Abstract
Classifications of the Campylobacter fetus subspecies fetus and venerealis were first described in 1959 and were based on the source of isolation (intestinal versus genital) and the ability of the strains to proliferate in the genital tract of cows. Two phenotypic assays (1% glycine tolerance and H2S production) were described to differentiate the subspecies. Multiple molecular assays have been applied to differentiate the C. fetus subspecies, but none of these tests is consistent with the phenotypic identification methods. In this study, we defined the core genome and accessory genes of C. fetus, which are based on the closed genomes of five C. fetus strains. Phylogenetic analysis of the core genomes of 23 C. fetus strains of the two subspecies showed a division into two clusters. The phylogenetic core genome clusters were not consistent with the phenotypic classifications of the C. fetus subspecies. However, they were consistent with the molecular characteristics of the strains, which were determined by multilocus sequence typing, sap typing, and the presence/absence of insertion sequences and a type I restriction modification system. The similarity of the genome characteristics of three of the phenotypically defined C. fetus subsp. fetus strains to C. fetus subsp. venerealis strains, when considering the core genome and accessory genes, requires a critical evaluation of the clinical relevance of C. fetus subspecies identification by phenotypic assays.
INTRODUCTION
Campylobacter fetus is an important veterinary pathogen and is associated with genital infections in cattle and sheep that result in abortion and infertility (1). Based on clinical and phenotypic observations, the species C. fetus was subdivided into two subspecies, C. fetus subsp. venerealis and C. fetus subsp. fetus (2). C. fetus subsp. venerealis was of venereal origin, had a strong ability to cause abortions, and persisted in the genital tracts of cattle and sheep, whereas C. fetus subsp. fetus was of intestinal origin, caused only sporadic abortions, and was cleared from the genital tract of the cow following the abortion. The two subspecies were phenotypically differentiated with the 1% glycine tolerance and H2S production tests; C. fetus subsp. fetus is positive in both tests (it is glycine tolerant and produces H2S), and C. fetus subsp. venerealis is negative in both tests (it does not grow in the presence of 1% glycine and does not produce H2S) (2). C. fetus strains that established themselves in the genital tract of a nonpregnant cow, like the venereal C. fetus subsp. venerealis strains, were isolated (3); however, the glycine resistance of these strains was reduced compared to that of most C. fetus subsp. fetus strains, and they were positive in the H2S test, like the intestinal C. fetus subsp. fetus strains (4). They were classified as an intermediate group (4) and designated Campylobacter fetus subsp. venerealis bv. Intermedius (1).
For molecular subspecies identification, several PCR assays have been described, but they lack specificity (5). The subspecies can be genetically differentiated with multilocus sequence typing (MLST) (6) and amplified fragment length polymorphism (AFLP) analyses (7), but these methods are laborious and therefore not useful for routine diagnostic methods. The rationale behind the differentiation between the C. fetus subspecies is the supposed difference in pathogenicity and disease epidemiology. C. fetus subsp. venerealis is described as the causative agent of bovine genital campylobacteriosis (BGC), a statutory disease in many countries of the world and listed by the World Organization for Animal Health (OIE); in contrast, C. fetus subsp. fetus is associated with sporadic abortions (8).
Comparative genomics of two C. fetus strains revealed several unique regions for both subspecies, as shown by Kienesberger et al. (9); C. fetus subsp. venerealis contained multiple unique regions representing insertion sequences and genomic islands with type IV secretion system components and phage-related/hypothetical proteins (9), and C. fetus subsp. fetus contained clustered regularly interspaced short palindromic repeat (CRISPR)-cas loci and unique genes involved in lipopolysaccharide (LPS) biosynthesis (9). These data suggest that C. fetus subspecies can be distinguished on genomic features, but comparative genomics of a larger set of C. fetus strains is lacking. The aim of this study is to characterize C. fetus strains of both subspecies based on whole-genome sequencing data and to compare the results of classification based on core and accessory genome analysis with the current C. fetus subspecies identification based on phenotypic assays.
MATERIALS AND METHODS
Bacterial strains: phenotyping and genotyping.
C. fetus strains (Table 1) were grown on heart infusion agar supplemented with 5% sheep blood (BioTrading, Mijdrecht, the Netherlands) for 2 days under microaerobic conditions (6% O2, 7% CO2, 7% H2, 80% N2, [Anoxomat; Mart Microbiology, Lichtenvoorde, the Netherlands]). The subspecies of the strains were phenotypically identified with the 1% glycine tolerance test and with a test of hydrogen sulfide (H2S) production in a medium with 0.02% cysteine-hydrochloride, as described previously (1). Molecular identification was performed with MLST (6) and AFLP analysis (7).
