Abstract
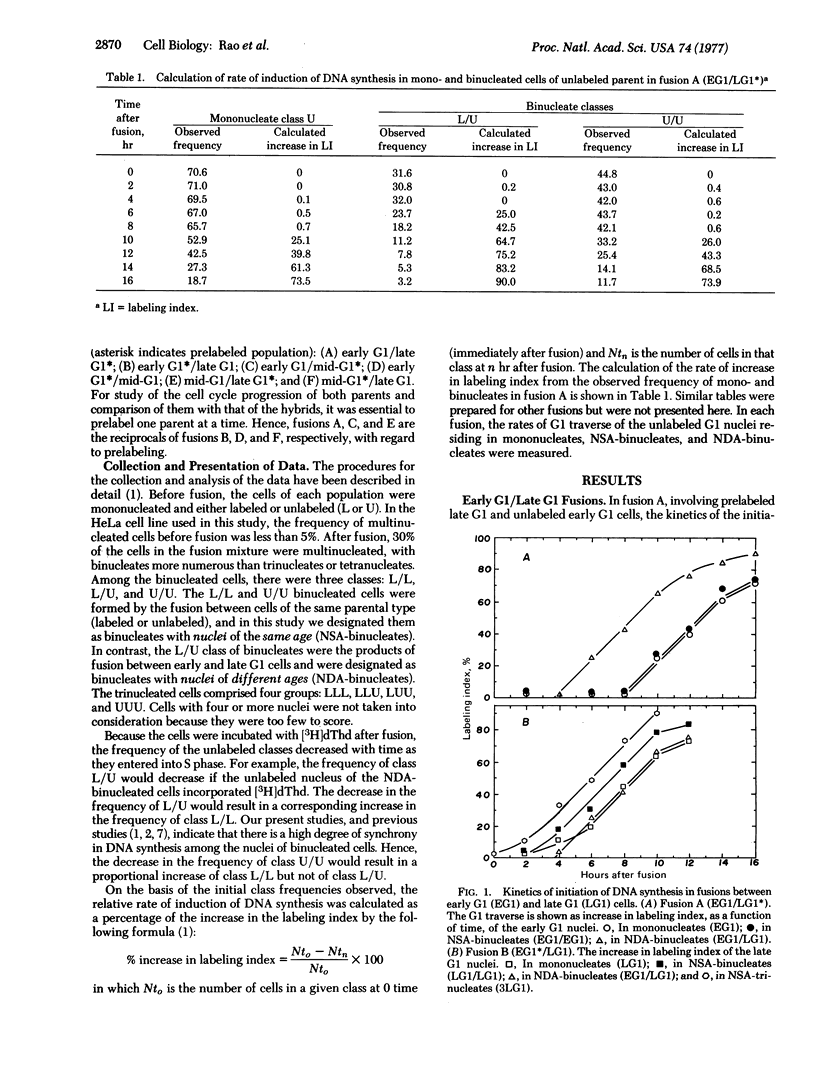
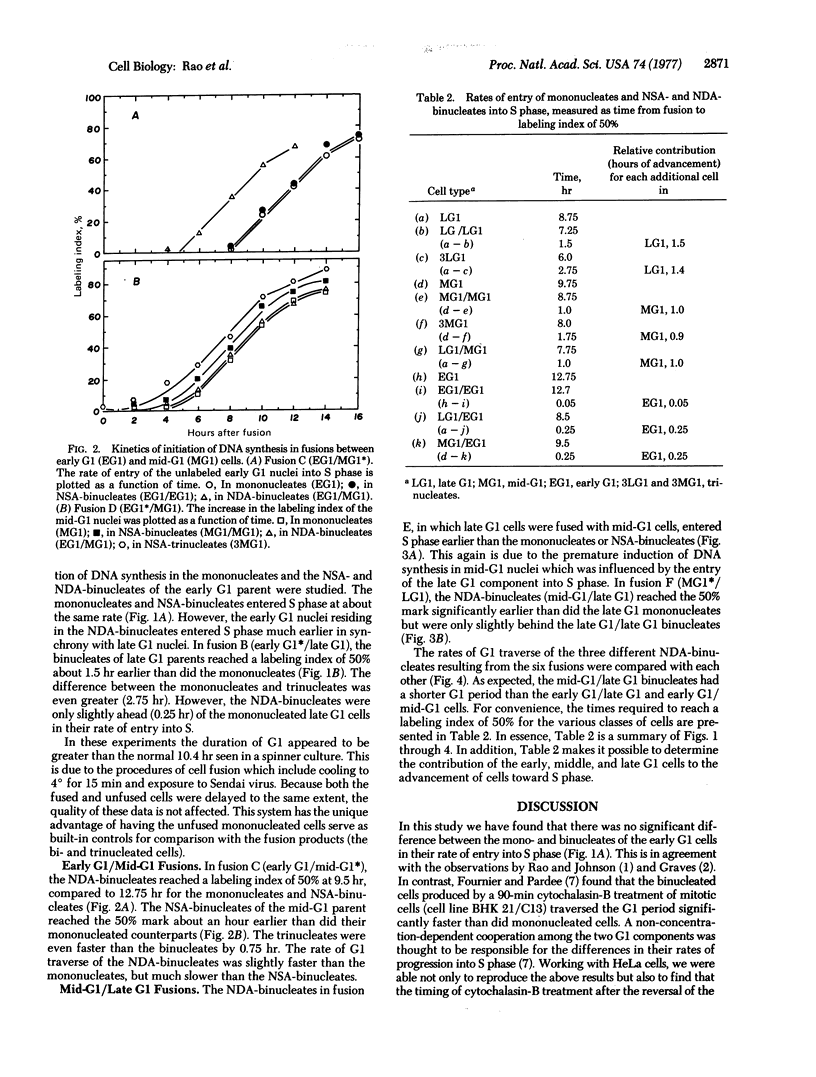
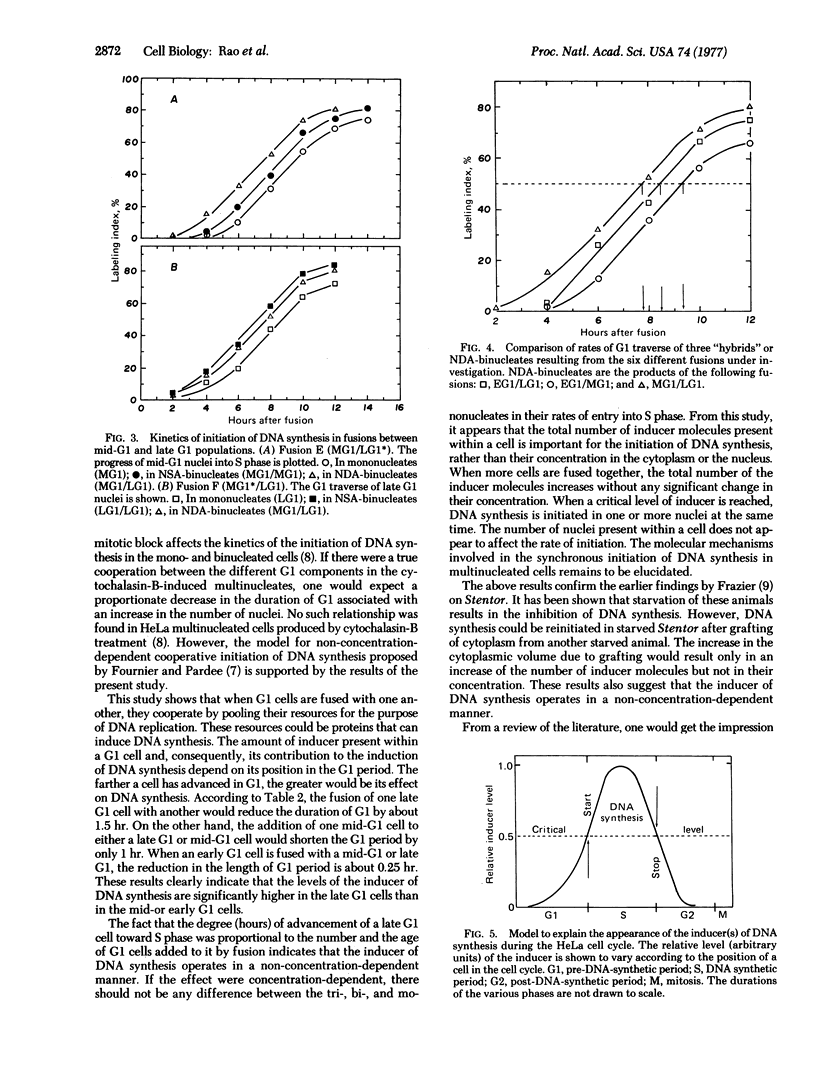
The object of this study was to determine whether the inducer(s) of DNA synthesis in mammalian cells accumulates gradually throughout the G1 period or becomes available suddenly at the G1-S transition. HeLa cells, synchronized at various points in the G1 period, were fused by using UV-inactivated Sendai virus. Early G1 cells were fused with mid-G1 or late G1 cells and late G1 cells were fused with mid-G1 cells. The G1 traverse of mono-, bi-, and trinucleated cells was studied. The bi- and trinucleated cells of mid-G1 and late G1 parents traversed the G1 period significantly faster than did their mononucleated counterparts. The reduction in the duration of the G1 period was proportional to the number and age of nuclei at the time of fusion. There was no significant difference between the mono- and binucleated cells of the early G1 parent in their rates of entry into S period. In light of these findings, a model is proposed in which the inducer(s) of DNA synthesis accumulates gradually throughout the G1 period, reaching a critical level at the G1-S boundary when DNA replication is initiated; after reaching