LETTER
Tick-borne encephalitis (TBE) is a viral zoonosis of increasing incidence in Eurasia, and diagnosis relies mainly on the detection of IgM antibodies in serum. We here present two cases of TBE, of four patients tested, in which we have detected TBE virus (TBEV) RNA in urine with real-time PCR during the encephalitic phase. This observation suggests a new diagnostic opportunity that could be evaluated in a larger cohort of patients.
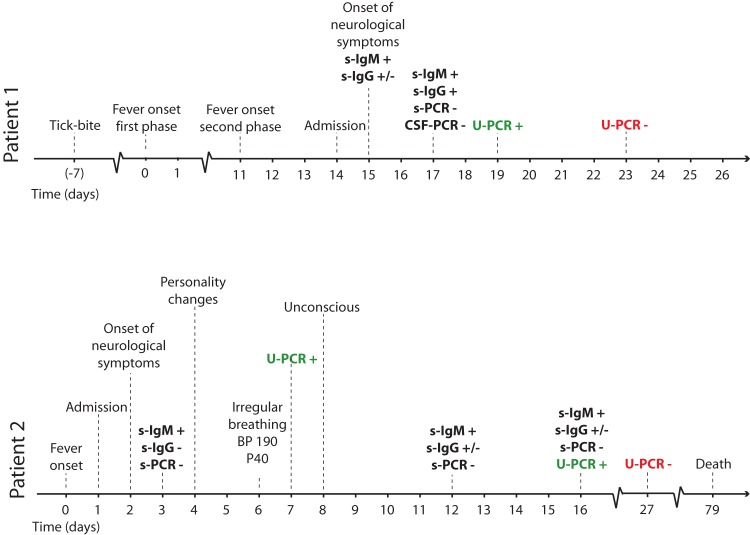
In the first case we report here, 1 week after a tick bite, a 61-year-old woman had high fever, nausea, and fatigue lasting for 5 to 6 days. She was asymptomatic for a week, after which the fever returned along with vomiting and severe headache. After admission, her condition worsened. She was unable to follow instructions, became somnolent and disoriented, and had signs of dysphasia. A cerebrospinal fluid (CSF) sample showed a leukocyte count of 69 × 106/liter (41% polymorphonuclear and 59% mononuclear) and elevated albumin. Specific TBE virus (TBEV) IgM antibodies were detected in serum drawn on day 2 after admission, and the patient seroconverted as regards IgG during the hospital stay. Before nucleic acid extraction using the EasyMag instrument (bioMérieux, Marcy l′Etoile, France), 250-μl amounts of serum, CSF, and urine samples were added to 2 ml of lysis buffer and incubated for 10 min at room temperature. TBEV RNA was detected from urine by TaqMan PCR performed as described previously (1) on day 19 after fever onset (Fig. 1). The patient's condition improved, and she was discharged after 10 days of hospitalization.
FIG 1.
Clinical course and diagnostic results of PCR and serology in two patients with TBE. Day 0, day of onset of first symptoms in both patients; s, serum samples; CSF, cerebrospinal fluid; U, urine; BP, blood pressure; P, pulse.
The second case was a 70-year-old woman who reported several tick bites during the preceding weeks and was admitted 2 days after the onset of high fever, nausea, and fatigue. She was on treatment with immunosuppressive drugs (mycophenolate, tacrolimus, and prednisolone) due to kidney transplantation. She developed paresis of the lower extremities and lost the strength in her hands. A CSF sample showed 488 × 106 leukocytes/liter (28% polymorphonuclear and 72% mononuclear), elevated albumin and lactate, and a slightly decreased glucose quotient. TBEV IgM antibodies (but not IgG) were detected in serum. On day 8 after admission, the patient became deeply unconscious, had a generalized status epilepticus, and was intubated. Magnetic resonance imaging (MRI) demonstrated high signals in the amygdala and hippocampus. Her neurological condition deteriorated, and she died 11 weeks after admission. TBEV RNA was detected from urine on day 7 and day 16 after onset (Fig. 1). The RNA quantities allowed sequencing of 10,432 nt from TBEV RNA amplicons, as described previously (2), which confirmed the PCR finding in urine, and phylogenetic analysis showed that the virus belonged to the TBE-Eu clade.
TBEV is a flavivirus causing an often biphasic disease, ranging from a subclinical infection to a severe meningoencephalitis with myelitis. Diagnosis is based on detection of IgM and IgG antibodies to TBEV in serum and CSF. The virus is seldom found in serum or CSF in the encephalitic phase, but TBEV can be isolated and viral RNA detected in blood during the first viremic phase. However, this is of little clinical value since infected persons rarely seek medical care during the first phase (3). The demonstration of TBEV RNA in urine samples from two patients during CNS manifestations of varying severity suggests an option for PCR-based diagnosis during at least 1 to 2 weeks after the onset of TBE, as shown in other flavivirus infections (4–8). These findings argue for prospective studies of TBEV RNA detection in urine in patients with TBE, with the aim of elucidating the value of this body fluid as a sample material for improved diagnosis.
ACKNOWLEDGMENTS
This work was supported by EU-Interreg IV A (project no. 167226), the Swedish Medical Research Council (grant no. 521-2011-3297), and the LUA-ALF Foundation of Sahlgrenska University Hospital (grant no. 145-841).
All authors report no conflicts of interest.
Footnotes
Published ahead of print 27 August 2014
REFERENCES
- 1.Brinkley C, Nolskog P, Golovjova I, Lundkvist Å, Bergström T. 2008. Tick-borne encephalitis virus natural foci emerge in western Sweden. Int. J. Med. Microbiol. 298(Suppl 1):73–80. 10.1016/j.ijmm.2007.12.005. [DOI] [Google Scholar]
- 2.Norberg P, Roth A, Bergström T. 2013. Genetic recombination of tick-borne flaviviruses among wild-type strains. Virology 440:105–116. 10.1016/j.virol.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Lindquist L, Vapalahti O. 2008. Tick-borne encephalitis. Lancet 371:1861–1871. 10.1016/S0140-6736(08)60800-4. [DOI] [PubMed] [Google Scholar]
- 4.Domingo C, Yactayo S, Agbenu E, Demanou M, Schulz AR, Daskalow K, Niedrig M. 2011. Detection of yellow fever 17D genome in urine. J. Clin. Microbiol. 49:760–762. 10.1128/JCM.01775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirayama T, Mizuno Y, Takeshita N, Kotaki A, Tajima S, Omatsu T, Sano K, Kurane I, Takasaki T. 2012. Detection of dengue virus genome in urine by real-time reverse transcriptase PCR: a laboratory diagnostic method useful after disappearance of the genome in serum. J. Clin. Microbiol. 50:2047–2052. 10.1128/JCM.06557-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonry JH, Brown CB, Cropp CB, Co JK, Bennett SN, Nerurkar VR, Kuberski T, Gubler DJ. 2005. West Nile virus detection in urine. Emerg. Infect. Dis. 11:1294–1296. 10.3201/eid1108.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathur A, Khanna N, Kulshreshtha R, Maitra SE, Chaturvedi UC. 1995. Viruria during acute Japanese encephalitis virus infection. Int. J. Exp. Pathol. 76:103–109. [PMC free article] [PubMed] [Google Scholar]
- 8.Barzon L, Pacenti M, Franchin E, Pagni S, Marcello T, Cattai M, Cusinato R, Palù G. 2013. Excretion of West Nile virus in urine during acute infection. J. Infect. Dis. 208:1086–1092. 10.1093/infdis/jit290. [DOI] [PubMed] [Google Scholar]