Abstract
We characterized 18 Vibrio isolates, including 15 recovered from human clinical specimens, and found that they clustered with two previously characterized Vibrio navarrensis isolates in a phylogenetic analysis. Four of the 18 strains may represent a new Vibrio species, distinct from V. navarrensis. The potential role of V. navarrensis in human disease needs further investigation.
TEXT
Identification of Vibrio isolates from human clinical specimens is essential for surveillance and epidemiology. The genus Vibrio includes species of great public health concern, such as Vibrio cholerae, which can cause large pandemics (1), and Vibrio vulnificus, which has a high case fatality rate (over 50% for septicemia) and is responsible for a large proportion of deaths related to seafood consumption (2). In 2008, the Centers for Disease Control and Prevention (CDC) received four Vibrio isolates recovered from human specimens that could not be identified to the species level with traditional phenotypic methods. The isolates were similar to sucrose-positive V. vulnificus (including positive test results for phenylalanine deaminase and cellobiose fermentation), but some characteristics were atypical for V. vulnificus (negative test results for lysine and ornithine decarboxylase and salicin fermentation). A preliminary sequence comparison using the rpoA sequence from one isolate matched the sequence from a Vibrio navarrensis strain. Vibrio navarrensis was first isolated in 1982 from sewage and river water of the Navarra Province in Spain (3), and V. navarrensis biotype pommerensis from the Baltic Sea was described in 2007 (4). The species has not previously been reported to be associated with human clinical specimens, so we surveyed our collection and found 13 unidentified isolates and one isolate submitted to CDC as V. vulnificus that were phenotypically similar to the 2008 isolates. We sought to further characterize the 18 isolates and place them in a phylogenetic framework with other Vibrio species, including the two isolates characterized in the original species description of V. navarrensis (3).
The 20 bacterial strains that we included (two V. navarrensis isolates, 17 unidentified isolates, and one potentially misclassified V. vulnificus isolate) had been characterized using a standard panel of 46 phenotypic tests that are routinely used for identification of enteric bacteria (5, 6) (Table 1). All isolates grew on thiosulfate-citrate-bile salts-sucrose (TCBS) agar, a selective medium for isolation of vibrios. The template for PCR was prepared as a crude lysate, according to the method described by Tarr et al. (7), from a single colony that had been grown overnight at 37°C on tryptic soy agar (TSA) II with 5% sheep blood (Difco, Franklin Lakes, NJ). We applied the multilocus sequence analysis (MLSA) approach of Thompson et al., which uses internal segments of housekeeping genes (pyrH, recA, rpoA, and 16S rRNA) (8). Amplicons were purified with the QIAquick PCR purification kit (Qiagen, Valencia CA) and were sequenced on an Applied Biosystems 3730 DNA analyzer (Life Technologies), following the manufacturer's instructions. Lasergene software (DNASTAR, Inc., Madison, WI) was used to analyze chromatograms. Genetic distances (d values) were calculated in MEGA4 (9) with the Kimura 2-parameter model (10), and the neighbor-joining algorithm was used to construct phylogenetic trees. The trees included sequences from GenBank for 53 Vibrio and 9 Vibrionaceae species. The robustness of each branch was estimated by the interior branch test (IBT) (11) with 1,000 replications.
TABLE 1.