a peak during early or mid-S period, it declines to below the critical level when DNA synthesis ceases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Terra N. MACRONUCLEAR DNA SYNTHESIS IN Stentor: REGULATION BY A CYTOPLASMIC INITIATOR. Proc Natl Acad Sci U S A. 1967 Mar;57(3):607–614. doi: 10.1073/pnas.57.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier R. E., Pardee A. B. Cell cycle studies of mononucleate and cytochalasin-B--nduced binucleate fibroblasts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):869–873. doi: 10.1073/pnas.72.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier E. A. DNA synthesis following gross alterations of the nucleocytoplasmic ratio in the ciliate Stentor coeruleus. Dev Biol. 1973 Sep;34(1):77–92. doi: 10.1016/0012-1606(73)90340-0. [DOI] [PubMed] [Google Scholar]
- Graves J. A. DNA synthesis in heterokaryons formed by fusion of mammalian cells from different species. Exp Cell Res. 1972 Jun;72(2):393–403. doi: 10.1016/0014-4827(72)90007-9. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. On the origin and persistence of a cytoplasmic state inducing nuclear DNA synthesis in frogs' eggs. Proc Natl Acad Sci U S A. 1967 Aug;58(2):545–552. doi: 10.1073/pnas.58.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., Mullinger A. M. The induction of DNA synthesis in the chick red cell nucleus in heterokaryons during the first cell cycle after fusion with HeLa cells. J Cell Sci. 1975 Aug;18(3):455–490. doi: 10.1242/jcs.18.3.455. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Rao P. N. Nucleo-cytoplasmic interactions in the acheivement of nuclear synchrony in DNA synthesis and mitosis in multinucleate cells. Biol Rev Camb Philos Soc. 1971 Feb;46(1):97–155. doi: 10.1111/j.1469-185x.1971.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. The cell cycle and the control of cellular reproduction. Adv Genet. 1976;18:99–177. doi: 10.1016/s0065-2660(08)60438-1. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970 Jan 10;225(5228):159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- Rao P. N. Mitotic synchrony in mammalian cells treated with nitrous oxide at high pressure. Science. 1968 May 17;160(3829):774–776. doi: 10.1126/science.160.3829.774. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Smith M. L. Regulation of DNA synthesis in cytochalasin B (CB)-induced binucleate HeLa cells. Exp Cell Res. 1976 Nov;103(1):213–218. doi: 10.1016/0014-4827(76)90257-3. [DOI] [PubMed] [Google Scholar]