TABLE 1.
General characteristics of C. fetus strains
| Strain | Countrya | Source | Phenotypeb |
Genotypec |
Core genome cluster | Accessory genesd,e |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1% glycine tolerance | H2S production | Phenotypic ID | AFLP | MLST (ST) | sap type | Prophage in sap locus | GI with T4SS | IS | Type I RM system | CRISPR-cas | ||||
| 82-40 | USA | Human | + | + | Cff | Cff | 6 | B | A | − | − | − | +/− | + |
| 110800-21-2 | NL | Bovine (bull) | + | + | Cff | Cff | 2 | B | A | − | + | − | +/− | − |
| BT 10/98 | UK | Ovine | + | + | Cff | Cff | 2 | B | A | − | − | − | +/− | − |
| 04/554 | AR | Bovine (fetus) | + | + | Cff | Cff | 5 | B | B | − | − | − | − | − |
| 98/v445 | UK | Bovine (bull) | + | + | Cff | Cff | 3 | B | B | − | + | − | − | + |
| 03/293 | AR | Bovine (fetus) | + | + | Cff | Cfvi | 4 | A | A | − | + | + | + | − |
| ADRI 1362 | AR | Bovine | + | + | Cff | Cfvi | 4 | A | A | + | + | + | + | − |
| Zaf 65 | SA | Bovine | + | + | Cff | Cfvi | 4 | A | A | − | − | + | + | − |
| 01/165 | AR | Bovine (mucus) | − | + | Cfvi | Cfvi | 4 | A | A | − | + | + | + | − |
| 02/298 | AR | Bovine (fetus) | − | + | Cfvi | Cfvi | 4 | A | A | − | + | + | + | − |
| 03/596 | AR | Bovine (fetus) | − | + | Cfvi | Cfvi | 4 | A | A | − | + | + | + | − |
| 92/203 | AR | Bovine (placenta) | − | + | Cfvi | Cfvi | 4 | A | A | − | + | + | + | − |
| 97/532 | AR | Bovine (mucus) | − | + | Cfvi | Cfvi | 4 | A | A | − | + | + | + | − |
| 98/25 | AR | Bovine (fetus) | − | + | Cfvi | Cfvi | 4 | A | A | − | + | + | + | − |
| WBT 011/09 | UK | unknown | − | + | Cfvi | Cfvi | 4 | A | A | − | + | + | + | − |
| Zaf 3 | SA | Bovine (fetus) | − | + | Cfvi | Cfvi | 4 | A | A | − | − | + | + | − |
| ADRI 513 | AU | unknown | − | + | Cfvi | Cfv | 4 | A | A | − | − | + | + | − |
| CCUG 33872 | CZ | unknown | − | + | Cfvi | Cfv | 4 | A | A | + | − | + | + | − |
| 84-112 | USA | Bovine | − | − | Cfv | Cfv | 4 | A | A | + | + | + | + | − |
| 97/608 | AR | Bovine (placenta) | − | − | Cfv | Cfv | 4 | A | A | + | + | + | + | − |
| B10 | USA | Bovine | − | − | Cfv | Cfv | 4 | A | A | + | + | + | + | − |
| CCUG 33900 | F | Bovine (abortion) | − | − | Cfv | Cfv | 4 | A | A | + | + | + | + | − |
| LMG 6570 | BE | Bovine | − | − | Cfv | Cfv | 4 | A | A | + | + | + | + | − |
AR, Argentina; AU, Australia; BE, Belgium; CZ, Czech Republic; F, France; NL, the Netherlands; SA, South Africa; UK, United Kingdom.
+, positive in assay; −, negative; Cff, C. fetus subsp. fetus; Cfv, C. fetus subsp. venerealis; Cfvi, C. fetus subsp. venerealis bv. Intermedius.
MLST (ST), multilocus sequence typing (sequence type).
GI with T4SS, genomic island with type IV secretion system; IS, insertion sequence; RM, restriction modification; CRISPR, clustered regularly interspaced short palindromic repeats.
+, genes are present; −, genes are absent.
Whole-genome sequencing.
