Abstract
Emerging canine coronavirus (CCoV) variants that are associated with systemic infections have been reported in the European Union; however, CCoV-associated disease in the United States is incompletely characterized. The purpose of this study was to correlate the clinicopathological findings and viral antigen distribution with the genotypic characteristics of CCoV in 11 puppies from nine premises in five states that were submitted for diagnostic investigation at Cornell University between 2008 and 2013. CCoV antigen was found in epithelial cells of small intestinal villi in all puppies and the colon in 2 of the 10 puppies where colon specimens were available. No evidence of systemic CCoV infection was found. Comparative sequence analyses of viral RNA extracted from intestinal tissues revealed CCoV-II genotype in 9 out of 11 puppies. Of the nine CCoV-IIs, five were subtyped as group IIa and one as IIb, while three CCoVs could not be subtyped. One of the CCoV-IIa variants was isolated in cell culture. Infection with CCoV alone was found in five puppies, of which two also had small intestinal intussusception. Concurrent infections with either parvovirus (n = 1), attaching-effacing Escherichia coli (n = 4), or protozoan parasites (n = 3) were found in the other six puppies. CCoV is an important differential diagnosis in outbreaks of severe enterocolitis among puppies between 4 days and 21 weeks of age that are housed at high population density. These findings will assist with the rapid laboratory diagnosis of enteritis in puppies and highlight the need for continued surveillance for CCoV variants and intestinal viral diseases of global significance.
INTRODUCTION
Canine coronavirus (CCoV) was first recognized as a pathogen of dogs in 1971 (1) and, together with transmissible gastroenteritis virus (TGEV) of swine and feline coronavirus (FCoV), is a member of the family Coronaviridae, subfamily Coronavirinae, genus Alphacoronavirus, species Alphacoronavirus-1 (2). Infection with CCoV is common in young dogs, particularly those housed in large groups, such as in kennels, shelters, and breeding facilities (3–7). Traditionally, CCoV has been reported to infect small intestinal villus absorptive epithelial cells, resulting in mild and self-limiting diarrheal disease (8, 9). Young dogs, particularly those coinfected with other enteropathogens, including parvovirus, can develop severe and often fatal disease (8, 10–12). The emergence of CCoV variants that are associated with severe clinical disease, mortality, and systemic infections of dogs has been reported from several countries in the European Union (EU) (13–18). Although fatal CCoV-associated disease without other pathogens was reported in two puppies in the United States in 2005, the CCoVs were not characterized (19).
CCoVs circulate as two distinct genotypes, CCoV-I and CCoV-II, and both viruses can be detected in feces and tissues obtained from infected dogs by reverse transcription-PCR (RT-PCR) (7, 20). These genotypes can be distinguished on the basis of antigenic and genetic differences in the gene encoding the surface spike protein (21, 22). The viral spike protein binds to the host cell receptor and triggers the fusion of the viral and cellular membranes, making it an important determinant of cellular tropism and pathogenicity (23). Genotype I CCoV cannot be propagated in cell culture; thus, it is understudied compared to genotype II CCoV, which is easily adapted to cell culture conditions. A similar situation exists with the closely related FCoV type I viruses and is suspected to be due to differential receptor requirements between genotypes (24). CCoV-II viruses use aminopeptidase N (APN) as the receptor (25), while the receptor for CCoV-I viruses has not been identified. CCoV-II viruses are classified into at least two subtypes, namely, CCoV-IIa and CCoV-IIb, based on the sequence of the first 300 amino acids of the spike protein, a region known as the N-terminal domain (NTD). The NTD is an important determinant of intestinal tropism in the closely related TGEV (26, 27). Although the CCoV-IIa and -IIb classification is not part of the official CCoV taxonomy, these subtypes are widely referenced in the literature. Moreover, CCoV-IIa viruses exist as two biotypes that differ in pathogenicity and tissue tropism and have an entirely CCoV-like NTD. The productive infection and replication of the classical CCoV-IIa biotype is restricted to intestinal epithelial cells. In contrast, an emergent pantropic CCoV-IIa biotype that can spread systemically is associated with profound leukopenia (28, 29) and has been detected by RT-PCR in the tonsils, thymus, heart, lungs, liver, pancreas, mesenteric lymph node, spleen, kidneys, urinary bladder, muscles, and brain of affected dogs (13–18). The isolation of virus from extraintestinal tissues also has been reported in some instances (13, 14, 16) but also failed in multiple other instances (18). The CCoV-IIb spike gene has a TGEV-like NTD (15, 30), and, like TGEV, it causes enteritis in neonatal animals. Although it generally is restricted to the small intestine, CCoV-IIb RNA has been detected in extraintestinal tissues of dogs coinfected with canine parvovirus (15–17) or with unknown comorbidity (18). Finally, a third CCoV-II variant with a CCoV-I NTD has been reported in both the United States and Sweden (25, 31).
The purpose of the present study was to characterize the genotype of CCoV associated with outbreaks of fatal disease in young dogs submitted to the Animal Health Diagnostic Center (AHDC) at Cornell University between 2008 and 2013. Following the localization of CCoV antigen in tissue sections by using immunostaining, the type and subtype of each virus was determined by sequencing of the NTD from purified viral RNA amplified by RT-PCR assay. Since changes in the proteolytic cleavage of the spike protein can modulate viral pathogenesis in FCoV (32), we also characterized the sequence of the spike protein cleavage motifs. Lastly, we used phylogenetic analysis to compare the sequences of the spike NTD obtained in the present study with those previously reported in the EU. The results of our study will assist with the rapid laboratory diagnosis of CCoV-associated enteritis in dogs and enhance surveillance for emerging intestinal viral variants of global significance.
MATERIALS AND METHODS
Diagnostic investigation.
The sample population consisted of dogs submitted to the AHDC at Cornell University between 2008 and 2013 with lesions of viral enteritis that were positive for the presence of CCoV antigen by immunohistochemical (IHC) staining. With the exception of puppies 4a and 4b, in which selected tissues were collected by the referring veterinarian during a field necropsy, all cases were processed for complete necropsy, including the collection of multiple segments of gastrointestinal tract. In addition to gross and histopathological examinations of a standard set of tissues, bacteriological culture of intestinal specimens, including Salmonella and Campylobacter species, and fluorescent antibody (FA) tests on fresh frozen tissue sections for CCoV, group A rotavirus, and canine parvovirus were performed on all cases. At the request of the referring veterinarian or according to the pathologist in charge, selected fresh tissues obtained from puppies 2, 4a, 4b, and 8 also were processed for virus isolation. Dogs with respiratory signs or lesions were examined for the presence of canine distemper virus, canine parainfluenza virus, and canine adenovirus by FA staining of frozen tissue sections.
Histopathology.
