Abstract
Cases of invasive mycosis due to Blastobotrys serpentis and B. proliferans identified by sequencing in a preterm patient and a rhabdomyosarcoma patient, respectively, are reported. Both species revealed elevated fluconazole and echinocandin MICs by the CLSI broth microdilution method. Additionally, B. serpentis exhibited high amphotericin B MICs, thus posing serious therapeutic challenges.
CASE REPORTS
Case 1.
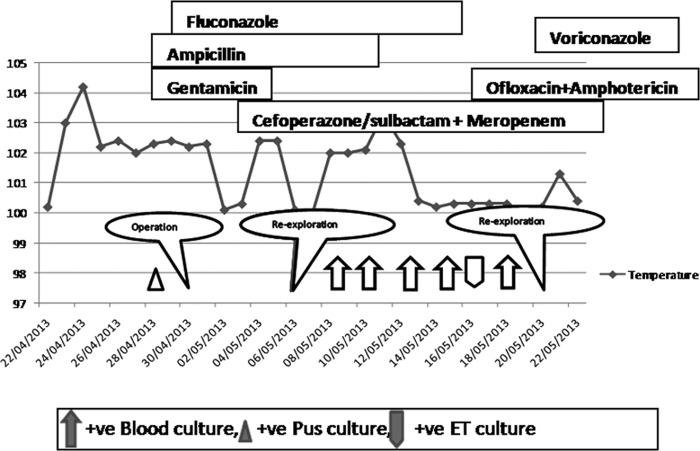
A 29-week gestation preterm male infant at birth was admitted on 24 April 2013 because of respiratory distress. The infant was born by normal vaginal delivery to a 24-year-old primigravida mother. On admission at day 1, the child was not active and had tachypnoea with a respiratory rate of 68/min and his chest X-ray showed mild haziness. Examination of the cardiovascular system revealed no abnormal heart sounds and equal bilateral peripheral pulses. The abdomen was soft, with no organomegaly noted. On day 5, a loud murmur was detected and an echocardiography revealed a large patent ductus arteriosus of 2.2 mm in size which was surgically closed. His total leukocyte count was elevated (24,000 cells/μl), and his C-reactive protein level was 0.1 mg/liter. Two days later, the infant developed abdominal distension and the X-ray revealed a pneumoperitoneum. This was managed with a peritoneal drainage followed by laparotomy. During surgery, a gangrenous gastric fundus with a sloughed greater curvature, leading to an approximately 40% to 45% loss of stomach volume, was seen. The necrotic tissues were removed; gastric anastomosis was done with placement of a gastrojejunal feeding tube via gastrotomy. The child underwent operation twice again, on days 14 and 29 of admission, because of anastomotic leakage and perforation due to a gangrenous bowel. Histopathological examination of tissue biopsy specimens of the distal ileum, terminal ileum, and stoma showed active inflammatory changes, but no granuloma and ganglion cells were present, ruling out Hirschsprung disease. The child was empirically treated with ampicillin and gentamicin starting from day 1 of admission along with prophylactic fluconazole (3 mg/kg of body weight twice weekly) due to a high risk for invasive candidiasis. Further during the course of treatment, meropenem therapy was included on day 3, cefoperazone-sulbactam therapy a week later, and ofloxacin therapy on day 17. The clinical course and the therapy instituted are depicted in Fig. 1. Cultures of the discharge from the laprotomy incision site on day 15 of admission grew yeasts identified as Stephanoascus ciferrii (identification, 86%) by the use of a Vitek2 yeast ID system (bioMérieux, Marcy l'Etoile, France) which exhibited a high fluconazole MIC (>64 μg/ml) by AST-YS06 (bioMérieux). Blood cultures in Bactec Peds Plus/F vials taken on days 16, 18, 21, 23, and 26 of admission grew fluconazole-resistant S. ciferrii after 2 to 3 days of incubation at 37°C. The endotracheal aspirate collected on day 23 also grew S. ciferrii. The patient had already received fluconazole (6 mg/kg) for 17 days; the treatment was then changed to amphotericin B (1.5 mg/kg) due to clinical deterioration. Since blood culture results continued to be positive, voriconazole (2 mg/kg twice daily) was added on day 5 to the amphotericin B therapy. However, the neonate succumbed to the complications of fungemia on day 31 of admission.
FIG 1.
The drugs administered and the clinical course of a case 1 neonate with invasive mycosis due to Blastobotrys serpentis. +ve, positive; ET, endotracheal aspirate.
