Abstract
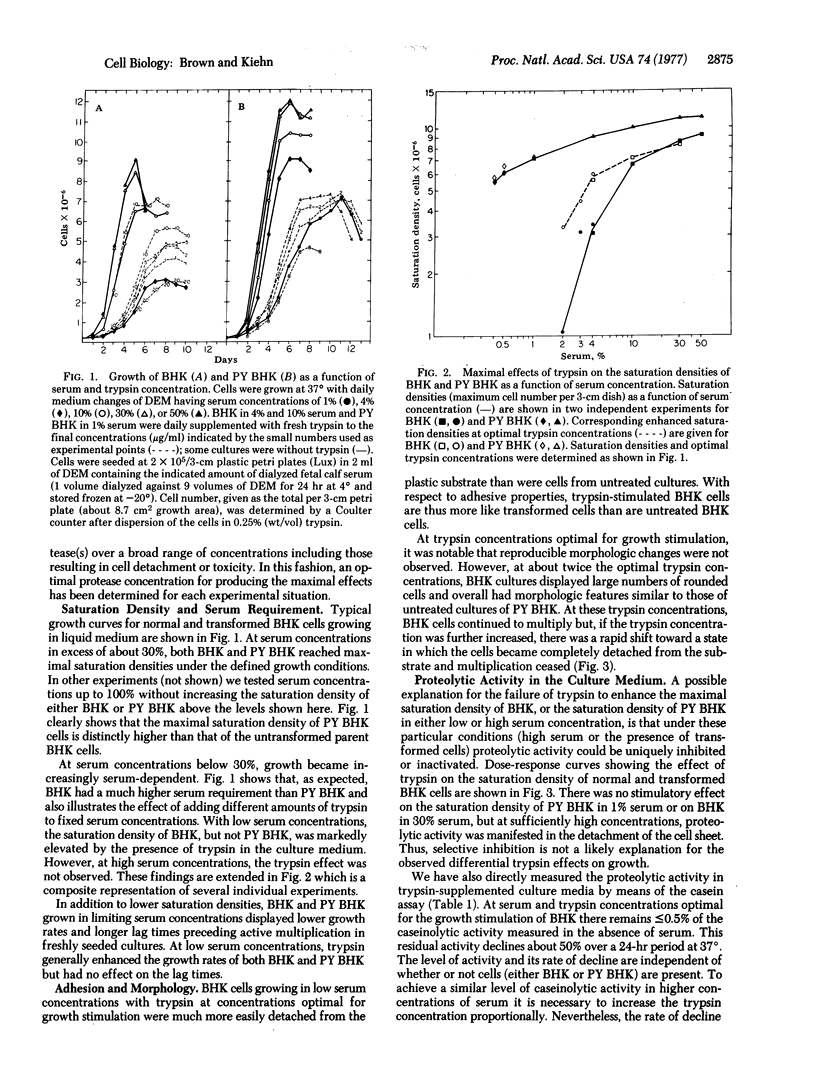
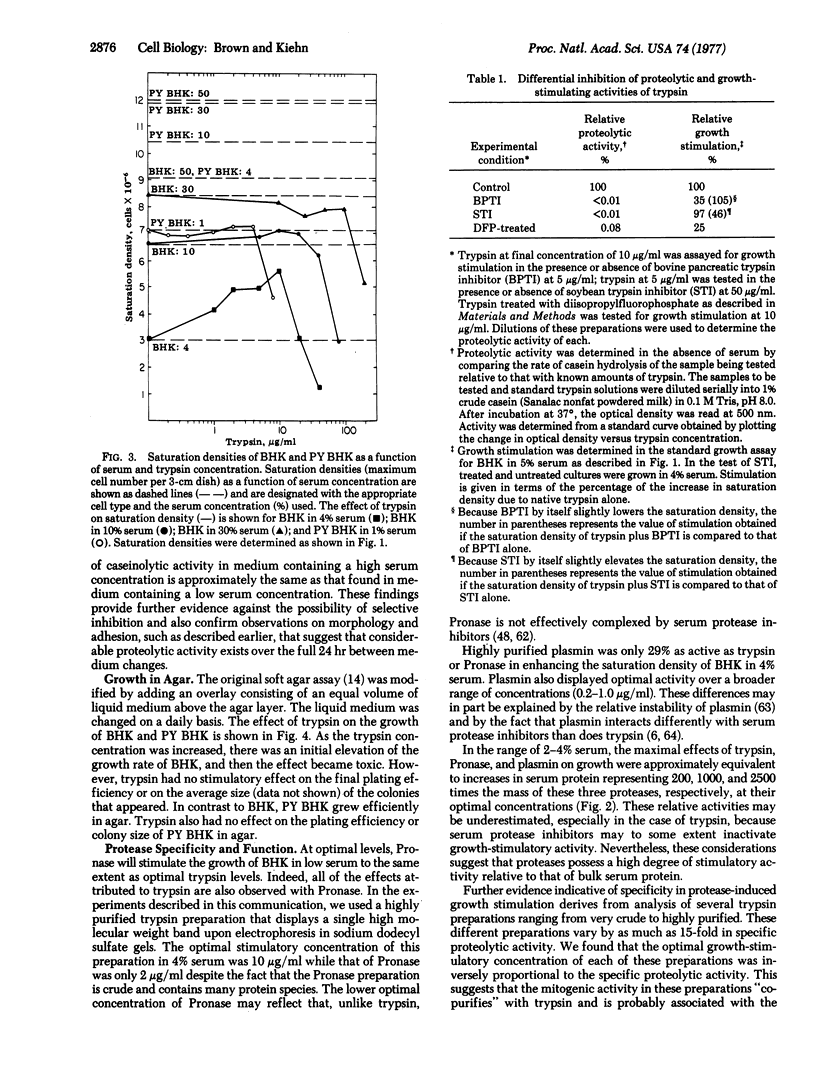
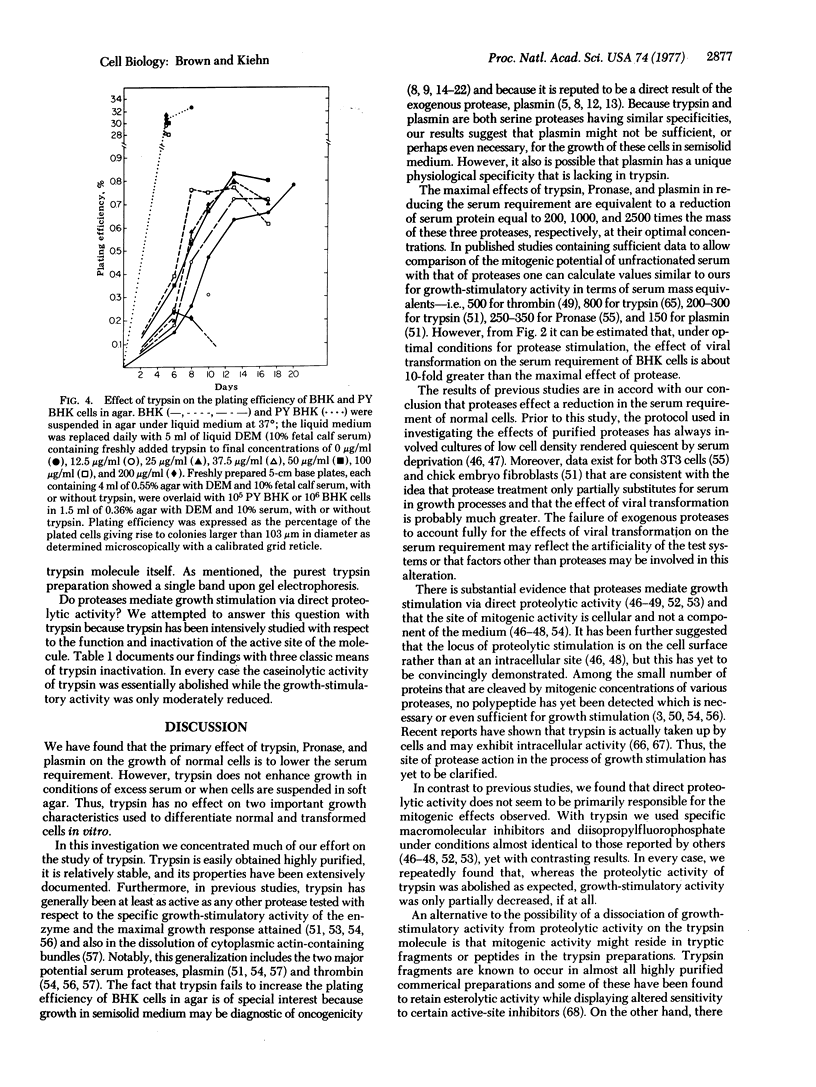
We have tested the effects of exogenous proteases on the growth of normal and transformed hamster fibroblasts in the classic culture assays for transformation. The results indicate that exogenous proteases act to decrease the serum requirement of normal cells but not nearly to the extent that occurs in the process of viral transformation. Proteases do not further decrease the serum requirement of transformed cells, nor do they affect the maximal saturation density or the plating efficiency in soft agar of either normal or transformed cells. Under conditions optimal for growth stimulation, proteases decrease the strength of cell-to-substrate adhesion but do not affect cellular morphology. In contrast to previous studies, experiments using highly purified trypsin and several different active-site inhibitors strongly suggest that the growth-stimulatory activity of trypsin is not directly related to the proteolytic activity of the molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Von Kaulla K. N. Dissimilarity of human vascular plasminogen activator and human urokinase. J Lab Clin Med. 1971 Sep;78(3):354–362. [PubMed] [Google Scholar]
- Astrup T. Tissue activators of plasminogen. Fed Proc. 1966 Jan-Feb;25(1):42–51. [PubMed] [Google Scholar]
- Beers W. H., Strickland S., Reich E. Ovarian plasminogen activator: relationship to ovulation and hormonal regulation. Cell. 1975 Nov;6(3):387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Bernik M. B., Kwaan H. C. Plasminogen activator activity in cultures from human tissues. An immunological and histochemical study. J Clin Invest. 1969 Sep;48(9):1740–1753. doi: 10.1172/JCI106140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Robbins P. W. Effect of proteases on activation of resting chick embryo fibroblasts and on cell surface proteins. Cell. 1975 Oct;6(2):137–147. doi: 10.1016/0092-8674(75)90004-5. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- CLIFFTON E. E., GROSSI C. E. Fibrinolytic activity of human tumors as measured by the fibrin-plate method. Cancer. 