Sections of brain, thymus, heart, trachea, lungs, liver, gallbladder, tongue, stomach, pancreas, small and large intestines, mesenteric lymph node, spleen, kidneys, adrenal glands, urinary bladder, skeletal muscles, and bone marrow were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4-μm thickness, and stained with hematoxylin and eosin. Selected sections of intestinal tract also were stained with tissue Gram stain and a modified Steiner silver stain to further characterize bacteria when suspected. For IHC staining, sections of tissues were deparaffinized and processed for antigen retrieval. After blocking endogenous peroxidase activity with 3% hydrogen peroxide and treatment with normal goat or normal rabbit serum for 5 min (Invitrogen, Carlsbad, CA), the slides were reacted with the coronavirus-specific mouse monoclonal antibody FIPV3-70 (Custom Monoclonal Antibodies International, Sacramento, CA), followed by biotinylated goat anti-mouse, streptavidin-peroxidase conjugate (Invitrogen), chromogen, 3,3-diaminobenzidine-tetra hydrochloride, and hematoxylin counterstain. Duplicate intestinal sections from each puppy were stained with the group A rotavirus-specific mouse monoclonal antibody 9-10 (33) and canine parvovirus-specific rabbit polyclonal antibody CPV vp1/vp2 (Colin Parrish, Baker Institute, Cornell University). For FIPV3-70 and 9-10 antibodies, heat antigen retrieval consisted of microwave in citrate buffer at pH 6.0 for 20 min, whereas for CPV vp1/vp2 antibody, antigen retrieval was accomplished by digestion with pronase for 30 min.
Virus isolation.
Ten percent tissue pools of lung, liver, spleen, and intestine from puppy 2, intestine from puppy 4a, lung and intestine from puppy 4b, and lung, liver, spleen, kidney, and brain from puppy 8 were prepared in Eagle's minimal essential medium (MEM-E) containing 0.5% bovine serum albumin and 10 μg/ml ciprofloxacin. After tissue disruption and low-speed centrifugation, 1 ml of the filtered supernatants from puppies 2, 4a, and 4b were inoculated onto monolayers of canine fibroblast-like A-72 cells (ATCC CRL-1542) and immortalized canine kidney cells (AHDC, Cornell University), while supernatant from puppy 8 was inoculated onto immortalized canine kidney cells and human colorectal adenocarcinoma HRT-18 (ATCC CCL-244) cells grown in 25-cm2 flasks as previously described (34). Supernatants from puppies 4a and 4b also were inoculated onto canine kidney MDCK cells (ATCC CCL-34) and HRT-18 cells, respectively. The extract was allowed to remain on the monolayer for 1 to 2 h and then rinsed with phosphate-buffered saline (PBS). Cells were cultured at 37°C in MEM-E containing 10% gamma-irradiated fetal bovine serum. At 5- to 7-day intervals, monolayers were disrupted with trypsin, and new monolayers established at a 1:3 split ratio. Cultures were monitored on a daily basis for the presence of cytopathic effect. CCoV isolation was confirmed by FA staining with the mouse monoclonal antibody FIPV3-70.
RT-PCR and genotyping.
RNA was extracted from formalin-fixed and paraffin-embedded (FFPE) tissues with a RecoverAll total nucleic acid isolation kit according to the manufacturer's instructions (Ambion, Foster City, CA, USA). The resulting RNA was reverse transcribed into cDNA using a SuperScript III first-strand synthesis system for RT-PCR (Life Technologies, Carlsbad, CA, USA). The RT reaction was primed with random hexamers. The presence of coronavirus cDNA within the sample was confirmed as previously described by PCR directed to a conserved region of the 3′ untranslated region (UTR) (35). Samples that tested positive for the presence of coronavirus RNA based on the 3′ UTR were further characterized as genotype I or II using oligonucleotide primers directed against S1/S2 and S2′ cleavage motifs (Fig. 1 and Table 1). Type II viruses were further characterized into subtypes on the basis of the NTD (Fig. 1 and Table 1). Previous studies on CCoV differentiate type I and type II CCoVs based on the sequence of the membrane (M) protein; however, it is unclear how changes in the M protein correlate with changes in the S protein. Therefore, we designed oligonucleotide primers against regions of the S protein that differ substantially between genotypes (Fig. 1 and Table 1). PCR was performed using Platinum Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturers' instructions with an annealing temperature of 55°C. PCR products were analyzed by electrophoresis on a 0.8% agarose gel. Products of the expected size were purified using the QIAquick gel extraction kit (Qiagen, Valencia, CA, USA).
FIG 1.
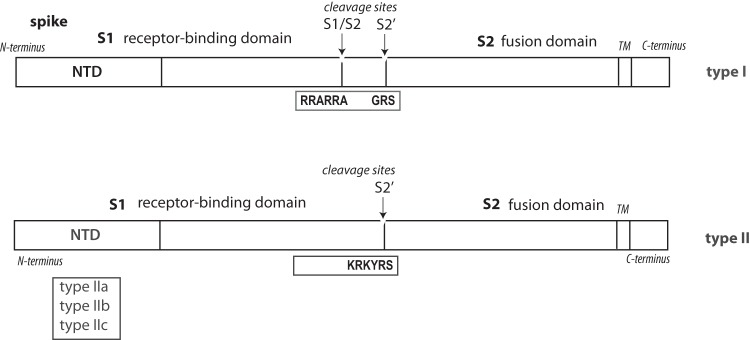
CCoV-I and CCoV-II spike genes with the location of the N-terminal domain (NTD), S1 receptor binding domain, S1/S2 cleavage site (S1/S2), S2′ cleavage site (S2′), S2 fusion domain, and transmembrane domain (TM). Note that the S1/S2 furin cleavage site is present only in CCoV-I viruses.
TABLE 1.
Oligonucleotide primers for amplification and sequencing of the 3′ UTR, NTD, S1/S2, and S2′
| Name | Specificity | Sense | Sequence (5′ to 3′) | Product size (bp) |
|---|---|---|---|---|
| CCV 1-1 | Type 1 S1/S2 cleavage site | + | CTGCTCAAGCTGCTGTAATT | 244 |
| − | TACTACTGTGTTGGTGGTGA | |||
| CCV 1-2 | Type 1 S2′ cleavage site | + | ATGTAATGACAGAAGTACA | 261 |
| − | TACATTGCCATCTTATTATCA | |||
| CCV 2-1 | Type 2 S1/S2 cleavage site | + | GCCATAGTTGGAGCTATGAC | 203 |
| − | CCTATTTACAAAGAATGGCC | |||
| CCV 2-2 | Type 2 S2′ cleavage site | + | ATGCCATTGTAATATTGTGC | 225 |
| − | CCATCAGAATGTGTGACGTTA | |||
| CCV-IIa | Type IIa NTD | + | ATGATTGTGATCGTAACTTG | 200 |
| − | TTGTACCACACCTCTGTAGG | |||
| CCV-IIb | Type IIb NTD | + | GAACTATAGGCAACCATTGG | 138 |
| − | TACAATGCTTTAAGATTTTC | |||
| 20179a | Type I NTD | + | GGCTCTATCACATAACTCAGTCCTAG | 163 |
| CCV-IIc | − | TACATACTAGCTTCAAATC |
Previously described oligonucleotide (20).