Case 2.
An 8-year-girl was referred to a Regional Cancer Centre after being diagnosed with rhabdomyosarcoma. The biopsy specimen from the nasal cavity showed a malignant round-cell neoplasm consistent with rhabdomyosarcoma infiltrating the respiratory mucosa and glandular tissue. During the course of chemotherapy, a peripherally inserted central catheter (PICC) was placed for administering chemotherapeutic drugs. Two months after PICC line insertion, the patient developed fever without any localizing features. Her blood culture sent in a BacT/Alert bottle grew a Candida species. The isolate was misidentified as Stephanoascus ciferrii with a low discrimination profile by the use of a Vitek2 yeast ID system. The patient's PICC line was removed, and oral fluconazole (10 mg/kg/day) was given for 2 weeks. Her fever subsided, and she was progressing well after 6 months of follow-up.
Mycological investigations.
The isolates originating from peritoneal and blood culture from case 1 (accession numbers VPCI 568/P/13 and VPCI 569/P/13) and blood culture from case 2 (accession number VPCI 573/P/13) were sent to the Mycology Reference Laboratory, V. P. Chest Institute, Delhi, India. They were identified as Blastobotrys serpentis and Blastobotrys proliferans from case 1 and case 2, respectively. A brief description of their morphological, biochemical, and molecular identification is given below.
Morphological and biochemical characteristics of Blastobotrys spp. (i) Blastobotrys serpentis.
The clinical isolates (VPCI 568/P/13 and VPCI 569/P/13), along with the type strain of B. serpentis (CBS 10541T), revealed wrinkled white yeast-like colonies which showed multilateral budding and occasionally spherical yeast cells after 4 days of incubation at 28°C on potato dextrose agar (PDA). They revealed abundant, well-differentiated pseudohyphae with blastoconidia after 5 days of incubation at 28°C on rice Tween 80 agar (Fig. 2a). Septate hyphae were not observed. Also, the isolates grew in the presence of 0.01% cycloheximide and grew weakly on 50% glucose whereas the growth was inhibited on 6.5% NaCl. The carbon and nitrogen assimilation profiles of the isolates were determined by the microplate technique with yeast nitrogen base and yeast carbon base (Difco) (http://www.cbs.knaw.nl/yeast), respectively. The microtiter plates containing the medium were inoculated with 50 μl of yeast inoculum (1:10 MacFarland standard no. 2) into each well and incubated at 25°C for 3 to 5 days without agitation. The readings were taken by spectrophotometer (SpectraMax Plus; Molecular Devices) at 405 nm after shaking. Table 1 lists the assimilation profiles of Blastobotrys serpentis isolates. Notably, Blastobotrys serpentis clinical isolates, along with the type strain, grew at 37°C and 40°C.
FIG 2.
(a) Slide cultures on malt extract agar of 5 days growth at 28°C of the Blastobotrys serpentis (VPCI 568/P/13) isolate collected from blood culture of case 1, showing well-differentiated pseudohyphae with abundant blastoconidia. Septate hyphae were not observed. Magnification, ×1,000. (b) Slide cultures on malt extract agar of 7 days growth at 28°C of the Blastobotrys proliferans (VPCI 573/P/13) isolate collected from blood culture of case 2, showing conidiophores bearing pear-shaped mother cells and crowned with secondary conidia (downward arrow) and lateral conidia (upward arrows). Magnification, ×400. (c) Thick-walled, terminal, and intercalary chlamydospores (arrows). Magnification, ×1,000.
TABLE 1.