1955 Nov-Dec;8(6):1146–1154. doi: 10.1002/1097-0142(1955)8:6<1146::aid-cncr2820080610>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ceccarini C., Eagle H. pH as a determinant of cellular growth and contact inhibition. Proc Natl Acad Sci U S A. 1971 Jan;68(1):229–233. doi: 10.1073/pnas.68.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibber B. A., Niles R. M., Prehn L., Sorof S. High extracellular fibrinolytic activity of tumors and control normal tissues. Biochem Biophys Res Commun. 1975 Jul 22;65(2):806–812. doi: 10.1016/s0006-291x(75)80216-6. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Silagi S., Newcomb E. W., Silverstein S. C., Acs G. Correlated suppression by 5-bromodeoxyuridine of tumorigenicity and plasminogen activator in mouse melanoma cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):47–50. doi: 10.1073/pnas.72.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman J. K., Silverstein S. C., Acs G. Immunological analysis of plasminogen activators from normal and transformed hamster cells. Evidence that the plasminogen activators produced by SV40 virus-transformed hamster embryo cells and normal hamster lung cells are antigenically identical. J Exp Med. 1975 Aug 1;142(2):419–434. doi: 10.1084/jem.142.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Eagle H., Foley G. E., Koprowski H., Lazarus H., Levine E. M., Adams R. A. Growth characteristics of virus-transformed cells. Maximum population density, inhibition by normal cells, serum requirement, growth in soft agar, and xenogeneic transplantability. J Exp Med. 1970 Apr 1;131(4):863–879. doi: 10.1084/jem.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. F., Aftonomos B. The use of pronase in tissue culture: a comparison with trypsin. J Cell Physiol. 1970 Apr;75(2):159–161. doi: 10.1002/jcp.1040750204. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Hale A. H., Weber M. J. Hydrolase and serum treatment of normal chick embryo cells: effects on hexose transport. Cell. 1975 Jul;5(3):245–252. doi: 10.1016/0092-8674(75)90099-9. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- Hodges G. M., Livingston D. C., Franks L. M. The localization of trypsin in cultured mammalian cells. J Cell Sci. 1973 May;12(3):887–902. doi: 10.1242/jcs.12.3.887. [DOI] [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- Hovi T., Vaheri A. Reversible release of chick embryo fibroblast cultures from density dependent inhibition of growth. J Cell Physiol. 