Comparative sequence analysis.
The products of the RT-PCR assays were sequenced by using the Sanger dideoxy sequencing method (Biotechnology Resource Facility, Cornell University). The CCoV RNA extracted from clinical samples was classified as CCoV-I or CCoV-II based on RT-PCR and sequencing of the spike S1/S2 and S2′ cleavage sites. Sequencing also was used to distinguish CCoV-IIa and CCoV-IIb variants by using a combination of new and previously published spike-specific primers targeting the NTD (20). Oligonucleotides specific for the CCoV-I NTD also were included in order to detect CCoV-I/CCoV-II recombinants (Fig. 1 and Table 1). We sequenced and aligned the CCoV-II S2′ cleavage site, which is adjacent to the conserved coronavirus fusion peptide (36), to look for deviations from the CCoV-II consensus cleavage motif, K-R-K-Y-R-S, where K is the amino acid lysine, R is the amino acid arginine, Y is the amino acid tyrosine, and S is the amino acid serine. This amino acid motif likely is cleaved by a variety of trypsin- and cathepsin-like proteases, with cleavage occurring between the R and S residues. Comparative analysis of PCR-amplified CCoV gene-specific sequences was performed on the N terminus of the S gene using Clustal X (Conway Institute, UCD, Dublin, Ireland) and viewed in Geneious v6.1.7 (Biomatters Ltd., Auckland, New Zealand). Neighbor-joining trees were constructed in Clustal X using 10,000 bootstrap trials and viewed in FigTree v1.4.0 (Institute of Evolutionary Biology, Edinburgh, United Kingdom).
Nucleotide sequence accession numbers.
The partial nucleotide sequences of CCoV spike genes have been deposited in the European Molecular Biology Laboratory Archives under the accession numbers LN624642 to LN624663.
RESULTS
Clinical findings.
The signalments and clinical presentations of 11 dogs from 9 premises investigated in the present study are presented in Table 2. No sex or breed predilections were noted. Affected puppies ranged in age from 4 days to 21 weeks with a median age of 7 weeks, and multiple puppies per litter and multiple litters were affected on most premises. The puppies were housed mostly in large groups that experienced severe clinical signs of intestinal illness and mortality in Indiana (n = 1), Kansas (n = 4), New York (n = 4), and Pennsylvania (n = 1). A litter in transit between shelters located in North Carolina and Rhode Island (n = 1) also was included.
TABLE 2.
Clinical history of puppies with canine coronavirus enteritis in this study
| Case | IDa | Location | Breed | Age | Description and/or clinical sign(s) |
|---|---|---|---|---|---|
| 1 | 08-149076 | NY | Golden retriever | 3 weeks | Breeder with 24 adults not affected; litter of 5 puppies with weakness, dehydration, vomiting, and diarrhea (2 died, 1 recovered); in other litters, 3 puppies were affected (1 died, 2 recovered) |
| 2 | 09-89334 | PA | Yorkshire terrier | 21 weeks | Central nervous system signs, including hypoglycemia; a littermate with diarrhea died 4 days earlier |
| 3 | 09-97736 | ID | Spaniel | 5 weeks | Breeder with 15–20 adults not affected; puppy with 4-day history of vomiting and diarrhea died during surgery for jejunoileal intussusception; 1 littermate with intussusception and 2 others ill |
| 4a | 09-107207 | KS | Maltese | 8 weeks | Distributor with 800 puppies with history of respiratory signs, vomiting, and diarrhea |
| 4b | 09-110089 | Basset hound | 8 weeks | ||
| 5 | 09-123567 | NY | Mixed | 7 weeks | Breeding/research facility; puppies with pale mucous membranes, depression, dehydration |
| 6a | 10-51534A | KS | Bichon frise | 8 weeks | Breeder with 200 adults not affected; 100 puppies with 20% mortality when 6 to 8 weeks old |
| 6b | 10-51534B | Mixed | 8 weeks | 1 to multiple puppies per litter died within 3–4 days of showing anorexia, vomiting, and diarrhea | |
| 7 | 12-120628 | RI | Mixed | 5 weeks | 8 rescued weaned puppies in transit from NC; 4 died with weakness, lethargy, dehydration, hypothermia |
| 8 | 12-159396 | NY | Shepherd mixed | 2 weeks | Rescue bitch from KY; 5 puppies died from a litter of 8 |
| 9 | 13-47387 | NY | German shepherd | 4 days | Breeding/boarding facility with 8 adults showing mild vomiting and diarrhea; 2 puppies died when 3 and 4 days old from a litter of 8 with abdominal pain and bloody stools |
ID, identifier code.
Laboratory findings.