Assimilation profile and growth characteristics of Blastobotrys serpentis and B. proliferans
| Characteristic or condition | Resulta |
|
|---|---|---|
| B. serpentis (n = 2 sources [peritoneal wound swab, blood]) | B. proliferans (n = 1 source [blood]) | |
| Carbon assimilation | ||
| d-Glucose | + | + |
| d-Galactose | + | + |
| l-Sorbose | + | + |
| N-Acetyl-glucosamine | + | + |
| d-Ribose | + | − |
| d-Xylose | + | + |
| l-Arabinose | + | + |
| l-Rhamnose | + | + |
| Sucrose | + | + |
| d-Maltose | + | + |
| d-Trehalose | + | + |
| d-Cellobiose | + | + |
| d-Melibiose | − | + |
| d-Mannose | + | + |
| d-Melezitose | − | − |
| Lactose | − | ± |
| d-Sorbitol | + | + |
| Raffinose | + | + |
| Gentiobiose | + | + |
| Palatinose | + | + |
| d-Melezitose | − | − |
| Glycerol | − | − |
| Erythritol | + | + |
| Xylitol | + | + |
| Inositol | + | + |
| d-Gluconate | + | + |
| l-Lactate | + | + |
| Citrate | − | − |
| l-Malate | + | + |
| d-Galactouronate | + | + |
| Acetate | + | + |
| Urease | − | + |
| Glucuronate | + | + |
| 2-Keto-d-gluconate | + | |
| Nitrogen assimilation | − | |
| Nitrate | − | − |
| l-Lysine | − | + |
| Leucine | + | + |
| Arginine | + | ± |
| Tyrosine | − | + |
| l-Proline | + | |
| Growth under different conditions | + | |
| 37°C | + | + |
| 40°C | + | + |
| 0.01% cycloheximide | + | − |
| 0.1% cycloheximide | − | Wc |
| 50% glucose | W | − |
| 6.5% NaCl | − | |
+, growth; −, no growth; W, weak growth.
(ii) Blastobotrys proliferans.
The VPCI 573/P/13 isolate showed dry, hairy, white colonies on potato dextrose agar (PDA) after 4 days of incubation at 28°C. Slide cultures of 7 days growth on malt extract agar at 28°C showed conidiophores bearing pear-shaped mother cells (Fig. 2b). The mother cells were single, and each was crowned with secondary conidia (Fig. 2b). In addition, globose, lateral conidia and hyaline, thick-walled terminal and intercalary chlamydospores were observed (Fig. 2c). Like B. serpentis, B. proliferans assimilated sugars such as d-glucose, d-galactose, d-xylose, maltose, etc., but failed to assimilate nitrate (Table 1). However, unlike B. serpentis, this species grew in the presence of melibiose. Also, the isolate revealed growth at 37°C.
In vitro antifungal susceptibility testing.
In vitro antifungal susceptibility testing of all isolates was done using the Clinical and Laboratory Standards Institute broth microdilution method, following the CLSI M27-A3 guidelines (1). The antifungals tested were amphotericin B (Sigma, St. Louis, MO), fluconazole (Pfizer, Groton, CT), itraconazole (Lee Pharma, Hyderabad, India), voriconazole (Pfizer), posaconazole (Merck, Whitehouse Station, NJ), isavuconazole (Basilea Pharmaceutica, Basel, Switzerland), 5-flucytosine (Sigma), caspofungin (Merck), micafungin (Astellas, Toyama, Japan), and anidulafungin (Pfizer). Candida krusei (ATCC 6258) and Candida parapsilosis (ATCC 22019) were used as quality control strains. The MIC endpoints were defined as the lowest drug concentrations that caused a prominent (50%) decrease in growth vis-à-vis the controls and were read visually after 24 h for azoles and echinocandins as recently validated by Pfaller et al. (2, 3, 4). For amphotericin B, the MIC was defined as the lowest concentration at which there was 100% inhibition of growth compared with the drug-free control well results. The in vitro antifungal susceptibility data for both the species are presented in Table 2. Blastobotrys serpentis (VPCI 568/P/13 and VPCI 569/P/13) isolates exhibited high fluconazole, amphotericin B, caspofungin, micafungin, and anidulafungin MICs. They had low isavuconazole, itraconazole, voriconazole, 5-flucytosine, and posaconazole MICs. However, B. proliferans VPCI 573/P/13 revealed reduced susceptibility to all the antifungals tested except amphotericin B (MIC, 0.125 μg/ml).
TABLE 2.
In vitro antifungal susceptibility profiles of the Blastobotrys species isolates
| Species identified | MIC (μg/ml)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMB | FLU | ITC | VRC | ISA | POS | CAS | MFG | AFG | 5-FC | |
| B. serpentis VPCI 568/P/13 | 2 | 32 | 0.25 | 0.5 | 0.25 | 0.5 | 1 | 0.25 | 0.5 | 0.25 |
| B. serpentis VPCI 569/P/13 | 1 | 32 | 0.25 | 0.25 | 0.125 | 0.25 | 1 | 0.25 | 0.5 | 0.5 |
| B. proliferans VPCI 573/P/13 | 0.125 | 64 | 1 | 4 | 4 | 2 | 4 | 1 | 4 | 32 |
Abbreviations used: AMB, amphotericin B; FLU, fluconazole; ITC, itraconazole; VRC, voriconazole; ISA, isavuconazole; POS, posaconazole; CAS, caspofungin; MFG, micafungin; AFG, anidulafungin; 5-FC, flucytosine.