Bacterial strains characterized in this study
| Strain | Original identification | Year | Source | Source type | Location |
|---|---|---|---|---|---|
| LMG 15976Ta,b | V. navarrensis | 1982 | Environment | Sewage | Spain |
| 2232a | V. navarrensis | 1982 | Environment | Sewage | Spain |
| 08-2461 | Vibrio species | 2008 | Human | Wound | USA |
| 08-2462 | Vibrio species | 2008 | Human | Blood | USA |
| 08-2466 | Vibrio species | 2008 | Human | Ear | USA |
| 08-2467 | Vibrio species | 2008 | Human | Wound | USA |
| 2462-79 | Vibrio species | 1979 | Human | Wound | USA |
| 2543-80 | Vibrio species | 1980 | NKc | NK | Venezuela |
| 2756-81 | Vibrio species | 1981 | Environment | River water | NK |
| 0053-83 | Vibrio species | 1983 | Human | Wound | USA |
| 1048-83 | Vibrio species | 1983 | Human | Blood | USA |
| 2421-86 | Vibrio species | 1986 | Human | Stool | USA |
| 2422-86 | Vibrio species | 1986 | Human | Stool | USA |
| 2481-86 | Vibrio species | 1986 | Human | Blood | USA |
| 2544-86 | Vibrio species | 1986 | Human | Blood | Singapore |
| 2578-87 | Vibrio species | 1987 | Animal | Dolphin | USA |
| 2538-88 | Vibrio species | 1988 | Human | Blood | USA |
| 2423-01 | Vibrio | 2001 | Human | Blood | USA |
| AM 36848 | Vibrio vulnificus | 2008 | Human | Blood | USA |
| AM 37820 | Vibrio species | 2009 | Human | Blood | USA |
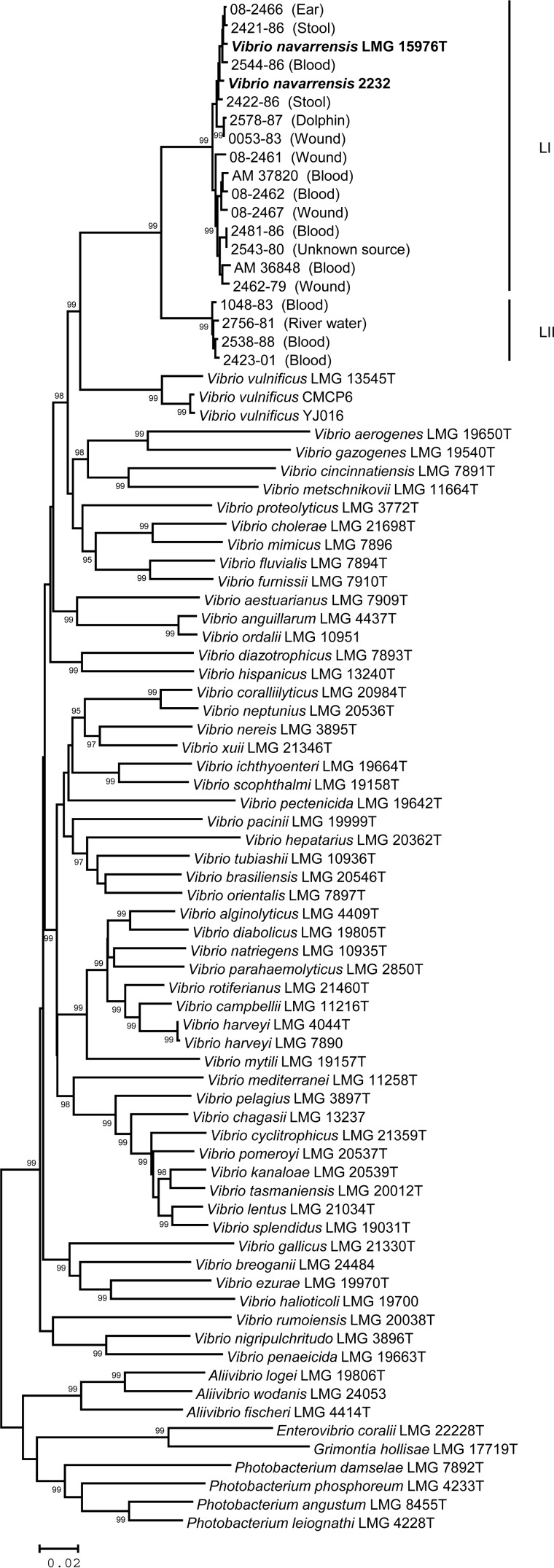
Of the four genes sequenced, three (pyrH, rpoA, and recA) produced concordant phylogenies (individual trees not shown); therefore, these three genes were concatenated and a single tree was constructed from the 1,443-bp alignment (Fig. 1). The 16S rRNA gene tree did not show concordance with that for the other genes (Fig. 2), and it was examined separately. The tree constructed from the concatenated alignment showed that all previously unidentified isolates and the apparently misclassified V. vulnificus isolate were more closely related to V. navarrensis than to