The results of IHC staining of formalin-fixed and paraffin-embedded (FFPE) tissue sections for the presence of CCoV antigen and other pathological, microbiological, and parasitological findings in dogs investigated in this study are presented in Table 3. All puppies had lesions consistent with viral enteritis characterized by various degrees of atrophy of small intestinal villi (villus/crypt ratio, approximately 1:2) that were lined with attenuated, low-cuboidal to squamous epithelial cells (Fig. 2A). Immunostaining confirmed the presence of CCoV antigen within the cytoplasm of small intestinal villus epithelial cells in all of the puppies (Table 3 and Fig. 2B and C). Infection with CCoV extended from the villus-crypt junction to the tip of villi diffusely along the small intestine in puppies 5, 7, 8, and 9, multifocally in groups of epithelial cells in puppies 1, 3, 4a, 4b, and 6b, and within scattered individual epithelial cells in puppies 2 and 6a. Of the 10 puppies in which colonic sections were available, only puppies 8 and 9 showed CCoV antigen within epithelial cells along the surface and crypts of the colon. Although lymphoid depletion of Peyer's patches was present in 7 puppies, none of 10 puppies where lymphoid tissues were examined by IHC showed positive staining for the presence of CCoV antigen. Rare individual CCoV antigen-positive cells, most likely antigen-presenting dendritic cells, were scattered within the mesenteric lymph nodes in puppies 6a, 6b, and 9. None of the puppies were positive for the presence of group A rotavirus by FA and IHC staining or Salmonella and Campylobacter species by bacteriological culture of intestinal specimens. However, concurrent intestinal pathogens were present in six puppies. Puppy 4a had severe acute multifocal crypt epithelial cell necrosis that was associated with canine parvovirus antigen as determined by FA and IHC staining. None of the other 10 puppies were positive for the presence of canine parvovirus antigen by FA and IHC staining. In addition to diffuse attenuation of villus epithelial cells, sections of small intestines from puppies 2 and 5 also showed multifocal epithelial cell necrosis and sloughing into the lumen that was associated with large and moderate numbers, respectively, of cytoplasmic coccidian parasites. Parasitological examination of fecal samples confirmed the presence of Isospora species and Cystoisospora ohioensis in puppies 2 and 5, respectively. The small intestine of puppy 2, a young Yorkshire terrier, also showed multifocal crypt ectasia, a finding associated with protein-losing enteropathy in this breed of dogs (37). The lumen of many colonic crypts in puppy 4b contained small numbers of pale eosinophilic, pear-shaped flagellated protozoan parasites consistent with mild trichomoniasis. Closely adherent Gram-negative coccobacilli consistent with attaching-effacing Escherichia coli (AEEC) were present multifocally along the apical membrane of villus epithelial cells in sections of small intestines from four puppies; large numbers of bacteria were present in puppy 4a, while puppies 5, 6a, and 6b had small numbers of adherent bacteria (38). The bacteriological culture of segments of jejunum taken from puppies 6a and 6b yielded E. coli isolates that were typed as O untypeable:H49 and O8:H14, respectively (E. coli Reference Center, The Pennsylvania State University). The isolate from puppy 6b also was positive for the presence of stxII, encoding Shiga-like toxin type II, and isolates from puppies 6a and 6b were negative for the presence of eae, encoding intimin-gamma. Consistent with clinical signs of weakness and vomiting, aspiration pneumonia was present in puppies 2, 5, 6a, and 7. Other lesions, including bronchopneumonia in puppies 4b and 6b and hepatocellular necrosis in puppies 8 and 9, were considered incidental findings. With the exception of puppy 4a, where lung tissue was not available, none of the lung sections taken from the remaining 10 puppies were positive for the presence of CCoV antigen by IHC staining.
TABLE 3.
Results of IHC staining of tissues for the presence of CCoV antigen and other findings in puppies investigated in this studya
| Case | ID | CCoV IHC |
Other finding(s) | ||
|---|---|---|---|---|---|
| SI | LI | Other tissues | |||
| 1 | 09-149076 | Pos. | Neg. | Neg.: lung, kidney, bone marrow | Peyer's patch lymphoid depletion |
| 2 | 09-89334 | Pos. | Neg. | Neg.: lung, urinary bladder | Protein-losing enteropathy, Isospora species, Peyer's patch lymphoid depletion, aspiration pneumonia |
| 3 | 09-97736 | Pos. | Neg. | Neg.: lung, liver | Jejunoileal intussusception, Peyer's patch lymphoid depletion |
| 4a | 09-107207 | Pos. | NA | NA | Canine parvovirus enteritis, AEEC |
| 4b | 09-110089 | Pos. | Neg. | Neg.: lung, MLN | Trichomoniasis, Peyer's patch lymphoid depletion, bronchointerstitial pneumonia |
| 5 | 09-123567 | Pos. | Neg. | Neg.: thymus, heart, trachea, lung, liver, pancreas, spleen | AEEC, Cystoisospora ohioensis, aspiration pneumonia |
| 6a | 10-51534A | Pos. | Neg. | Pos.: MLN (rare); Neg.: thymus, lung, stomach, pancreas | AEEC, aspiration pneumonia, bone marrow depletion, thymic and MLN lymphoid depletion |
| 6b | 10-51534B | Pos. | Neg. | Pos.: MLN (rare); Neg.: thymus, lung | AEEC, bronchopneumonia, bone marrow depletion, thymic, MLN and Peyer's patch lymphoid depletion |
| 7 | 12-120628 | Pos. | Neg. | Neg.: lung, tongue, stomach, MLN, liver, gallbladder, pancreas, kidney | Jejunojejunal intussusception, ulcerative gastritis, Peyer's patch lymphoid depletion, aspiration pneumonia |
| 8 | 12-159396 | Pos. | Pos. | Neg.: thymus, lung, liver, stomach, spleen, kidney | Mild, multifocal, acute hepatocellular necrosis |
| 9 | 13-47387 | Pos. | Pos. | Pos.: MLN (rare); Neg.: lung, stomach | Peyer's patch lymphoid depletion, mild, multifocal, subacute hepatocellular necrosis |
SI, small intestine; LI, large intestine; Pos., positive; Neg., negative; NA, not available; MLN, mesenteric lymph node; AEEC, attaching-effacing Escherichia coli.
FIG 2.
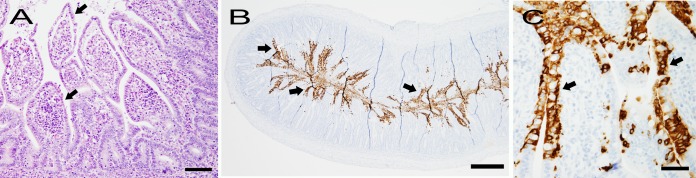
Photomicrographs of small intestine from puppy 8 with typical lesions of CCoV infection. (A) The villus epithelial cells are diffusely disorganized, attenuated, and low cuboidal (arrows). Note that the space between the epithelial cells and the lamina propria is an artifact of processing (hematoxylin and eosin stain; bar, 100 μm). (B) Cross-section of small intestine showing CCoV antigen in villus epithelial cells (arrows; immunohistochemical stain; bar, 500 μm). (C) Higher magnification showing CCoV antigen in the cytoplasm of villus epithelial cells (arrows; immunohistochemical stain; bar, 50 μm).
Virus isolation.
CCoV was isolated from puppy 4b, and canine parvovirus was isolated from puppy 4a. CCoV cytopathic effect (CPE), consisting of cell rounding, cell death, and syncytium formation, was observed 24 h postinoculation of canine A-72 cells (Fig. 3A and B). The other two cell lines did not yield CCoV. Infected A-72 cells were positive for the presence of CCoV antigen by FA staining (Fig. 3C). The sequencing of CCoV RNA extracted from infected cell culture lysates further confirmed the infection of puppy 4b with CCoV-IIa that corresponded to the RT-PCR assay and sequencing results from corresponding FFPE intestinal specimens.
FIG 3.
