of cysts in others tissues (8). Their results suggested that OT should not be considered a local event.
In our study, we found 3 false-positive reactions (1 SV-PCR and 2 LV-PCR) for the patients from the control group who had negative PCR results in the ocular samples. These patients had evidence of chronic toxoplasmic infection (presence of antitoxoplasmic IgG without specific IgM) but had biological tests that argued against OT (i.e., negative immunoblot and GWC of <2) and no evidence for other localizations of toxoplasmosis. Moreover, the patients did not develop T. gondii acute or reactivated disease over a 6-month follow-up period, supporting the fact that the positive PCR result represented merely transient DNAemia detection. The LV-PCR false-positive results occurred in samples from immunocompromised patients. Martino et al. also described positive PCR results for immunocompromised individuals who did not develop toxoplasmic disease, i.e., with no evidence of organ involvement (19). In agreement with Martino et al., our results suggest that some cyst reactivation may occur and lead to DNA detection in the blood, especially for immunocompromised patients, but that this reactivation may occur silently, with no clinical impact. They may also suggest that even in cases of OT, a reactivation of cysts from other tissues may occur independently of the ocular disease. More recently, Silveira et al. also reported that parasitemia was present in their patients with acute or chronic toxoplasmosis regardless of the presence of ocular lesions (20).
Parenthetically, in our study, LV-PCR was not more often positive in the samples of the OT group (n = 36) than in those of the control group (n = 13 if patients with no serological evidence of recent/chronic toxoplasmosis are excluded) (P = 0.745). We do recognize, however, that the number of patients here is relatively small, and in contrast, positive SV-PCR results were significantly more frequent in the OT group (5/121 samples) than in the control group (1/284) (P < 0.005). Thus, the fact that the reactivation of cysts in the eye may or may not be an event distinct from the reactivation of cysts in other tissues is still a moot point. Performing PCR with ocular samples furnishes results different from those with PCR performed with whole peripheral blood. If we consider the patients of the OT group in whom LV peripheral blood and ocular fluid were both tested, blood PCR allows the diagnosis of 9 of 36 cases, while ocular PCR allows the diagnosis of 16 of 36 cases (P = 0.034). Moreover, while the specificity is 100% for PCR with ocular samples, it is only 89.5% for PCR with LV blood. The volume of the ocular sample might have been insufficient in our study, but we did not systematically note the ocular sample volume and thus cannot comment further on that point. It should also be noted that aqueous humor may not be the most relevant ocular fluid because it is not in contact with the posterior part of the eye (in contrast to vitreous humor), where the infection occurs. Moreover, in the absence of a specific kit for DNA extraction from ocular samples, we used the QIAmp DNA Blood Mini kit; it cannot be ruled out that the lack of a tissue lysis buffer in this kit may have influenced our results. Additional studies should be performed to compare aqueous and vitreous humors in this setting. Taking these data into account, it seems that toxoplasmic retinochoroiditis is first a local event. Detection of parasitic DNA in the blood might be considered either a random event or (especially in immunocompromised patients) a release of toxoplasmic DNA from the eye into the blood.
Toxoplasma gondii strains have different capacities of dissemination according to their genetic background (16), which may explain why the sensitivity of PCR with peripheral blood was lower here than in studies performed in Brazil (20–22). In France, genotype II is most frequently responsible for OT (23); additional studies in other geographical zones should be performed to assess locally the performance of blood PCR in OT.
Finally, whatever the origin of the sample (ocular or blood), PCR methods appear to be more effective for immunocompromised patients than for their immunocompetent counterparts.
Conclusion.
For now, PCR for Toxoplasma genome detection using whole blood does not offer sufficient sensitivity to be a globally contributive tool for the diagnosis of OT. Performing PCR using a large volume of blood could increase sensitivity but alters specificity. The continuing development of molecular tools, such as large-volume PCR extraction protocols, may preclude the need for invasive ocular fluid sampling in the future, Nonetheless, our results show that the analysis of ocular samples by PCR, the calculation of the Goldmann-Witmer coefficient, and immunoblotting are still required to establish a relevant OT diagnosis.