other Vibrio species; however, 14 of the isolates clustered with the Vibrio navarrensis type strain, while the remaining four isolates fell into another, closely related cluster (Fig. 1). Each cluster was highly supported by the IBT (99%). For convenience, the cluster containing the V. navarrensis type strain was designated lineage I (LI), whereas the second, closely related group was referred to as lineage II (LII). The average distance between the 14 isolates in LI and the type strain (LMG 15976T) was d = 0.01, whereas the average distance between the isolates in LII and LMG 15976T was d = 0.062. The average divergence between the two lineages (d = 0.063) was similar to the distance between closely related species pairs, such as V. cholerae and Vibrio mimicus (d = 0.074) and Vibrio furnissii and Vibrio fluvialis (d = 0.065). Based on the concatenated gene tree, we concluded that LI isolates could be identified as V. navarrensis, but LII isolates could represent a separate species. We conducted BLAST searches with LII sequences and, consistent with the phylogenies shown here, we did not find any match closer than V. navarrensis. The BLAST results suggest that, if LII is a species distinct from V. navarrensis, then it could be a novel undescribed species; however, more information is needed to make this determination.
FIG 1.
Phylogenetic tree constructed from a 1,443-bp alignment of concatenated pyrH-recA-rpoA sequences. Previously characterized V. navarrensis strains are indicated in bold. Numbers above the branches indicate the probability that the branch length is greater than zero based on the interior branch test; only values of ≥95 are shown. Scale bar, 0.02 nucleotide substitutions per site.
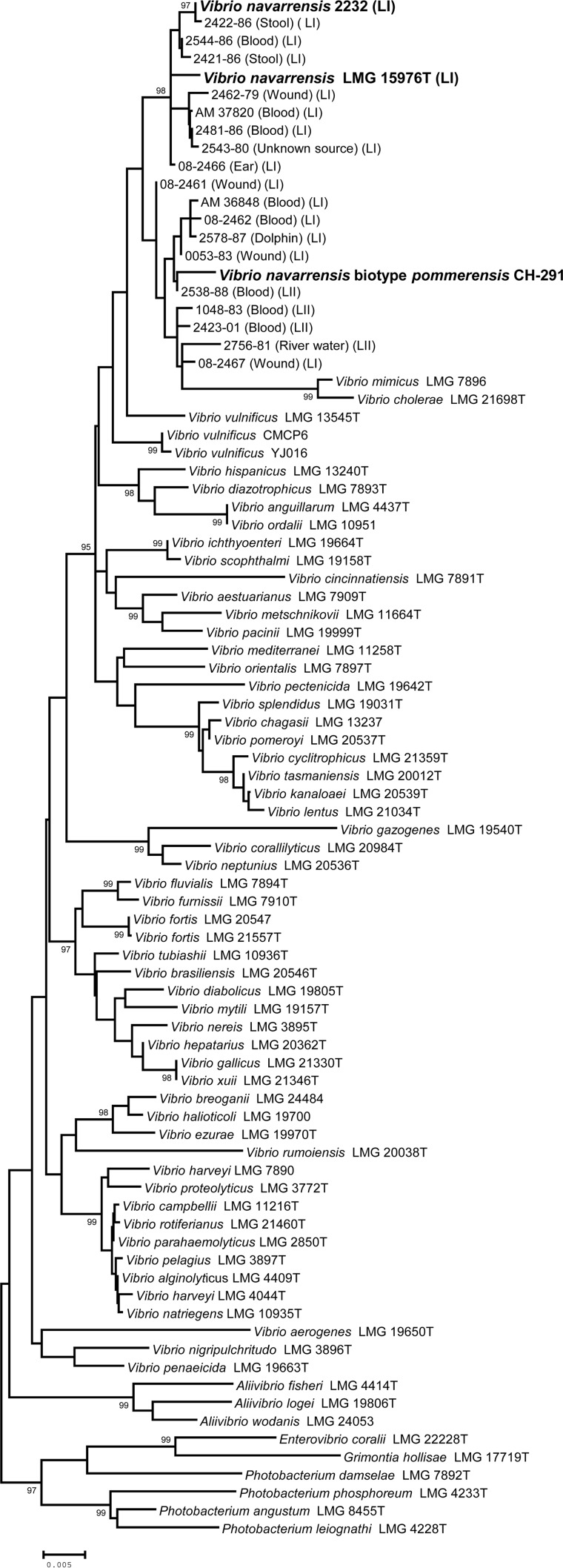
FIG 2.
Gene tree constructed from a 1,041-bp alignment of 16S rRNA gene sequences. Previously characterized V. navarrensis strains are indicated in bold. Numbers above the branches indicate the probability that the branch length is greater than zero based on the interior branch test; only values of ≥95 are shown. Scale bar, 0.02 nucleotide substitutions per site.