1975 Dec;87(2):245–252. doi: 10.1002/jcp.1040870213. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Laug W. E., Gardner A., Nye C. A., Fink L. M., Benedict W. F. In vitro correlates of transformation in C3H/10T1/2 clone 8 mouse cells. Cancer Res. 1976 Aug;36(8):2863–2867. [PubMed] [Google Scholar]
- Kakunaga T., Kamahora J. Properties of hamster embryonic cells transformed by 4-nitroquinoline-1-oxide in vitro and their correlations with the malignant properties of the cells. Biken J. 1968 Dec;11(4):313–332. [PubMed] [Google Scholar]
- Kucinski C. S., Fletcher A. P., Sherry S. Effect of urokinase antiserum on plasminogen activators: demonstration of immunologic dissimilarity between plasma plasminogen activator and urokinase. J Clin Invest. 1968 Jun;47(6):1238–1253. doi: 10.1172/JCI105816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LADEHOFF A. The fibrinolytic activity of tumors of the kidney and bladder. Acta Pathol Microbiol Scand. 1962;55:273–280. doi: 10.1111/j.1699-0463.1962.tb04126.x. [DOI] [PubMed] [Google Scholar]
- Laug W. E., Jones P. A., Benedict W. F. Relationship between fibrinolysis of cultured cells and malignancy. J Natl Cancer Inst. 1975 Jan;54(1):173–179. doi: 10.1093/jnci/54.1.173. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Maizel A., Nicolini C., Baserga R. Effect of cell trypsinization on nuclear proteins of WI-38 fibroblasts in culture. J Cell Physiol. 1975 Aug;86(1):71–82. doi: 10.1002/jcp.1040860109. [DOI] [PubMed] [Google Scholar]
- Mak T. W., Rutledge G., Sutherland D. J. Androgen-dependent fibrinolytic activity in a murine mammary carcinoma (Shionogi SC-115 cells) in vitro. Cell. 1976 Feb;7(2):223–226. doi: 10.1016/0092-8674(76)90021-0. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Reed G. Colonial growth in agar of cells derived from neoplastic and non-neoplastic tissues of children. Pediatr Res. 1968 Sep;2(5):356–360. doi: 10.1203/00006450-196809000-00004. [DOI] [PubMed] [Google Scholar]