Sequencing of ITS and D1/D2 regions.
Genomic DNA was extracted from all of the test yeast isolates as described by Xu et al. (5). Extracted DNA was amplified using the ITS-1 (5′-TCCGTAGGTGAACCTTGCGG-3′) and ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) primers, which amplify the internal transcribed spacer (ITS) region of the ribosomal subunit, and the NL-1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL-4 (5′-GGTCCGTGTTTCAAGACGG-3′) primers, which amplify the ∼600-bp D1/D2 region of the large ribosomal subunit (6, 7). Amplified DNA was sequenced in both strands on an ABI 3130XL Genetic Analyzer (Applied Biosystems, Foster City, CA) using a BigDye Terminator kit, v3.1 (Applied Biosystems). Sequences were aligned using Sequencing Analysis 5.3.1 software (Applied Biosystems). GenBank basic local alignment search tool (BLAST) searches (http://www.ncbi.nlm.nih.gov/BLAST/Blast.cgi) were performed for species identification. ITS and D1/D2 region sequences of the two isolates VPCI 568/P/13 and VPCI 569/P/13 from case 1 showed 99% homology (query coverage, 99%) with Candida sp. strain YS W113A (GenBank accession no. AM410670 [ITS] and AM410667 [D1/D2]), which was named species nov. Blastobotrys serpentis in 2008 (8). However, the ITS and D1/D2 region sequences of isolate VPCI 573/P/13 showed 99% homology (query coverage, 99%) with those of Blastobotrys proliferans CBS 522.75T (accession no. EU343812 [ITS] and DQ442684 [D1/D2]).
For phylogenetic analyses, the D1/D2 region sequences of the type and reference strains of B. serpentis CBS 10541T and W113B and B. proliferans CBS 522.75T, along with those of B. chiropterorum CBS 6064T and Trichomonascus ciferrii CBS 5295 (synonym Stephanoascus ciferrii), were retrieved from the NCBI database. The sequences of the test and reference isolates were aligned using the ClustalW program (version 1.82) (9), and the final alignments were edited manually. A maximum-likelihood tree based on D1/D2 gene sequences using 2,000 bootstrap replications was constructed using MEGA version 5 (10). The phylogenetic tree enabled the differentiation of B. serpentis and B. proliferans from each other and from the closely related species B. chiropterorum with a good bootstrap support value (99%). Trichomonascus ciferrii was also classified apart from clusters of both of the species (Fig. 3).
FIG 3.
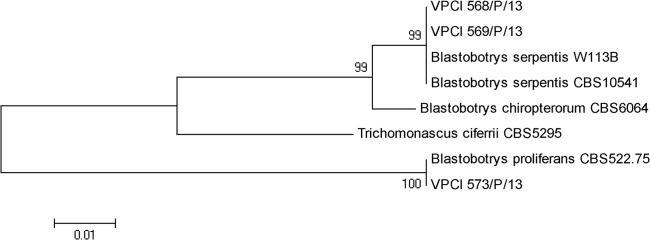
Phylogenetic tree based on D1/D2 sequences of the type/reference strains of B. serpentis CBS 10541T and W113B, B. proliferans CBS 522.75T, B. chiropterorum CBS 6064T, and Trichomonascus ciferrii CBS 5295, obtained by using maximum-likelihood phylogenetic analyses with 2,000 bootstrap replications.
Fungal infections due to species of Blastobotrys are extremely rare, and a solitary case of peritonitis due to B. proliferans in a patient undergoing continuous ambulatory peritoneal dialysis is on record (11). Recently, the role of B. rhaffinosifermentans causing a significant airway inflammation in a cystic fibrosis patient before lung transplant and pleural space infection post-lung transplant was highlighted (12). The genus Blastobotrys von Klopotek (1967) was originally treated as a hyphomycete, and it was believed to be closely related to the genus Sporothrix, from which it was distinguished on the basis of morphological criteria (13). On the basis of the D1/D2 large-subunit (LSU) rRNA gene sequences, Kurtzman and Robnett placed the genus Blastobotrys in the saccharomycetales and suggested that Blastobotrys, Arxula, and Sympodiomyces may represent the same anamorphic genus (14). Later, this suggestion was confirmed using multilocus analysis and the species Arxula and Sympodiomyces were therefore transferred to the Blastobotrys genus (teleomorph Trichomonascus) (14). The two species of Blastobotrys reported here, viz., B. serpentis and B. proliferans, were initially misidentified as Stephanoascus ciferrii by the use of a Vitek2 system. Kurtzman and Robnett, by using multigene analysis in 2007, showed that Stephanoascus cifferii (synonym Candida cifferii) is in the Trichomonascus clade, and the genus Stephanoascus became a synonym of Trichomonascus (14). However, this taxonomic yeast nomenclature change has clearly not been updated in the Vitek2 system database.