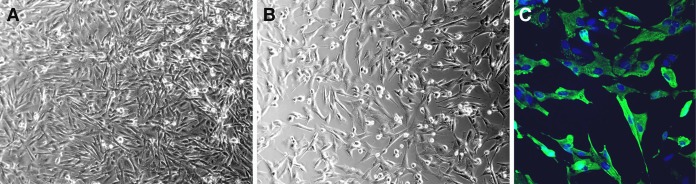
Canine fibroblast-like A-72 cells, 24 h postinfection, with puppy 4b CCoV. Phase-contrast microscopy of uninfected cell culture monolayer (A) and CCoV-infected cell culture monolayer with cytopathic effect characterized by rounding and death of individual cells (B) (10× original magnification). Immunofluorescence microscopy of CCoV-infected cell culture monolayer (mouse monoclonal antibody FIPV3-70 followed by Alexa Fluor 488-conjugated anti-IgG; 20× original magnification).
RT-PCR, genotyping, and comparative sequence analysis.
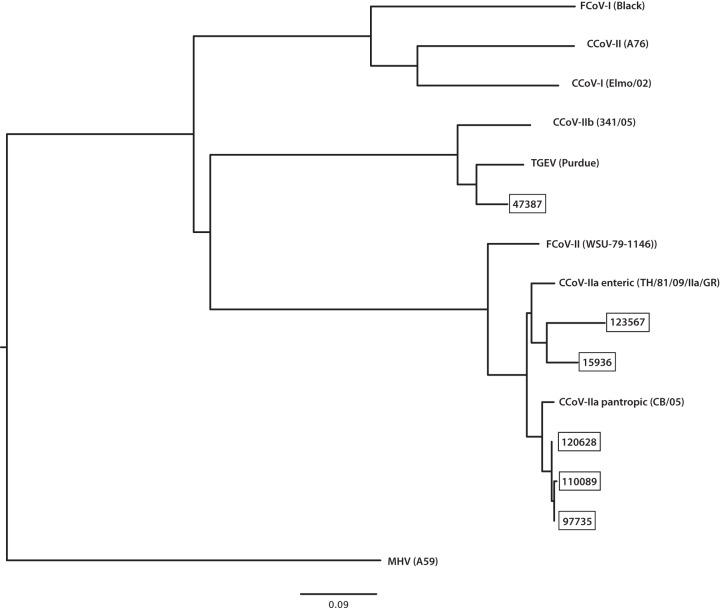
Coronavirus RNA was detected by RT-PCR in 9 out of 11 puppies; insufficient CCoV RNA was recovered from puppies 1 and 6b (Table 4). Comparative sequence analysis revealed CCoV-II in all 9 cases. Consequently, the S1/S2 cleavage site was not analyzed, because it is present only in the CCoV-I genotype (Fig. 1) (39). The CCoVs from puppies 3, 4b, 5, 7, and 8 were subtyped as CCoV-IIa, while the CCoV from puppy 9 was subtyped as CCoV-IIb; those from puppies 2, 4a, and 6a could not be subtyped. The S2′ site was sequenced in eight out of the nine puppies from which CCoV RNA was successfully extracted. No variations in amino acid sequence at the cleavage site were detected (Table 4). Based on a neighbor-joining phylogenic tree of available CCoV NTDs (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov/), CCoV-IIa viruses from puppies 3, 4b, 5, 7, and 8 clustered with other CCV-IIa viruses, including those associated with previous reports of pantropic CCoV in the EU, while the CCoV-IIb from puppy 9 clustered with CCoV-IIb from the EU (Fig. 4).
TABLE 4.
Results of CCoV genotyping and subtyping with S2′ and S1/S2 cleavage site sequences
| CCoVa (case) | Genotype | Subtypec | Cleavage site sequenceb |
|
|---|---|---|---|---|
| S2′ | S1/S2 | |||
| 89334-09 (2) | II | ND | KRKYRS | ARTR—–G |
| 97736-09 (3) | II | a | KRKYRS | ERTR—–G |
| 107207 (4a) | II | ND | KRKYRS | DRTR—–G |
| 110089-09 (4b) | II | a | KRKYRS | ARTR—–G |
| 123567-09 (5) | II | a | KRKYRS | ERTR—–G |
| 51534-10A (6a) | II | ND | KRKYRS | ARTR—–G |
| 120628-12 (7) | II | a | KRKYRS | ND |
| 159396-12 (8) | II | a | KRKYRS | ERTR—–G |
| 47387-13 (9) | II | b | KRKYRS | ARTR—–G |
| Reference | ||||
| CB/05 | II | a | KRKYRS | ARTR—–G |
| 1-71 | II | a | KRKYRS | ERTR—–G |
| 341/05 | II | b | KRKYRS | ARTR—–G |
| Elmo/02 | I | NA | QPGGRS | VRRARRAVQG |
The reference CCoVs are the following: CB/05, pantropic CCoV-IIa (AAZ91437.1); 1-71, enteric CCoV-IIa (AAV65515.1); 341/05, CCoV-IIb (ACJ63231.1); and Elmo/02, CCoV-I (AAP72149). They are included to highlight the conserved nature of the S2′ cleavage site within genotype II viruses.
Basic residues suspected to be important for cleavage activation of the spike protein are in boldface.
ND, not determined. NA, not applicable.
FIG 4.
Phylogenetic tree based on the spike protein N-terminal domain (NTD). Sequences are identified as CCoV-IIa or -IIb, followed by the case number.
DISCUSSION
Although closely related to pantropic CCoV-IIa, the CCoV-IIa viruses associated with neonatal mortality in our study were restricted to the intestinal tract; therefore, the infections they caused were consistent with classical enteric CCoV infection (19, 40). The use of immunostaining to confirm CCoV disease in our study might explain the lack of detection of pantropic CCoV. Previous work on pantropic CCoV has relied mainly on RT-PCR to detect systemic spread. RT-PCR is very sensitive and can detect viral genome in tissues without productive viral replication or infection being present. IHC is arguably a better method for detecting clinically relevant infection because it requires high levels of viral antigen and, as a result, viral replication to yield a positive result. In addition, IHC can localize antigen to biologically relevant cellular compartments, such as the cytoplasm of infected villus epithelial cells (Fig. 2B and C). Because the detection of viral antigen by immunostaining is highly time dependent, early infection with concentrations of CCoV antigen below the detection limit of the assay cannot be ruled out completely as a reason for the lack of detection of CCoV in extraintestinal tissues of our puppies. The confirmation of pantropic CCoV in previous reports relied primarily on RT-PCR assays; however, whether viral replication or infection is present in individual tissues cannot be conclusively confirmed by this method alone. Conversely, the isolation of CCoVs from extraintestinal tissues has been reported, but the clinical significance of this finding is unclear without the colocalization of extraintestinal lesions with viral antigen. Previous reports with FCoV have confirmed viral RNA by RT-PCR in tissues obtained from otherwise healthy cats (41). As with CCoV, immunostaining generally is negative in these cases. In the same studies, the positive RT-PCR results were attributed to viremia, a finding that is common among cats experiencing asymptomatic enteric infection with FCoV (42). The possibility that viremia is associated with low-level virus replication within tissues of healthy animals cannot be ruled out completely (41).