Whole-genome sequence data of 21 C. fetus strains were obtained using Roche GS-FLX Titanium sequencing. Roche 454 reads were assembled into contigs using the Newbler assembler (v2.6). The genomes of two C. fetus strains, 04/554 and 97/608, were closed through assembly of the Roche 454 contigs into scaffolds by using Perl scripts. To validate the assembly of the contigs and to determine the orientations and order of the scaffolds, a circular high-resolution AflII restriction map of the genome was generated by optical mapping (Argus Optical Mapper; OpGen, Inc., Gaithersburg, MD). The assembly of the sap locus, genomic islands, regions with insertion sequences, and repeats was confirmed with PacBio Continuous Long Reads (Keygene N.V., Wageningen, the Netherlands). All base calls and polymeric tracts were validated using the high-depth Illumina MiSeq reads. The genomes of strains 04/554 and 97/608 were annotated as described previously (10).
Phylogenetic analysis of core and accessory genomes.
Three available closed C. fetus genomes were used as references: strain 82-40 (GenBank accession number CP000487), strain 84-112 (GenBank accession numbers HG004426 and HG004427), and strain 03/293 (GenBank accession numbers CP006999 to CP007002). The amino acid sequences of the open reading frames (ORFs) encoded by five genomes (the three reference genomes plus two genomes [04/554 and 97/608] sequenced in this study) were used as input for an all-versus-all sequence similarity search using BLASTp (−e 0.0001, >80% similarity cutoff). ORFs that exist in each of the five strains (>80% identity over at least 80% of the protein length) were considered to be part of the C. fetus core genome. The ORF sequences of strain 82-40 were used as reference sequences of the core genes. Regions encoding the sap locus, genomic islands, restriction modification (RM) systems, prophages, and insertion sequences were considered accessory genes.
The accessory genes in the Roche 454 contigs of 21 C. fetus strains were identified with a local BLASTn analysis (−e 0.0001, >80% similarity cutoff) against the identified accessory genes of the five closed C. fetus strains. The strains were considered positive for the specific accessory regions if the BLASTn match was >80% over at least 80% of the region.
The phylogenetic analysis of the core genomes was performed as follows. The nucleotide sequences of the predicted genes of the Roche 454 contigs were generated using GeneMark v2.8 (11). For each core gene, the corresponding nucleotide sequence of each strain was extracted and aligned on a gene-by-gene basis using MUSCLE (12). The alignments were concatenated into a contiguous sequence for each C. fetus strain. From this concatenated alignment, a phylogenetic maximum-likelihood tree was built using RAxML v7.2.8 under the GTRCAT model.
Nucleotide sequence accession numbers.
The genome sequence of C. fetus subsp. fetus strain 04/554 has been deposited in GenBank under the accession numbers CP008808 and CP008809, and the sequence of C. fetus subsp. venerealis strain 97/608 has been deposited under the accession numbers CP008810 to CP008812.
RESULTS
Genome features.
The genome of C. fetus subsp. fetus strain 04/554 is a circular chromosome of 1,800,764 bp with an average G+C content of 33.2% and one megaplasmid of 25,862 bp. The genome of C. fetus subsp. venerealis strain 97/608 has a circular chromosome of 1,935,028 bp with an average G+C content of 33.3% and contains two megaplasmids of 38,272 bp and 27,124 bp. The general features of the assembled genomes are shown in Table 2. The genome features of strains 03/293, 82-40, and 84-112 have been described previously (9, 10) and are summarized in Table 2.
TABLE 2.
Features of assembled C. fetus genomes
| Feature | Data for strain (reference or source): |
||||
|---|---|---|---|---|---|
| 04/554 (this study) | 97/608 (this study) | 03/293 (10) | 82-40 (9)a | 84-112 (9)a | |
| Genome size (bp) | 1,800,764 | 1,935,028 | 1,866,009 | 1,773,615 | 1,926,886 |
| G+C content (%) | 33.2 | 33.3 | 33.3 | 33.3 | 33.3 |
| No. of rRNA genes | 3 | 3 | 3 | 3 | 3 |
| No. of tRNA genes | 43 | 43 | 43 | 43 | 43 |
| No. of homopolymeric G+C tracts (>8 bp) | 29 | 24 | 31 | 30 | 34 |
| No. of open reading frames (no. of pseudogenes) | 1,684 (68) | 1,879 (60) | 1,773 (48) | 1,769 | 1,992 |
| Plasmids | 1 | 2 | 3 | 0 | 1 |
| Size (bp) | 25,862 | 38,272/27,124 | 91,400/35,326/3,993 | 61,141 | |
| G+C content (%) | 29.0 | 31.3/28.1 | 29.4/33.0/31.4 | 31.5 | |
| sap locus type | B | A | A | A | A |
| Insertion elements (no. of copies) | 0 | 14 | 13 | 0 | 5 |
| Restriction or modification locus type | None | I | I | None | I |
| CRISPR-cas system | No | No | No | Yes | No |
With modifications from original publication.