- Mott D. M., Fabisch P. H., Sani B. P., Sorof S. Lack of correlation between fibrinolysis and the transformed state of cultured mammalian cells. Biochem Biophys Res Commun. 1974 Nov 27;61(2):621–627. doi: 10.1016/0006-291x(74)91002-x. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Noonan K. D. Role of serum in protease-induced stimulation of 3T3 cell division past the monolayer stage. Nature. 1976 Feb 19;259(5544):573–576. doi: 10.1038/259573a0. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Quigley J. P., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Requirement of plasminogen for correlated changes in cellular morphology, colony formation in agar, and cell migration. J Exp Med. 1973 Nov 1;138(5):1056–1064. doi: 10.1084/jem.138.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Nelson-Rees W. A., Springer E. L. Epithelial cell cultures from normal and cancerous human tissues. J Natl Cancer Inst. 1976 Apr;56(4):843–849. doi: 10.1093/jnci/56.4.843. [DOI] [PubMed] [Google Scholar]
- Pearlstein E., Hynes R. O., Franks L. M., Hemmings V. J. Surface proteins and fibrinolytic activity of cultured mammalian cells. Cancer Res. 1976 Apr;36(4):1475–1480. [PubMed] [Google Scholar]
- Peterson H. I., Kjartansson I., Korsan-Bengtsen K., Rudenstam C. M., Zettergren L. Fibrinolysis in human malignant tumours. Acta Chir Scand. 1973;139(3):219–223. [PubMed] [Google Scholar]
- Pollack R., Risser R., Conlon S., Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin R., Chou I. N., Black P. H. Proteolytic enzymes, cell surface changes, and viral transformation. Adv Cancer Res. 1975;22:203–260. doi: 10.1016/s0065-230x(08)60178-5. [DOI] [PubMed] [Google Scholar]
- Rubin H. pH and population density in the regulation of animal cell multiplication. J Cell Biol. 1971 Dec;51(3):686–702. doi: 10.1083/jcb.51.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford K. K. Biologic manifestations of oncogenesis in vitro: a critique. J Natl Cancer Inst. 1974 Nov;53(5):1481–1485. doi: 10.1093/jnci/53.5.1481. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. S., Owens R. B., Hiller A. J., Nelson-Rees W. A., Johnston J. O. The biology of human cells in tissue culture. I. Characterization of cells derived from osteogenic sarcomas. Int J Cancer. 1976 Feb 15;17(2):219–234. doi: 10.1002/ijc.2910170211. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Desmond W., Chuman L. M., Sato G., Saier M. H. Growth control of heterologous tissue culture cells in the congenitally athymic nude mouse. Cancer Res. 1976 Apr;36(4):1353–1360. [PubMed] [Google Scholar]