The negative virus isolation result from pooled samples of lung and intestine from puppy 2 might be attributed to the low level of CCoV, as suggested from the small numbers of epithelial cells with viral antigen in immunostained sections of small intestine from this puppy. Conversely, negative CCoV isolation results from multiple nonintestinal tissues taken from puppies 2, 4a, and 8 further suggest a lack of viral replication in extraintestinal tissues from these puppies.
Viral tropism extended to the large intestines in puppies 8 and 9, which were infected with CCoV-IIa and CCoV-IIb, respectively. To our knowledge, colonic infection has been documented in only one of five experimentally infected 10-week-old puppies, 10 days postinoculation with the C54 reference CCoV-II (40, 43). Given that concurrent pathogens were not found, extensive intestinal infection with CCoV alone most likely accounted for the demise of puppies 8 and 9. In support of this interpretation was the presence of hepatocellular necrosis in these puppies, a common finding in animals with extensive loss of intestinal barrier integrity which results in the showering of the portal circulation with toxic products. Interestingly, these were the youngest puppies (4 days and 2 weeks), and host factors such as age may have contributed to CCoV infection of colonic epithelial cells.
Lymphopenia is a common clinical finding in reports of dogs with pantropic CCoV infection. Although hemograms were not available, lymphoid depletion of the thymus, mesenteric lymph nodes, or intestinal Peyer's patches was present in eight out of 10 puppies that had lymphoid tissues available. Similar lymphoid depletion was found in two puppies with fatal CCoV-associated enteritis previously described in the United States (19). Consistent with the previous report, CCoV infection of lymphoid tissues was not found in our study. Infections with other coronaviruses, including Middle East respiratory syndrome (MERS) coronavirus, severe acute respiratory syndrome (SARS) coronavirus, equine coronavirus (ECoV), and FCoV are associated with lymphopenia (44–47). Where the cause of lymphopenia has been investigated, it is attributed to indirect mechanisms secondary to the viral infection, such as cytokine-mediated apoptosis (48, 49).
All but one of the puppies in our study originated from high-density housing where outbreaks of enterocolitis were ongoing. Over half (6/11, or 54%) of our cases had concurrent intestinal infections with various combinations of pathogens. Coinfection with canine parvovirus, a known risk factor for CCoV-associated mortality, was found only in puppy 4a. Severe intestinal damage can result in the translocation of toxic products to extraintestinal tissues, particularly the lungs and liver. Consistent with this observation, bronchopneumonia and hepatocellular necrosis were present in four puppies. Additionally, aspiration pneumonia, a common clinical complication seen in young debilitated puppies that are vomiting, also was present in four puppies. The clinical significance of intestinal infection with AEEC in four puppies is unclear; however, the presence of small intestinal epithelial colonization by these organisms likely contributed to clinical signs of intestinal dysfunction, leading to mortality in these cases. Clearly, host and environmental factors, such as overcrowding of puppies, coinfections with intestinal pathogens, pathogen load, and degree of maternal immunity can determine the outcome of CCoV-associated enteritis. Although mutations in the S2′ cleavage site were not found, it remains possible that unidentified viral factors also contributed to the fatal outcomes. These factors may include variations in the NTD, a region that is known to be important for enterotropism in TGEV. The subtyping of CCoV-II was based on the amino acid sequence of the NTD (Fig. 1). Because both CCoV-IIa and -IIb viruses were associated with fatal outcomes, it appears that viruses with a CCoV-like NTD (subtype CCoV-IIa) or a TGEV-like NTD (CCoV-IIb) have the potential to cause fatal enteritis.
Intussusception was observed in the small intestine of 2 of the 11 puppies with fatal CCoV-associated enteritis (Table 3). Interestingly, puppy 3 was from a breeding facility where a littermate with small intestinal intussusception recovered following surgical resection and anastomosis. This breeder recalled having over a dozen other puppies with small intestinal intussusception over the last 2 years following the introduction of several breeders acquired from Sweden, where CCoV outbreaks were documented around the same period (31). Interestingly, a similar association between CCoV infection and small intestinal intussusception was reported in one of the two puppies previously described in the United States (19). The pathogenesis of intestinal intussusception associated with enteric viral infection is not well understood; however, in human infants, intestinal intussusception has been associated with adenovirus and enterovirus infections as well as vaccination with a discontinued live-attenuated rhesus-human reassortant rotavirus tetravalent vaccine (50–52). The observation that CCoV-associated enteritis can sometimes be present with small intestinal intussusception suggests a similar pathogenesis.
Our study provides detailed pathological findings in 11 puppies with fatal CCoV-associated enteritis that originated from nine premises in five states in the United States between 2008 and 2013. Key regions of the viral spike gene were sequenced to determine if viral factors played a role in these CCoV-associated mortalities. Owing to the retrospective nature of the present study with available FFPE tissue samples collected at necropsy with variance in postmortem intervals, sample quality, and degree of RNA cross-linking by formalin fixation, combined with the relatively low ratio of viral to cellular RNA within tissues and the natural variability of the CCoV S gene, limited our ability to use next-generation sequencing methods to capture a more complete assessment of the viral populations involved in these cases. Another limitation of the present study was our inability to subtype the CCoV-II viruses from three puppies, which likely was attributable to divergent NTD that could not be amplified by our PCR primers. Lastly, approximately 50% prevalence of CCoV-I and CCoV-II coinfections has been reported previously; however, we were unable to detect any CCoV-I infections in our study. Our CCoV-I primers were based on multiple alignments of previously published CCoV-I virus sequences in the region of the S1/S2 and S2′ cleavage sites. It is possible that the puppies in our study were infected with divergent CCoV-I viruses that were not amplified by our S-specific primers.
This study revealed the presence of CCoV-IIb variants in the United States and highlighted the potential of CCoV-IIa and CCoV-IIb to cause morbidity and mortality in puppies. Extended tissue tropism of CCoV to the large intestine was found in two puppies; however, pantropic CCoV infections were not identified. The isolation of CCoV-IIa from puppy 4b confirmed the validity of genotyping results obtained from the corresponding FFPE tissue sections. CCoVs should be considered a differential diagnosis and specifically sought in outbreaks of severe enteritis among puppies up to 21 weeks of age, particularly when housed in high population density but also in cases with small intestinal intussusception or enteritis associated with infections caused either by parvovirus, AEEC, or protozoan parasites.
ACKNOWLEDGMENTS
We thank Kelly L. Sams and Wendy O. Wingate for technical assistance and Jean K. Millet for critical review of the manuscript. We also thank Deanna Shaffer for insights.
This work was supported by funds from the College of Veterinary Medicine at Cornell University.