In contrast, the 16S rRNA gene tree did not separate the 20 isolates into two distinct lineages (Fig. 2), and the tree placed V. cholerae and V. mimicus in the V. navarrensis cluster. The 16S rRNA gene sequences from the 20 isolates contained a number of unresolved positions, presumably the result of polymorphism among multiple operons, a phenomenon that has been described previously for Vibrio (12, 13). There were 1,155 nucleotide sites in the alignment; of those, 22 were polymorphic, and only one of those could be unambiguously determined in all 20 sequences. We concluded that the fragment of the 16S rRNA gene we used here was not useful for phylogenetic clustering and identification of the closely related Vibrio isolates we examined in this study.
We summarized phenotypic profiles separately for the two lineages (Table 2). We did not find a clear diagnostic difference between the two lineages and could not separate them into two species or different biotypes based on phenotype. We did compare the LII profile to published data for V. navarrensis biotype pommerensis (4). Although sample sizes for both groups were small and the phenotypic test panels only partially overlapped, there were four characteristics for which the two lineages differed (Table 2). Thus, we concluded that it is unlikely that LII represents V. navarrensis biotype pommerensis. A 16S rRNA gene sequence was available for a biotype pommerensis strain (Fig. 2), but the tree could not shed light on the relationships among isolates. Further study is ongoing, including genome sequencing, to help resolve the taxonomic status of the LII isolates; however, a comprehensive comparison of Vibrio strains is still needed, since sequences are not available for all described Vibrio species.
TABLE 2.
