Abstract
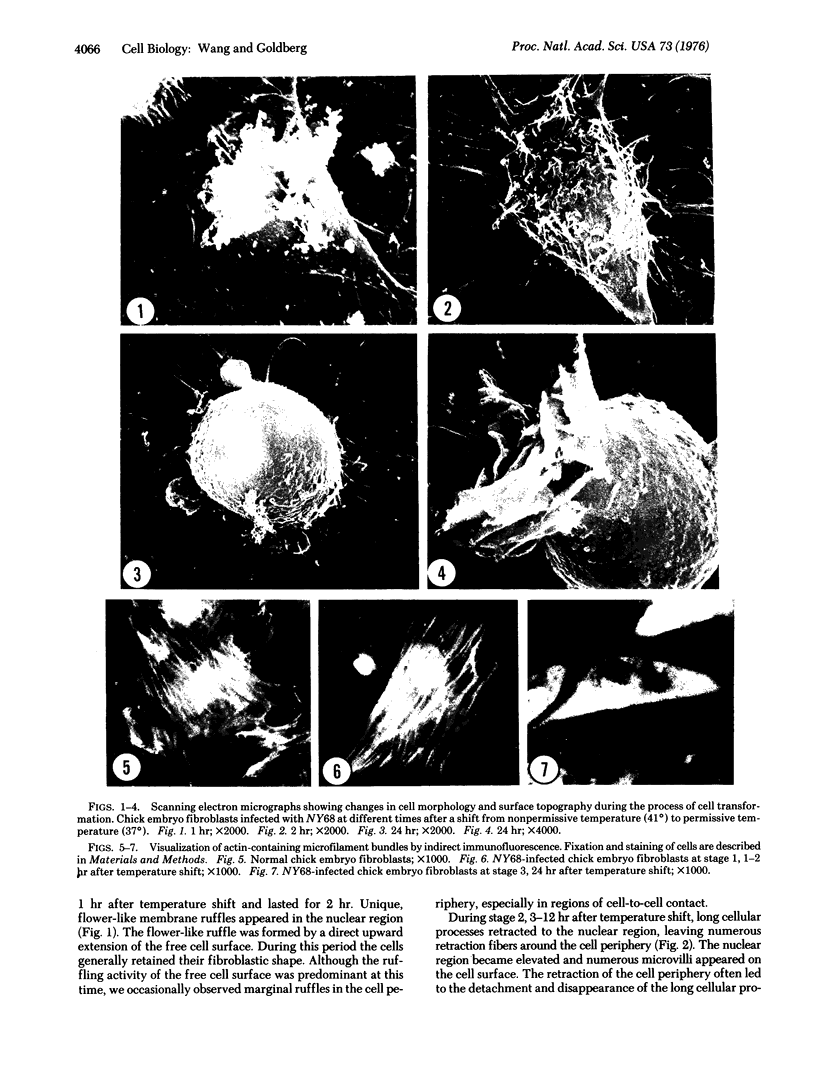
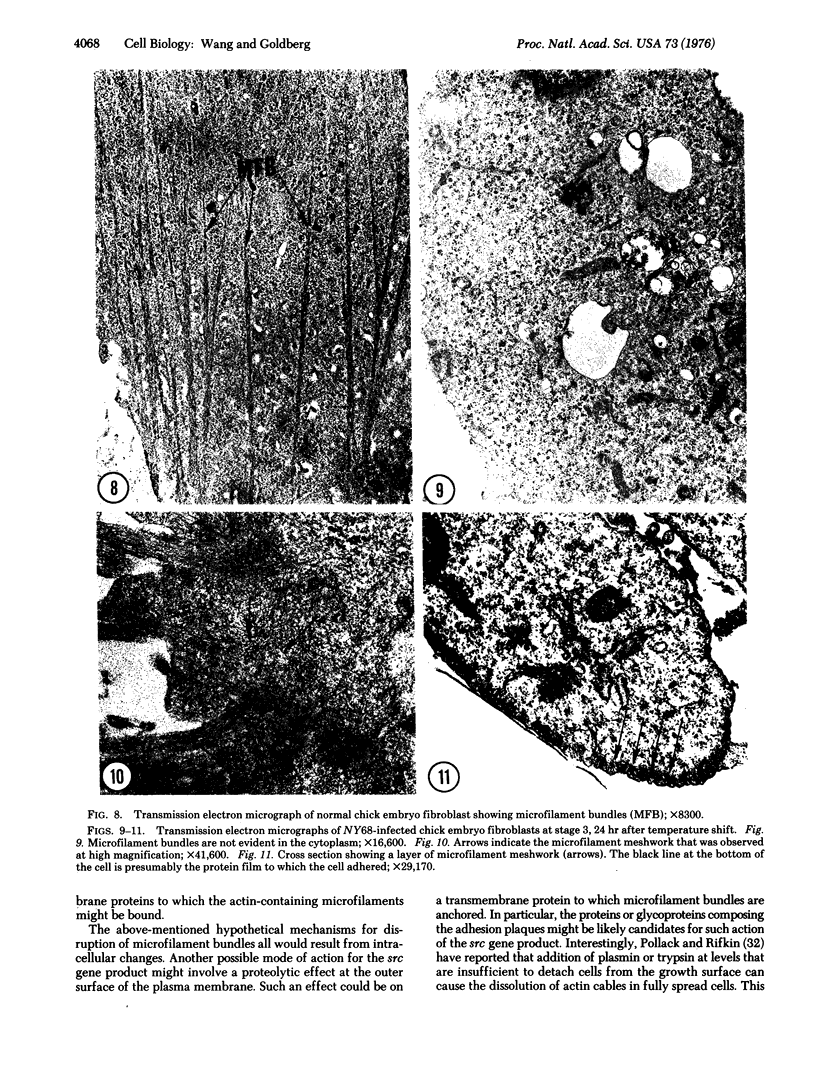
A series of morphological changes occurred when chick embryo fibroblasts infected with the NY68 mutant of Rous sarcoma virus were shifted from nonpermissive temperature (41degrees) to permissive temperature (37 degrees). We observed three distinct stages in cell morphology and surface topography that were correlated with a reduction in the organization and assembly of actin-containing microfilament bundles. Our observations suggest that control of microfilament organization and surface topography are responsive to the presence of a functioning transforming gene (src) product of Rous sarcoma virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V. R., Chen L. B., Buchanan J. M. Surface ruffles as markers for studies of cell transformation by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3144–3148. doi: 10.1073/pnas.72.8.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Cytoplasmic fibrils in living cultured cells. A light and electron microscope study. Protoplasma. 1967;64(4):349–380. doi: 10.1007/BF01666538. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. O., Sheppard J. R., Burger M. M. Cyclic membrane changes in animal cells: transformed cells permanently display a surface architecture detected in normal cells only during mitosis. Proc Natl Acad Sci U S A. 1971 Jan;68(1):244–247. doi: 10.1073/pnas.68.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Berg G., Bushnell A., Chang C. M., Dickerman L., Hopkins N., Miller M. L., Pollack R., Wang E. Fibrillar systems in cell motility. Ciba Found Symp. 1973;14:83–107. doi: 10.1002/9780470719978.ch5. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The use of heavy meromyosin binding as an ultrastructural cytochemical method for localizing and determining the possible functions of actin-like microfilaments in nonmuscle cells. J Histochem Cytochem. 1975 Jul;23(7):529–542. doi: 10.1177/23.7.1095652. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M., Krishnan N. Hot alcoholic phosphotungstic acid and uranyl acetate as routine stains for thick and thin sections. J Cell Biol. 1971 Aug;50(2):550–557. doi: 10.1083/jcb.50.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. doi: 10.1083/jcb.56.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from SV40-transformed cells. II. Ultrastructural study. J Cell Biol. 1971 Sep;50(3):691–708. doi: 10.1083/jcb.50.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr T. S., Hall D. E., Allison A. C. Role of contractile microfilaments in the release of histamine from mast cells. Nature. 1972 Apr 14;236(5346):350–351. doi: 10.1038/236350a0. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Hoch P., Ho D. Adhesion of culture cells to their substratum. Exp Cell Res. 1974 Mar 15;84(1):207–218. doi: 10.1016/0014-4827(74)90398-x. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring. I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z Zellforsch Mikrosk Anat. 1970;109(4):431–449. [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ludueña M. A. Surface movements, microfilaments and cell locomotion. Ciba Found Symp. 1973;14:53–82. doi: 10.1002/9780470719978.ch4. [DOI] [PubMed] [Google Scholar]