Footnotes
Published ahead of print 24 September 2014
REFERENCES
- 1. Binn LN, Lazar EC, Keenan KP, Huxsoll DL, Marchwicki RH, Strano AJ. 1974. Recovery and characterization of a coronavirus from military dogs with diarrhea, p 359–366 Proceedings of the 18th annual meeting of the United States Animal Health Association. U.S. Animal Health Association, St Joseph, MO. [PubMed] [Google Scholar]
- 2. King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed). 2012. Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 3. Bandai C, Ishiguro S, Masuya N, Hohdatsu T, Mochizuki M. 1999. Canine coronavirus infections in Japan: virological and epidemiological aspects. J. Vet. Med. Sci. 61:731–736. 10.1292/jvms.61.731. [DOI] [PubMed] [Google Scholar]
- 4. Naylor MJ, Monckton RP, Lehrbach PR, Deane EM. 2001. Canine coronavirus in Australian dogs. Aust. Vet. J. 79:116–119. 10.1111/j.1751-0813.2001.tb10718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulz BS, Strauch C, Mueller RS, Eichhorn W, Hartmann K. 2008. Comparison of the prevalence of enteric viruses in healthy dogs and those with acute haemorrhagic diarrhoea by electron microscopy. J. Small Anim. Pract. 49:84–88. 10.1111/j.1748-5827.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stavisky J, Pinchbeck G, Gaskell RM, Dawson S, German AJ, Radford AD. 2012. Cross sectional and longitudinal surveys of canine enteric coronavirus infection in kennelled dogs: a molecular marker for biosecurity. Infect. Genet. Evol. 12:1419–1426. 10.1016/j.meegid.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ntafis V, Mari V, Decaro N, Papanastassopoulou M, Pardali D, Rallis TS, Kanellos T, Buonavoglia C, Xylouri E. 2013. Canine coronavirus, Greece. Molecular analysis and genetic diversity characterization. Infect. Genet. Evol. 16:129–136. 10.1016/j.meegid.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keenan KP, Jervis HR, Marchwicki RH, Binn LN. 1976. Intestinal infection of neonatal dogs with canine coronavirus 1-71: studies by virologic, histologic, histochemical, and immunofluorescent techniques. Am. J. Vet. Res. 37:247–256. [PubMed] [Google Scholar]
- 9. Saif LJ. 1990. Comparative aspects of enteric viral infections, p 9–31 In Saif LJ, Thiel KW. (ed), Viral diarrheas of man and animals. CRC Press, Inc, Boca Raton, FL. [Google Scholar]
- 10. Appel MJG. 1988. Does canine coronavirus augment the effects of subsequent parvovirus infection? Vet. Med. 83:360–366. [Google Scholar]
- 11. Pratelli A, Tempesta M, Roperto FP, Sagazio P, Carmichael L, Buonavoglia C. 1999. Fatal coronavirus infection in puppies following canine parvovirus 2b infection. J. Vet. Diagn. Investig. 11:550–553. 10.1177/104063879901100615. [DOI] [PubMed] [Google Scholar]
- 12. Greene CE, Decaro N. 2011. Canine viral enteritis. In Greene CE. (ed), Infectious diseases of the dog and cat, 4th ed. Elsevier Health Sciences, Philadelphia, PA. [Google Scholar]
- 13. Buonavoglia C, Decaro N, Martella V, Elia G, Campolo M, Desario C, Castagnaro M, Tempesta M. 2006. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 12:492–494. 10.3201/eid1203.050839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zappulli V, Caliari D, Cavicchioli L, Tinelli A, Castagnaro M. 2008. Systemic fatal type II coronavirus infection in a dog: pathological findings and immunohistochemistry. Res. Vet. Sci. 84:278–282. 10.1016/j.rvsc.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Decaro N, Mari V, Campolo M, Lorusso A, Camero M, Elia G, Martella V, Cordioli P, Enjuanes L, Buonavoglia C. 2009. Recombinant canine coronaviruses related to transmissible gastroenteritis virus of swine are circulating in dogs. J. Virol. 83:1532–1537. 10.1128/JVI.01937-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ntafis V, Mari V, Decaro N, Papanastassopoulou M, Papaioannou N, Mpatziou R, Buonavoglia C, Xylouri E. 2011. Isolation, tissue distribution and molecular characterization of two recombinant canine coronavirus strains. Vet. Microbiol. 151:238–244. 10.1016/j.vetmic.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zicola A, Jolly S, Mathijs E, Ziant D, Decaro N, Mari V, Thiry E. 2012. Fatal outbreaks in dogs associated with pantropic canine coronavirus in France and Belgium. J. Small Anim. Pract. 53:297–300. 10.1111/j.1748-5827.2011.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Decaro N, Cordonnier N, Demeter Z, Egberink H, Elia G, Grellet A, Le Poder S, Mari V, Martella V, Ntafis V, von Reitzenstein M, Rottier PJ, Rusvai M, Shields S, Xylouri E, Xu Z, Buonavoglia C. 2013. European surveillance for pantropic canine coronavirus. J. Clin. Microbiol. 51:83–88. 10.1128/JCM.02466-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Evermann JF, Abbott JR, Han S. 2005. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J. Vet. Diagn. Investig. 17:610–614. 10.1177/104063870501700618. [DOI] [PubMed] [Google Scholar]
- 20. Decaro N, Mari V, Elia G, Addie DD, Camero M, Lucente MS, Martella V, Buonavoglia C. 2010. Recombinant canine coronaviruses in dogs, Europe. Emerg. Infect. Dis. 16:41–47. 10.3201/eid1601.090726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Decaro N, Buonavoglia C. 2008. An update on canine coronaviruses: viral evolution and pathobiology. Vet. Microbiol. 132:221–234. 10.1016/j.vetmic.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Poder S. 2011. Feline and canine coronaviruses: common genetic and pathobiological features. Adv. Virol. 2011:609465. 10.1155/2011/609465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perlman S, Gallagher T, Snijder EJ. 2008. Nidoviruses. ASM Press, Washington, DC. [Google Scholar]