Phenotypic test results for Vibrio navarrensis (LI) and associated lineage (LII) with Vibrio vulnificus for comparison
| Phenotypic testa | % positive by day 7 |
Reaction resultb |
|||
|---|---|---|---|---|---|
| Vibrio vulnificusc | Lineage I (n = 16) | Lineage II (n = 4) | V. navarrensis LMG 15976T | V. navarrensis biotype pommerensisd | |
| Indole production (HIB)e | 97 | 56 | 75 | + | + |
| Methyl Rede | 80 | 100 | 100 | + | + |
| Voges-Proskauere | 0 | 0 | 0 | − | − |
| Citrate, Simmons' agar | 75 | 75 | 25 | + (5) | − |
| H2S-TSI | 0 | 0 | 0 | − | − |
| Urea hydrolysis | 1 | 19 | 0 | − | − |
| Phenylalanine deaminase | 35 | 94 | 100 | + | − |
| Lysine, Moeller's mediume | 99 | 0 | 0 | − | − |
| Arginine, Moeller's mediume | 0 | 0 | 0 | − | − |
| Ornithine, Moeller's mediume | 55 | 0 | 0 | − | − |
| Motility | 99 | 81 | 100 | − | + |
| Malonate utilization | 0 | 19 | 0 | − | − |
| d-Glucose, acid production | 100 | 100 | 100 | + | + |
| d-Glucose, gas production | 0 | 0 | 0 | − | − |
| Acid production from | |||||
| d-Adonitol | 0 | 0 | 0 | − | − |
| l-Arabinose | 0 | 6 | 0 | − | − |
| d-Arabitol | 0 | 0 | 0 | − | − |
| Cellobiose | 99 | 94 | 100 | + | + |
| Dulcitol | 0 | 0 | 0 | − | − |
| Erythritol | 0 | 0 | 0 | − | ND |
| d-Galactose | 96 | 56 | 25 | − | ND |
| Glycerol | 1 | 0 | 0 | − | + |
| myo-Inositol | 0 | 0 | 0 | − | − |
| Lactose | 0 | 12 | 0 | − | + |
| Maltose | 100 | 100 | 100 | + (5) | + |
| Mannitol | 45 | 100 | 100 | + | + |
| Mannose | 98 | 94 | 100 | + (5) | ND |
| Melibiose | 40 | 6 | 0 | − | ND |
| α-Methyl-d-glucoside | 0 | 0 | 25 | − | ND |
| Raffinose | 0 | 0 | 0 | − | − |
| l-Rhamnose | 0 | 6 | 100 | − | − |
| Salicin | 95 | 19 | 50 | − | ND |
| d-Sorbitol | 0 | 12 | 75 | − | − |
| Sucrose | 15 | 100 | 100 | + | + |
| Trehalose | 100 | 100 | 100 | + | + |
| d-Xylose | 0 | 0 | 0 | − | − |
| Mucate | 0 | 0 | 0 | − | − |
| Esculin hydrolysise | 40 | 75 | 75 | − | ND |
| Acetate utilization | 7 | 56 | 100 | − | ND |
| Nitrate reduction to nitritee | 100 | 94 | 100 | + | + |
| Oxidase | 100 | 100 | 100 | + | + |
| DNase (25°C) | 50 | 94 | 100 | + (5) | ND |
| ONPG test | 75 | 50 | 0 | + | ND |
| Tyrosine clearing | 75 | 94 | 75 | + | + |
| Growth in nutrient broth with | |||||
| 0% NaCl | 0 | 0 | 0 | − | − |
| 1% NaCl | 99 | 100 | 100 | + | + |
HIB, heart infusion broth; TSI, triple sugar iron agar; ONPG, o-nitrophenyl-β-galactopyranoside.
Numbers in parentheses indicate the number of days of incubation required to observe a positive reaction. +, positive reaction after 48 h of incubation at 36°C; −, negative result after 48 h of incubation; ND, not determined.
Strain data were obtained from reference 6.
Phenotypic results were obtained from reference 4.
Tests were performed with NaCl at a final concentration of 1%.
Without the knowledge that V. navarrensis can be recovered from human specimens, the phenotypic characteristics of the species could make it difficult to differentiate from sucrose-positive V. vulnificus. A positive reaction for phenylalanine deaminase is rare among clinically relevant Vibrionaceae strains, but it was nearly ubiquitous in the isolates that we examined and is also fairly common among V. vulnificus strains. Positive results for esculin hydrolysis and cellobiose fermentation were also common among our study isolates, and these characteristics are typical of V. vulnificus but are unusual among the other Vibrionaceae species that are commonly isolated in clinical laboratories. One notable distinction is that the V. navarrensis isolates showed negative test results for lysine and ornithine decarboxylase and arginine dihydrolase. The clinically relevant Vibrionaceae species, including V. vulnificus, generally utilize one or more of these pathways, with the exception of Grimontia hollisae (formerly Vibrio hollisae) (5); however, G. hollisae utilizes few of the substrates in the test panel and so would not be confused with V. navarrensis. We note that the aforementioned shared characteristics were the basis for inclusion in the study, and so V. navarrensis isolates exhibiting different characteristics would not have been included in the study.