Phylogeny of the C. fetus core genome.
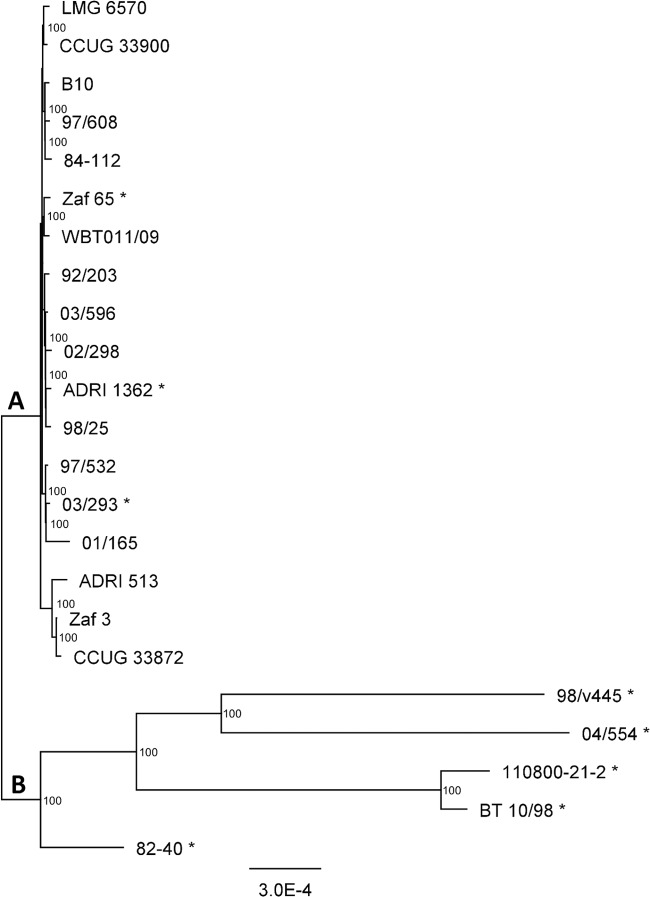
A comparison of five closed C. fetus genomes (of strains 82-40, 84-112, 04/554, 97/608, and 03/293) revealed highly syntenic genomes, which shared >90% sequence identity. The core genome of C. fetus was defined on the ORFs present in the closed genomes of five C. fetus strains and consisted of 1,409,454 bp and 1,509 ORFs. The core genome was then identified in an additional 18 C. fetus strains, and the genetic distances of all 23 core genomes were visualized with a phylogenetic maximum-likelihood tree (Fig. 1). The phylogenetic tree is arranged in two clusters of strains, designated clusters A and B. The majority (n = 18) of the strains were located in cluster A, whereas cluster B consisted of five strains with greater genetic distances.
FIG 1.
Phylogenetic tree of C. fetus strains based on the core genomes. Strains that are phenotypically identified as C. fetus subsp. fetus (Cff) are marked with an asterisk. Bootstrap supports are indicated on the branches. The scale represents the mean number of nucleotide substitutions per site.
Accessory genes of C. fetus strains.
Major differences among the five closed C. fetus genomes were found in the accessory genes. Table 1 shows the identified genes belonging to the sap loci, insertion sequences, genomic islands, type I restriction-modification systems, prophages, and CRISPR-cas systems of the 23 analyzed C. fetus strains.
sap locus.
All analyzed C. fetus strains contained a sap locus, but the composition of this region differed between strains. sap type B strains belonged to the core genome cluster B, whereas sap type A strains were found in genome clusters A and B (Table 1). The sap loci of strains 84-112 and 97/608 contained transposable elements and a set of phage-related genes and hypothetical genes, indicating the presence of a prophage. These prophage sequences shared 100% identity but were inserted at different positions in the sap locus.
Genomic islands.