- Stiles C. D., Desmond W., Chuman L. M., Sato G., Saier M. H., Jr Relationship of cell growth behavior in vitro to tumorigenicity in athymic nude mice. Cancer Res. 1976 Sep;36(9 PT1):3300–3305. [PubMed] [Google Scholar]
- Stiles C. D., Desmond W., Jr, Sato G., Saier M. H., Jr Failure of human cells transformed by simian virus 40 to form tumors in athymic nude mice. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4971–4975. doi: 10.1073/pnas.72.12.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Bo Chen L. The role of surface proteins in cell proliferation as studied with thrombin and other proteases. Proc Natl Acad Sci U S A. 1975 Feb;72(2):413–417. doi: 10.1073/pnas.72.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Rucoslahti E., Nordling S. Neuraminidase stimulates division and sugar uptake in density-inhibited cell cultures. Nat New Biol. 1972 Aug 16;238(85):211–212. doi: 10.1038/newbio238211a0. [DOI] [PubMed] [Google Scholar]
- Weiss G., Beller F. K. Localization of fibrinolytic activity in uterine cancer. Am J Obstet Gynecol. 1969 Apr 1;103(7):1023–1027. doi: 10.1016/s0002-9378(16)34454-4. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Veselý P., Sindelarova J. Growth regulation and tumour formation of normal and neoplastic rat cells. Int J Cancer. 1973 Jan 15;11(1):77–89. doi: 10.1002/ijc.2910110109. [DOI] [PubMed] [Google Scholar]
- Wiblin C. N., MacPherson I. A. The transformation of BHK 21 hamster cells by simian virus 40. Int J Cancer. 1972 Sep 15;10(2):296–309. doi: 10.1002/ijc.2910100210. [DOI] [PubMed] [Google Scholar]
- Wolf B. A., Goldberg A. R. Rous-sarcoma-virus-transformed fibroblasts having low levels of plasminogen activator. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3613–3617. doi: 10.1073/pnas.73.10.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke J. Phenotypic variation and its control in polyoma-transformed BHK21 cells. Exp Cell Res. 1971 May;66(1):209–223. doi: 10.1016/s0014-4827(71)80031-9. [DOI] [PubMed] [Google Scholar]
- Zetter B. R., Chen L. B., Buchanan J. M. Effects of protease treatment on growth, morphology, adhesion, and cell surface proteins of secondary chick embryo fibroblasts. Cell. 1976 Mar;7(3):407–412. doi: 10.1016/0092-8674(76)90170-7. [DOI] [PubMed] [Google Scholar]