Case 1 reported here involved a severely immunocompromised neonate who had undergone multiple surgeries due to intestinal perforation and developed persistent fungemia due to B. serpentis, which was isolated from multiple blood cultures, peritoneal drainage, and endotracheal aspirate. Blastobotrys serpentis has never previously been reported as an etiologic agent of invasive mycoses in humans. However, the species was first described in 2008 as a novel member of genus Blastobotrys isolated from the intestine of a dead trinket snake in India (8). Notably, both species of Blastobotrys reported here, i.e., B. proliferans and B. serpentis, had reduced susceptibility to commonly used antifungals, i.e., fluconazole and caspofungin. Additionally, B. serpentis isolates had relatively high anidulafungin (0.5 μg/ml) and micafungin (0.25 μg/ml) MICs, considering that the recent epidemiological cutoff values for 5 Candida species, barring C. parapsilosis, for both the echinocandins range from 0.03 to 0.25 μg/ml (4). Furthermore, B. serpentis also exhibited high amphotericin B MICs, thus raising serious concerns about the therapeutic options for patients with Blastobotrys infections. Due to the rarity of clinical cases of infection by Blastobotrys, there is paucity of information on the susceptibility data of this genus. In the present report of case 1, the newborn developed breakthrough fungemia due to B. serpentis while he was on fluconazole prophylaxis and could not be successfully treated with amphotericin B, for which the isolate had high MICs. The neonate had all the risk factors reported for development of candidemia, including prematurity, prolonged antibiotic treatment, total parenteral nutrition, mechanical ventilation, and an extended stay in the neonatal intensive care unit (15). Case 2 with fungemia due to B. proliferans probably responded to removal of the PICC line. Although the patient was also treated with fluconazole, the isolate showed a high (64 μg/ml) fluconazole MIC. Previously, Quirin et al. reported that B. proliferans isolated from a case of peritonitis showed decreased susceptibility to all antifungals tested, except amphotericin B (11). Similarly to our experience, the removal of the Tenckhoff catheter and administration of oral fluconazole resulted in a successful outcome in the case reported by Quirin et al. (11). Notably, B. rhaffinosifermentans also had reduced susceptibility to fluconazole in a recently reported case (12). The patient was treated successfully with aggressive antifungal therapy, including multiple courses of intravenous caspofungin, oral voriconazole, and nebulized l-amphotericin B extending for 22 months (12). The sources of both Blastobotrys species in the present cases remain enigmatic, especially in the newborn, who possibly acquired the infection while in the hospital, whereas in case 2, the child may have obtained the fungus from her surroundings, as B. proliferans has been previously isolated from garden soil and house dust (13). However, B. serpentis has so far been reported to have been isolated only from the gut of a dead snake in a zoo in India, suggesting that the yeasts of the genus Blastobotrys may be acquired by humans and animals from the environment (8).
Of the 21 species of Blastobotrys previously described in a taxonomic study of yeasts (13), 12 were reported to grow at 37°C, and these three species, namely, B. proliferans, B. chiropterorum, and B. rhaffinosifermentans, have been isolated from humans (11–13, 16). Notably, a report in the latest edition of a monograph describing yeasts (13) asserting that growth of the B. serpentis type strain (CBS 10541) is negative at 37°C is erroneous, as it, along with the two clinical strains (VPCI 568/P/13 and VPCI 569/P/13), showed luxuriant growth at 37°C in the present study. Furthermore, species of the genus Blastobotrys reveal diverse morphological and biochemical characteristics and are therefore difficult to identify by standard mycological procedures in routine laboratories. Additionally, they exhibit reduced susceptibility to antifungals. Therefore, any significant clinical yeast isolate originating from sterile sites which is identified as Stephanoascus ciferrii by the Vitek2 system should be evaluated further by molecular techniques and in vitro antifungal susceptibility testing should be performed. Finally, the emergence of Blastobotrys spp. as opportunistic fungal pathogens capable of causing invasive disease, with reduced susceptibility to antifungals, is noteworthy, as it poses serious diagnostic and therapeutic challenges.