Two genomic islands (GIs) that encoded a type IV secretion system (T4SS), as defined by Kienesberger et al. (9, 13), were identified in the chromosomes of the closed C. fetus genomes. These two chromosomally located T4SS regions were present in strains that were distributed over the two core genome clusters (Table 1).
Insertion sequences.
Insertion sequences (ISs) were found in the chromosomes as well as in plasmids of the closed genomes of strains 84-112, 97/608, and 03/293. The identified ISs belonged to the IS605, IS607, and IS200 families. The ISs were found only in the C. fetus strains belonging to core genome cluster A (Table 2). Each IS-positive strain contained all of the identified IS families.
Restriction modification system.
Three of the closed C. fetus genomes, of strains 84-112, 97/608, and 03/293, contained a type I restriction modification (RM) system. This type I RM system consists of hsd genes with intervening ORFs, similar to the type I RM systems described for C. jejuni (14). The complete type I RM system is found only in C. fetus strains belonging to core genome cluster A (Table 2). The three other C. fetus strains of sap type A, 82-40, 110800-21-2, and BT 10/98, contained a remnant of this type, as the type I RM system of these strains lacked the hsdS2 gene. The genomes of the sap type B strains did not contain any type I RM-encoding genes.
CRISPR-cas system.
CRISPRs were present in all C. fetus strains, but only two C. fetus subsp. fetus strains, 82-40 and 98/v445, contained cas genes. These strains were not linked with the same core genome cluster.
Core genome clusters compared with accessory genes.
The presence of specific components of the accessory genes encoding prophages, genomic islands, and the CRISPR-cas system was not associated with a specific core genome cluster. The IS elements and complete type I RM system were exclusively found in the strains of cluster A. Strains of cluster B did not contain IS elements or a complete type I RM system, and they have different sap types.
Core genome clusters compared to subspecies identification.
The subspecies were not consistently subdivided phenotypically or genotypically (Table 1). The results of the genotypic method MLST were consistent with those of the obtained core genome clustering; strains of cluster A all were MLST sequence type 4 (ST4), whereas cluster B consisted of strains with other MLST STs. Cluster B included two strains with similar MLST ST2s, and these strains had lower genetic distances than the other strains in cluster B. AFLP distinguished the strains within cluster A with a minor difference in fingerprints as C. fetus subsp. venerealis and C. fetus subsp. venerealis bv. Intermedius (6), but this discrimination was not observed with the phylogenetic analysis of the core genomes. Strains that were phenotypically classified as C. fetus subsp. venerealis and C. fetus subsp. venerealis bv. Intermedius belonged to core genome cluster A, but the eight phenotypically classified C. fetus subsp. fetus strains were dispersed among both genome clusters. This is represented in Fig. 1, in which all phenotypically identified C. fetus subsp. fetus strains are marked with an asterisk.
DISCUSSION
The original classification of the C. fetus subspecies is based on differences in the colonization of different niches and phenotypic characteristics (2–4). The two C. fetus subspecies are highly syntenic, sharing 92.9% sequence identity (9), and the subspecies cannot be distinguished by DNA-DNA hybridization (15), which questions the validity of subspecies differentiation and hampers an adequate taxonomic positioning of the subspecies. Furthermore, the reliability of the 1% glycine tolerance test can be influenced by the transduction of glycine tolerance by phages (16). Several molecular assays for the identification of the C. fetus subspecies have been published (5); however, none of the molecular assays corresponded fully to the phenotypic identification of the C. fetus subspecies (5, 6).
In this study, phylogenetic analysis of the core genomes subdivided the C. fetus strains into two clusters. All strains that were phenotypically identified as C. fetus subsp. venerealis (including C. fetus subsp. venerealis bv. Intermedius) clustered in one core genome cluster, contained only strains with MLST ST4, and harbored IS elements and a type I RM system. The strains phenotypically identified as C. fetus subsp. fetus were assigned to both clusters. Three C. fetus subsp. fetus strains, 03/293, Zaf 65, and ADRI 1362, were assigned to the core genome cluster with C. fetus subsp. venerealis and C. fetus subsp. venerealis bv. Intermedius strains, despite their phenotypic identification of C. fetus subsp. fetus. The similarity of the MLST identification and core genome clusters can be explained by the fact that MLST is a small-scale reflection of the core genome. The phylogenetic analysis showed an obvious resemblance to the MLST STs of the strains; the genetic distances between strains with the same STs were very low, as shown in strains of ST4 and ST2, and the genetic distances increased for strains with different STs.