Footnotes
Published ahead of print 10 September 2014
REFERENCES
- 1.Bodaghi B, Touitou V, Fardeau C, Paris L, LeHoang P. 2012. Toxoplasmosis: new challenges for an old disease. Eye (Lond.) 26:241–244. 10.1038/eye.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. 1996. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am. J. Ophthalmol. 121:35–46. [DOI] [PubMed] [Google Scholar]
- 3.Montoya JG, Remington JS. 1996. Toxoplasmic chorioretinitis in the setting of acute acquired toxoplasmosis. Clin. Infect. Dis. 23:277–282. 10.1093/clinids/23.2.277. [DOI] [PubMed] [Google Scholar]
- 4.Delair E, Monnet D, Grabar S, Dupouy-Camet J, Yera H, Brezin AP. 2008. Respective roles of acquired and congenital infections in presumed ocular toxoplasmosis. Am. J. Ophthalmol. 146:851–855. 10.1016/j.ajo.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Garweg JG, Garweg SD, Flueckiger F, Jacquier P, Boehnke M. 2004. Aqueous humor and serum immunoblotting for immunoglobulin types G, A, M, and E in cases of human ocular toxoplasmosis. J. Clin. Microbiol. 42:4593–4598. 10.1128/JCM.42.10.4593-4598.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fekkar A, Bodaghi B, Touafek F, Le Hoang P, Mazier D, Paris L. 2008. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. J. Clin. Microbiol. 46:1965–1967. 10.1128/JCM.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talabani H, Asseraf M, Yera H, Delair E, Ancelle T, Thulliez P, Brezin AP, Dupouy-Camet J. 2009. Contributions of immunoblotting, real-time PCR, and the Goldmann-Witmer coefficient to diagnosis of atypical toxoplasmic retinochoroiditis. J. Clin. Microbiol. 47:2131–2135. 10.1128/JCM.00128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bou G, Figueroa MS, Marti-Belda P, Navas E, Guerrero A. 1999. Value of PCR for detection of Toxoplasma gondii in aqueous humor and blood samples from immunocompetent patients with ocular toxoplasmosis. J. Clin. Microbiol. 37:3465–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contini C, Seraceni S, Cultrera R, Incorvaia C, Sebastiani A, Picot S. 2005. Evaluation of a real-time PCR-based assay using the LightCycler system for detection of Toxoplasma gondii bradyzoite genes in blood specimens from patients with toxoplasmic retinochoroiditis. Int. J. Parasitol. 35:275–283. 10.1016/j.ijpara.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Cassaing S, Bessieres MH, Berry A, Berrebi A, Fabre R, Magnaval JF. 2006. Comparison between two amplification sets for molecular diagnosis of toxoplasmosis by real-time PCR. J. Clin. Microbiol. 44:720–724. 10.1128/JCM.44.3.720-724.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Groot-Mijnes JD, Rothova A, Van Loon AM, Schuller M, Ten Dam-Van Loon NH, De Boer JH, Schuurman R, Weersink AJ. 2006. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am. J. Ophthalmol. 141:313–318. 10.1016/j.ajo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Westeneng AC, Rothova A, de Boer JH, de Groot-Mijnes JD. 2007. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann-Witmer coefficient in aqueous analysis. Am. J. Ophthalmol. 144:781–785. 10.1016/j.ajo.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Mesquita RT, Vidal JE, Pereira-Chioccola VL. 2010. Molecular diagnosis of cerebral toxoplasmosis: comparing markers that determine Toxoplasma gondii by PCR in peripheral blood from HIV-infected patients. Braz. J. Infect. Dis. 14:346–350. 10.1016/S1413-8670(10)70073-8. [DOI] [PubMed] [Google Scholar]
- 14.Wallon M, Franck J, Thulliez P, Huissoud C, Peyron F, Garcia-Meric P, Kieffer F. 2010. Accuracy of real-time polymerase chain reaction for Toxoplasma gondii in amniotic fluid. Obstet. Gynecol. 115:727–733. 10.1097/AOG.0b013e3181d57b09. [DOI] [PubMed] [Google Scholar]
- 15.Lee SE, Hong SH, Lee SH, Jeong YI, Lim SJ, Kwon OW, Kim SH, You YS, Cho SH, Lee WJ. 2012. Detection of ocular Toxoplasma gondii infection in chronic irregular recurrent uveitis by PCR. Korean J. Parasitol. 50:229–231. 10.3347/kjp.2012.50.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ajzenberg D. 2010. Is PCR testing on blood samples useful or not in the diagnosis of Toxoplasma encephalitis? Trans. R. Soc. Trop. Med. Hyg. 104:569–570. 10.1016/j.trstmh.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Colombo FA, Vidal JE, Penalva de Oliveira AC, Hernandez AV, Bonasser-Filho F, Nogueira RS, Focaccia R, Pereira-Chioccola VL. 2005. Diagnosis of cerebral toxoplasmosis in AIDS patients in Brazil: importance of molecular and immunological methods using peripheral blood samples. J. Clin. Microbiol. 43:5044–5047. 10.1128/JCM.43.10.5044-5047.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correia CC, Melo HR, Costa VM. 2010. Influence of neurotoxoplasmosis characteristics on real-time PCR sensitivity among AIDS patients in Brazil. Trans. R. Soc. Trop. Med. Hyg. 104:24–28. 10.1016/j.trstmh.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Martino R, Bretagne S, Einsele H, Maertens J, Ullmann AJ, Parody R, Schumacher U, Pautas C, Theunissen K, Schindel C, Munoz C, Margall N, Cordonnier C. 2005. Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin. Infect. Dis. 40:67–78. 10.1086/426447. [DOI] [PubMed] [Google Scholar]
- 20.Silveira C, Vallochi AL, Rodrigues da Silva U, Muccioli C, Holland GN, Nussenblatt RB, Belfort R, Rizzo LV. 2011. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br. J. Ophthalmol. 95:396–400. 10.1136/bjo.2008.148205. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira IM, Vidal JE, de Mattos CDCB, de Mattos LC, Qu D, Su C, Pereira-Chioccola VL. 2011. Toxoplasma gondii isolates: multilocus RFLP-PCR genotyping from human patients in Sao Paulo State, Brazil identified distinct genotypes. Exp. Parasitol. 129:190–195. 10.1016/j.exppara.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Mattos CC, Meira CS, Ferreira AI, Frederico FB, Hiramoto RMGC, Jr, Mattos LC, Pereira-Chioccola VL. 2011. Contribution of laboratory methods in diagnosing clinically suspected ocular toxoplasmosis in Brazilian patients. Diagn. Microbiol. Infect. Dis. 70:362–366. 10.1016/j.diagmicrobio.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Fekkar A, Ajzenberg D, Bodaghi B, Touafek F, Le Hoang P, Delmas J, Robert PY, Darde ML, Mazier D, Paris L. 2011. Direct genotyping of Toxoplasma gondii in ocular fluid samples from 20 patients with ocular toxoplasmosis: predominance of type II in France. J. Clin. Microbiol. 49:1513–1517. 10.1128/JCM.02196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