- 24. Dye C, Temperton N, Siddell SG. 2007. Type I feline coronavirus spike glycoprotein fails to recognize aminopeptidase N as a functional receptor on feline cell lines. J. Gen. Virol. 88:1753–1760. 10.1099/vir.0.82666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Regan AD, Millet JK, Tse LP, Chillag Z, Rinaldi VD, Licitra BN, Dubovi EJ, Town CD, Whittaker GR. 2012. Characterization of a recombinant canine coronavirus with a distinct receptor-binding (S1) domain. Virology 430:90–99. 10.1016/j.virol.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schultze B, Krempl C, Ballesteros ML, Shaw L, Schauer R, Enjuanes L, Herrler G. 1996. Transmissible gastroenteritis coronavirus, but not the related porcine respiratory coronavirus, has a sialic acid (N-glycolylneuraminic acid) binding activity. J. Virol. 70:5634–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krempl C, Schultze B, Laude H, Herrler G. 1997. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 71:3285–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Decaro N, Campolo M, Lorusso A, Desario C, Mari V, Colaianni ML, Elia G, Martella V, Buonavoglia C. 2008. Experimental infection of dogs with a novel strain of canine coronavirus causing systemic disease and lymphopenia. Vet. Microbiol. 128:253–260. 10.1016/j.vetmic.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marinaro M, Mari V, Bellacicco AL, Tarsitano E, Elia G, Losurdo M, Rezza G, Buonavoglia C, Decaro N. 2010. Prolonged depletion of circulating CD4+ T lymphocytes and acute monocytosis after pantropic canine coronavirus infection in dogs. Virus Res. 152:73–78. 10.1016/j.virusres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wesley RD. 1999. The S gene of canine coronavirus, strain UCD-1, is more closely related to the S gene of transmissible gastroenteritis virus than to that of feline infectious peritonitis virus. Virus Res. 61:145–152. 10.1016/S0168-1702(99)00032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Escutenaire S, Isaksson M, Renstrom LH, Klingeborn B, Buonavoglia C, Berg M, Belak S, Thoren P. 2007. Characterization of divergent and atypical canine coronaviruses from Sweden. Arch. Virol. 152:1507–1514. 10.1007/s00705-007-0986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Licitra BN, Millet JK, Regan AD, Hamilton BS, Rinaldi VD, Duhamel GE, Whittaker GR. 2013. Mutation in spike protein cleavage site and pathogenesis of feline coronavirus. Emerg. Infect. Dis. 19:1066–1073. 10.3201/eid1907.121094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White AK, Hansen-Lardy L, Brodersen BW, Kelling CL, Hesse RA, Duhamel GE. 1998. Enhanced immunohistochemical detection of infectious agents in formalin-fixed, paraffin-embedded tissues following heat-mediated antigen retrieval. J. Vet. Diagn. Investig. 10:214–217. 10.1177/104063879801000225. [DOI] [PubMed] [Google Scholar]
- 34. Dubovi EJ, Hawkins M, Griffin RA, Jr, Johnson DJ, Ostlund EN. 2013. Isolation of Bluetongue virus from canine abortions. J. Vet. Diagn. Investig. 25:490–492. 10.1177/1040638713489982. [DOI] [PubMed] [Google Scholar]
- 35. Herrewegh AA, de Groot RJ, Cepica A, Egberink HF, Horzinek MC, Rottier PJ. 1995. Detection of feline coronavirus RNA in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase PCR. J. Clin. Microbiol. 33:684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Madu IG, Roth SL, Belouzard S, Whittaker GR. 2009. Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide. J. Virol. 83:7411–7421. 10.1128/JVI.00079-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simmerson SM, Armstrong PJ, Wunschmann A, Jessen CR, Crews LJ, Washabau RJ. 2014. Clinical features, intestinal histopathology, and outcome in protein-losing enteropathy in Yorkshire Terrier dogs. J. Vet. Intern. Med. 28:331–337. 10.1111/jvim.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wales AD, Woodward MJ, Pearson GR. 2005. Attaching-effacing bacteria in animals. J. Comp. Pathol. 132:1–26. 10.1016/j.jcpa.2004.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pratelli A, Martella V, Decaro N, Tinelli A, Camero M, Cirone F, Elia G, Cavalli A, Corrente M, Greco G, Buonavoglia D, Gentile M, Tempesta M, Buonavoglia C. 2003. Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. J. Virol. Methods 110:9–17. 10.1016/S0166-0934(03)00081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tennant BJ, Gaskell RM, Kelly DF, Carter SD, Gaskell CJ. 1991. Canine coronavirus infection in the dog following oronasal inoculation. Res. Vet. Sci. 51:11–18. 10.1016/0034-5288(91)90023-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kipar A, Meli ML, Baptiste KE, Bowker LJ, Lutz H. 2010. Sites of feline coronavirus persistence in healthy cats. J. Gen. Virol. 91:1698–1707. 10.1099/vir.0.020214-0. [DOI] [PubMed] [Google Scholar]
- 42. Can-Şahna K, Ataseven Pınar VS, Oğuzoğlu TÇD. 2007. The detection of feline coronaviruses in blood samples from cats by mRNA RT-PCR. J. Feline Med. Surg. 9:369–372. 10.1016/j.jfms.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stavisky J, Pinchbeck GL, German AJ, Dawson S, Gaskell RM, Ryvar R, Radford AD. 2010. Prevalence of canine enteric coronavirus in a cross-sectional survey of dogs presenting at veterinary practices. Vet. Microbiol. 140:18–24. 10.1016/j.vetmic.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Groot-Mijnes JDF, van Dun JM, van der Most RG, de Groot RJ. 2005. Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease. J. Virol. 79:1036–1044. 10.1128/JVI.79.2.1036-1044.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiwanitkit V. 2007. Lymphopenia in severe acute respiratory syndrome: a summary on its frequency. Nepal Med. Coll. J. 9:132–133. [PubMed] [Google Scholar]
- 46. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, Makhdoom HQ, Zumla AI, Memish ZA. 2013. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 13:752–761. 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pusterla N, Mapes S, Wademan C, White A, Ball R, Sapp K, Burns P, Ormond C, Butterworth K, Bartol J, Magdesian KG. 2013. Emerging outbreaks associated with equine coronavirus in adult horses. Vet. Microbiol. 162:228–231. 10.1016/j.vetmic.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haagmans BL, Egberink HF, Horzinek MC. 1996. Apoptosis and T-cell depletion during feline infectious peritonitis. J. Virol. 70:8977–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chan PK, Chen GG. 2008. Mechanisms of lymphocyte loss in SARS coronavirus infection. Hong Kong Med. J. 14(Suppl 4):21–26. [PubMed] [Google Scholar]
- 50. Iskander J, Haber PP, Murphy T, Chen R, Sabin M. 2004. Suspension of rotavirus vaccine after reports of intussusception–United States, 1999. MMWR Morb. Mortal. Wkly. Rep. 53:879. [PubMed] [Google Scholar]
- 51. Chia AY, Chia JK. 2009. Intestinal intussusception in adults due to acute enterovirus infection. J. Clin. Pathol. 62:1026–1028. 10.1136/jcp.2008.063610. [DOI] [PubMed] [Google Scholar]
- 52. Arbizu RA, Aljomah G, Kozielski R, Baker SS, Baker RD. 21 January 2013. Intussusception associated with adenovirus. J. Pediatr. Gastroenterol. Nutr. 10.1097/MPG.0b013e3182868971. [DOI] [PubMed] [Google Scholar]