Campylobacter fetus subsp. venerealis bv. Intermedius is described as a phenotypic variant of C. fetus subsp. venerealis (1). The phenotypically identified C. fetus subsp. venerealis bv. Intermedius strains are all positioned together with C. fetus subsp. venerealis strains in cluster A of the phylogenetic tree. The accessory genes of C. fetus subsp. venerealis and C. fetus subsp. venerealis bv. Intermedius strains showed no consistent presence of a C. fetus subsp. venerealis- or C. fetus subsp. venerealis bv. Intermedius-specific region. However, it is remarkable that, except for strain ADRI 513, all of the analyses with AFLP identified C. fetus subsp. venerealis strains that contained a prophage in the sap locus and that this prophage is absent in the majority of C. fetus subsp. venerealis bv. Intermedius strains. Almost all proteins of this prophage are hypothetical and have an unknown function, but one may speculate that the presence of this prophage influences the phenotypic difference, such as the difference in H2S production between the C. fetus subsp. venerealis and C. fetus subsp. venerealis bv. Intermedius strains.
The differentiation between C. fetus subspecies goes beyond only taxonomic interest. Clinically, the subspecies have been described as different. C. fetus subsp. venerealis (including bv. Intermedius) is described as the causative agent of bovine genital campylobacteriosis (BGC). There is a generally accepted association between the C. fetus subspecies and their specific clinical features, epidemiological characteristics, and host niche specificities. Bovine products for trade must be checked for the absence of C. fetus subsp. venerealis, as stated in the Terrestrial Animal Health Code by the World Organization of Animal Health (OIE) (17). When C. fetus is detected in such a screening, subspecies identification is generally made by phenotypic assays, as described in the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (18). However, there is no evidence that the phenotypic markers (glycine tolerance and H2S production) are linked to the virulence characteristics of the C. fetus subspecies. Future diagnostics of C. fetus should preferably detect genomic characteristics associated with virulence and different host niches. The virulence genes of genomic islands present in C. fetus strains were described as C. fetus subsp. venerealis specific and were proposed as targets for diagnostic assays (19, 20). However, these genes are not consistently present in C. fetus subsp. venerealis genomes (13, 21) and therefore are not useful for a diagnostic assay. Pending the identification of virulence-associated genes, one should be aware that the current association between phenotype and virulence is questionable, since several phenotypically defined C. fetus subsp. fetus strains have the same genomic characteristics as C. fetus subsp. venerealis strains on the basis of core genome and accessory gene similarity, as shown in this study. The inconsistency of the phenotypes and genomic characteristics of C. fetus strains encourages a critical evaluation of the clinical relevance of C. fetus subspecies identification by phenotypic assays.
ACKNOWLEDGMENTS
We thank Brian Brooks and John Devenish (Canadian Food Inspection Agency) for providing strains. We also thank Mary Chapman and Nathaniel Simon for the generation of Illumina MiSeq reads, and we thank James Bono for the generation of PacBio RS reads.