Nucleotide sequence accession numbers.
VPCI 568/P/13, VPCI 569/P/13, and VPCI 573/P/13 ITS sequences were submitted to GenBank with accession numbers KM056339, KM056340, and KM056341, respectively, and LSU sequences with accession numbers KM056336, KM056337, and KM056338, respectively. The 2 strains B. serpentis VPCI 568/P/13 and B. proliferans VPCI 573/P/13 were deposited in the CBS-KNAW Fungal Biodiversity Centre, The Netherlands, with accession numbers CBS 13871 and CBS 13872, respectively.
ACKNOWLEDGMENTS
J.F.M. received grants from Astellas, Basilea, and Merck. He has been a consultant to Astellas, Basilea, and Merck and received speaker's fees from Merck and Gilead. All of the other authors report no potential conflicts of interest.
We alone are responsible for the content and writing of the paper.
Footnotes
Published ahead of print 27 August 2014
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts—. 3rd ed: approved standard M27-A3. CLSI, Wayne, PA. [Google Scholar]
- 2.Pfaller MA, Boyken LB, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2008. Validation of 24-hour fluconazole MIC readings versus the CLSI 48-hour broth microdilution reference method: results from a global Candida antifungal surveillance program. J. Clin. Microbiol. 46:3585–3590. 10.1128/JCM.01391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfaller MA, Boyken LB, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2011. Validation of 24-hour posaconazole and voriconazole MIC readings versus the CLSI 48-hour broth microdilution reference method: application of epidemiological cutoff values to results from a global Candida antifungal surveillance program. J. Clin. Microbiol. 49:1274–1279. 10.1128/JCM.02437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller M, Boyken L, Hollis R, Kroeger J, Messer S, Tendolkar S, Diekema D. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Candida species to anidulafungin, caspofungin, and micafungin. J. Clin. Microbiol. 49:624–629. 10.1128/JCM.02120-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Ramos AR, Vilgalys R, Mitchell TG. 2000. Clonal and spontaneous origins of fluconazole resistance in Candida albicans. J. Clin. Microbiol. 38:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, 315–322 In Innis MA, Gelfand DH, Sninsky JJ, White TJ. (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 7.Kurtzman CP, Robnett CJ. 1997. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 35:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhadra B, Singh PK, Rao RS, Shivaji S. 2008. Blastobotrys serpentis sp. nov., isolated from the intestine of a Trinket snake (Elaphe sp., Colubridae). FEMS Yeast Res. 8:492–498. 10.1111/j.1567-1364.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 9.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 10.Tamura K, Peterson D, Peterson N, Stetcher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quirin N, Desnos-Ollivier M, Cantin JF, Valery JC, Doussy Y, Goursaud R, Dromer F, Tivollier JM. 2007. Peritonitis due to Blastobotrys proliferans in a patient undergoing continuous ambulatory peritoneal dialysis. J. Clin. Microbiol. 45:3453–3455. 10.1128/JCM.00967-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong JY, Chambers AL, Fuller J, Lacson A, Mullen J, Lien D, Humar A. 2014. Successful lung transplant in a child with cystic fibrosis and persistent Blastobotrys rhaffinosifermentans infection. Pediatr. Transplant. 18:E169–E173. 10.1111/petr.12294. [DOI] [PubMed] [Google Scholar]
- 13.Smith MT, de Hoog GS, Statzell-Tallman A, Kurtzman CP. 2011. Blastobotrys von Klopotek, p 959–977 In Kurtzman CP, Fell JW, Boekhout T. (ed), The yeasts, a taxonomic study, 5th ed. Elsevier Science BV, Amsterdam, The Netherlands. [Google Scholar]
- 14.Kurtzman CP, Robnett CJ. 2007. Multigene phylogenetic analysis of the Trichomonascus, Wickerhamiella and Zygoascus yeast clades, and the proposal of Sugiyamaella gen. nov. and 14 new species combinations. FEMS Yeast Res. 7:141–151. 10.1111/j.1567-1364.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 15.Burwell LA, Kaufman D, Blakely J, Stoll BJ, Fridkin SK. 2006. Antifungal prophylaxis to prevent neonatal candidiasis: a survey of perinatal physician practices. Pediatrics 118:e1019–e1026. 10.1542/peds.2006-0446. [DOI] [PubMed] [Google Scholar]
- 16.Furman RM, Ahearn DG. 1983. Candida ciferrii and Candida chiropterorum isolated from clinical specimens. J. Clin. Microbiol. 18:1252–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]