In summary, a key finding of this study was the identification of human clinical isolates as V. navarrensis. Our characterization of V. navarrensis uncovered a second distinct lineage (LII), which likely represents a distinct and possibly novel Vibrio species. Genome sequencing could identify virulence mechanisms through comparisons with other pathogenic vibrios. Vibrio navarrensis was isolated from diverse human sources, including blood samples, strongly suggesting that it is a human pathogen. Further studies are required to demonstrate its role in human disease and to learn more about its epidemiology and prevalence and the clinical outcomes associated with infection.
Nucleotide sequence accession numbers.
The sequences generated in this study were deposited in GenBank under accession numbers KJ807092 to KJ807171.
ACKNOWLEDGMENTS
We thank Anne Whitney in the Division of High-Consequence Pathogens and Pathology Genomics Unit Core Facility at the CDC for technical assistance. We are grateful to P. Gerner-Smidt, P. Fields, C. Fitzgerald, and three anonymous reviewers for comments on an earlier draft of the manuscript.
The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 3 September 2014
REFERENCES
- 1.Faruque SM, Albert MJ, Mekalanos JJ. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones MK, Oliver JD. 2009. Vibrio vulnificus: disease and pathogenesis. Infect. Immun. 77:1723–1733. 10.1128/IAI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urdaci MC, Marchand M, Ageron E, Arcos JM, Sesma B, Grimont PA. 1991. Vibrio navarrensis sp. nov., a species from sewage. Int. J. Syst. Bacteriol. 41:290–294. 10.1099/00207713-41-2-290. [DOI] [PubMed] [Google Scholar]
- 4.Jores J, Appel B, Lewin A. 2007. Vibrio navarrensis biotype pommerensis: a new biotype of V. navarrensis isolated in the German Baltic Sea. Syst. Appl. Microbiol. 30:27–30. 10.1016/j.syapm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Abbott SL, Janda JM, Johnson JA, Farmer JJ., III 2007. Vibrio and related organisms, p 723–733 In Murray PR. (ed), Manual of clinical microbiology, 9th ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 6.Farmer JJ, III, Janda JM, Brenner FW, Cameron DN, Birkhead KM. 2005. Genus I. Vibrio, p 494 In Garrity GM. (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2, part B Springer, New York, NY. [Google Scholar]
- 7.Tarr CL, Patel JS, Puhr ND, Sowers EG, Bopp CA, Strockbine NA. 2007. Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J. Clin. Microbiol. 45:134–140. 10.1128/JCM.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson FL, Gevers D, Thompson CC, Dawyndt P, Naser S, Hoste B, Munn CB, Swings J. 2005. Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. Appl. Environ. Microbiol. 71:5107–5115. 10.1128/AEM.71.9.5107-5115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599. 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 10.Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111–120. 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 11.Sitnikova T, Rzhetsky A, Nei M. 1995. Interior-branch and bootstrap tests of phylogenetic trees. Mol. Biol. Evol. 12:319–333. [DOI] [PubMed] [Google Scholar]
- 12.Acinas SG, Marcelino LA, Klepac-Ceraj V, Polz MF. 2004. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J. Bacteriol. 186:2629–2635. 10.1128/JB.186.9.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno C, Romero J, Espejo RT. 2002. Polymorphism in repeated 16S rRNA genes is a common property of type strains and environmental isolates of the genus Vibrio. Microbiology 148:1233–1239. [DOI] [PubMed] [Google Scholar]