Footnotes
Published ahead of print 17 September 2014
REFERENCES
- 1.Véron M, Chatelain R. 1973. Taxonomy study of the genus Campylobacter Sebald and Verón and designation of the neotype strain for the type species, Campylobacter fetus (Smith and Taylor) Sebald and Verón. Int. J. Syst. Bacteriol. 23:122–134. 10.1099/00207713-23-2-122. [DOI] [Google Scholar]
- 2.Florent A. 1959. Les deux vibriosis génitales; la vibriose due à V. fetus venerealis et la vibriose d'origine intestinale due à V. fetus intestinalis. Meded. Veeartsenijsch. Rijksuniv. Gent. 3:1–60. [Google Scholar]
- 3.Park RWA, Munro IB, Melrose DR, Stewart DL. 1962. Observations on the ability of two biochemical types of Vibrio fetus to proliferate in the genital tract of cattle and their importance with respect to infertility. Br. Vet. J. 118:411. [Google Scholar]
- 4.Florent A. 1963. A propos des vibrions responsables de la vibriose génitale des bovins et des ovins. Bull. Off. Int. Epiz. 60:1063–1074. [Google Scholar]
- 5.van der Graaf-van Bloois L, van Bergen MA, van der Wal FJ, de Boer AG, Duim B, Schmidt T, Wagenaar JA. 2013. Evaluation of molecular assays for identification Campylobacter fetus species and subspecies and development of a C. fetus specific real-time PCR assay. J. Microbiol. Methods 95:93–97. 10.1016/j.mimet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 6.van Bergen MAP, Dingle KE, Maiden MC, Newell DG, van der Graaf-Van Bloois L, van Putten JP, Wagenaar JA. 2005. Clonal nature of Campylobacter fetus as defined by multilocus sequence typing. J. Clin. Microbiol. 43:5888–5898. 10.1128/JCM.43.12.5888-5898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagenaar JA, van Bergen MAP, Newell DG, Grogono-Thomas R, Duim B. 2001. Comparative study using amplified fragment length polymorphism fingerprinting, PCR genotyping, and phenotyping to differentiate Campylobacter fetus strains isolated from animals. J. Clin. Microbiol. 39:2283–2286. 10.1128/JCM.39.6.2283-2286.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia MM, Eaglesome MD, Rigby C. 1983. Campylobacters important in veterinary medicine. Vet. Bull. 53:793–818. [Google Scholar]
- 9.Kienesberger S, Sprenger H, Wolfgruber S, Halwachs B, Thallinger GG, Perez-Perez GI, Blaser MJ, Zechner EL, Gorkiewicz G. 2014. Comparative genome analysis of Campylobacter fetus subspecies revealed horizontally acquired genetic elements important for virulence and niche specificity. PLoS One 9:e85491. 10.1371/journal.pone.0085491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Graaf-van Bloois L, Miller WG, Yee E, Bono JL, Rijnsburger M, Campero C, Wagenaar JA, Duim B. 2014. First closed genome sequence of Campylobacter fetus subsp. venerealis bv. Intermedius. Genome Announc. 2(1):e01246-13. 10.1128/genomeA.01246-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukashin AV, Borodovsky M. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 26:1107–1115. 10.1093/nar/26.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113. 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorkiewicz G, Kienesberger S, Schober C, Scheicher SR, Gully C, Zechner R, Zechner EL. 2010. A genomic island defines subspecies-specific virulence features of the host-adapted pathogen Campylobacter fetus subsp. venerealis. J. Bacteriol. 192:502–517. 10.1128/JB.00803-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller WG, Pearson BM, Wells JM, Parker CT, Kapitonov VV, Mandrell RE. 2005. Diversity within the Campylobacter jejuni type I restriction-modification loci. Microbiology 151:337–351. 10.1099/mic.0.27327-0. [DOI] [PubMed] [Google Scholar]
- 15.Roop RM, Smibert RM, Johnson JL, Krieg NR. 1984. Differential characteristics of catalase-positive campylobacters correlated with DNA homology groups. Can. J. Microbiol. 30:938–951. 10.1139/m84-147. [DOI] [PubMed] [Google Scholar]
- 16.Chang W, Ogg JE. 1971. Transduction and mutation to glycine tolerance in Vibrio fetus. Am. J. Vet. Res. 32:649–653. [PubMed] [Google Scholar]
- 17.OIE. 2013. Terrestrial animal health code. Office International des Epizooties, Paris, France. [Google Scholar]
- 18.OIE. 2012. Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees), 7th ed, p 652 Office International des Epizooties, Paris, France. [Google Scholar]
- 19.Iraola G, Hernandez M, Calleros L, Paolicchi F, Silveyra S, Velilla A, Carretto L, Rodriguez E, Perez R. 2012. Application of a multiplex PCR assay for Campylobacter fetus detection and subspecies differentiation in uncultured samples of aborted bovine fetuses. J. Vet. Sci. 13:371–376. 10.4142/jvs.2012.13.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moolhuijzen PM, Lew-Tabor AE, Wlodek BM, Aguero FG, Comerci DJ, Ugalde RA, Sanchez DO, Appels R, Bellgard M. 2009. Genomic analysis of Campylobacter fetus subspecies: identification of candidate virulence determinants and diagnostic assay targets. BMC Microbiol. 9:86. 10.1186/1471-2180-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abril C, Brodard I, Perreten V. 2010. Two novel antibiotic resistance genes, tet(44) and ant(6)-Ib, are located within a transferable pathogenicity island in Campylobacter fetus subsp. fetus. Antimicrob. Agents Chemother. 54:3052–3055. 10.1128/AAC.00304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]