Abstract
Rapid and effective diagnosis of brucellosis is a challenge for clinicians. Even when diagnosis is on time and therapy is initiated, meticulous follow-up appointments are crucial for ensuring the efficacy of the treatment. Due to shortcomings of serological methods, molecular diagnosis, especially real-time PCR, is becoming a main approach in laboratory diagnostics. Thus, the development of efficient procedures and standardization of the PCR tests will have a great impact on the precise detection and quantification of bacterial DNA loads, which is valuable for the medical management of brucellosis patients. We developed a new TaqMan real-time PCR directed to bcsp31, a shared gene of the brucellae. The bcsp31 gene fragment was cloned into pJET1.2. Recombinant pJET1.2-bcsp31 was linearized by HindIII digestion, and the product was used for the preparation of a standard curve. A panel of Brucella spp. and non-Brucella pathogens was tested. No bacterial genomes other than those of the brucellae were detected. According to the results, specificity of the method was 100%. In a clinical assessment, the positive-control group comprised 37 patients with microbiologically confirmed brucellosis, and 25 healthy individuals served as the negative-control group. By the end of the treatment period, there was a significant decrease in the DNA load of the 37 brucellosis patients, which persisted for the 4 weeks of monitoring after treatment, suggesting that our proposed method is an efficient monitoring tool. Serum samples prior to any treatment were collected from the 25 serologically suspicious patients and assessed by our method; 72% of these patients tested positive for brucellosis.
INTRODUCTION
The brucellae are facultative, intracellular, Gram-negative bacteria that infect animals and humans (1). Although it has been eradicated in some developed countries, brucellosis still remains a major problem worldwide. Diagnosis of the disease presents a major challenge to clinical and veterinary services (2, 3). Brucella spp. are able to survive within host cells, leading to relapses and focal complications (1). Early diagnosis of brucellosis results in early initiation of therapy, which plays a key role in the success of the control and eradication programs; nonetheless, accurate diagnosis requires reliable and sensitive diagnostic tools (4). The intracellular localization of the bacteria and the slow evolution of the disease hamper the usefulness of blood cultures (5). Despite being the gold standard, isolation of brucellae from blood culture or other sterile body fluids has a sensitivity of about 70% (4, 6). Serodiagnostic assays are principally based on antibodies against the Brucella lipopolysaccharide (LPS) and have high sensitivity but low specificity. The low specificity may be due to the cross-reaction of antibodies with LPS from other bacterial species and subsensitive or high-immunity reactions, depending on the subclinical or endemic prevalence of the disease (4, 6, 7). The presence of IgG and IgM for months after therapy complicates the interpretation of serological tests in patients from areas where brucellosis is endemic and limits the accuracy of diagnosis and posttreatment follow-up (8, 9).
Molecular diagnostic techniques, including real-time PCR, have higher efficiencies than that of culture methods and serological tests and thus offer a more promising performance (10). Real-time PCR systems exhibit improved sensitivity, specificity, and speed; due to their combined use of fluorogenic dyes and direct detection, these methods further eliminate the need for postamplification detection procedures (11). In addition, quantitative real-time PCR is a useful tool for differentiating between inactive but serologically positive brucellosis and active brucellosis (12, 13). The goals of our present study were to design a quantitative TaqMan-based real-time PCR assay and to evaluate the performance of this assay using human serum samples.
MATERIALS AND METHODS
Bacterial strains and DNA extraction.
Brucella abortus strains S19, 544, 133, and RB51, Brucella melitensis strains 16M and Rev-1, 15 clinical isolates of B. abortus, and 12 clinical isolates of B. melitensis were used as positive controls for the assay. Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Enterococcus faecalis (ATCC 25212), Bacillus cereus (ATCC 9634), Listeria monocytogenes, Legionella pneumophila (clinical isolate), Helicobacter pylori (ATCC 26695), Pseudomonas aeruginosa (ATCC 8821), Neisseria meningitidis type B, Klebsiella pneumoniae (clinical isolate), Salmonella enterica serovar Typhimurium, Salmonella enterica, Mycobacterium tuberculosis H37Rv (genome isolated previously), and Yersinia enterocolitica were used as non-Brucella species. DNA was extracted from a fresh culture of each strain with the QIAamp DNA minikit (Qiagen, USA) according to the manufacturer's instructions.
Patients and clinical specimens.
Serum samples used in this study from three groups, patients with suspected brucellosis, a positive-control group, and a negative-control group. Twenty-five serum samples from the patients with suspected brucellosis from the western areas of Iran were collected from 2010 through 2012. Eight of these cases had a history of previous infection. According to our current guidelines and published procedures for the diagnosis of human brucellosis, suspected patients were screened by serological tests. Seropositive results were further confirmed by a blood culture method and also by complementary serological tests, if needed (14, 15).
For the negative- and positive-control groups, 25 serum samples from healthy volunteers with no history of brucellosis and 37 serum samples from patients with confirmed brucellosis, respectively, were collected. Of the patients in the positive-control group confirmed to have brucellosis, seven experienced relapse of brucellosis. Patients with brucellosis had been confirmed on the basis of clinical manifestations, serological evidence, and bacteriological findings. As described above, one blood sample was collected for culture, and multiple serum samples were collected for tracing bacterial DNA from each patient. An initial serum sample was collected before treatment, one was collected at week 4 during therapy, a sample was obtained after completion of the therapy (at week 6), and a final serum sample was obtained 4 weeks posttreatment. The therapeutic regimen consisted of a combination of doxycycline (200 mg/day by mouth for 6 weeks) plus rifampin (600 to 900 mg/day by mouth for 6 weeks). All clinical experiments involving human patients were performed according to Tarbiat Modares Institutional Guidelines for Clinical Studies and also Ethical Guidelines for Clinical Studies by IR Ministry of Health and Medical Education (document BP-QP-106-01).
Blood culture and serological method.
Blood cultures were performed using the Bactec alert automated system. A standard tube agglutination (STA) test was carried out for all serum samples; titers that were ≥1:160 were considered positive (16, 17).
DNA extraction from serum samples.
Serum samples were preserved at −70°C. DNA extraction from serum and from clinical isolates was performed with the QIAamp DNA minikit (Qiagen) in accordance with the manufacturer's instructions. The quality of each sample was monitored by a spectrophotometer (NanoDrop 2000), after which the samples were stored at −20°C until tested.
Primers and probe.
AlleleID software, version 7.0 (PREMIER Biosoft) was used to design a TaqMan probe and primers. The TaqMan real-time PCR assay was designed based on the genus-specific bcsp31 gene, which encodes an immunogenic outer membrane protein of 31 kDa. The 166-bp amplicon was amplified with the primers Mbm1 (5′-ATCGTTCTTGAAGCCTAC-3′) and Mbm2 (5′-AAATACCGTTCGAGATGG-3′). We designed a 24-bp TaqMan probe, MbmTq (5′-ATATCAAGGCTGAACACCTGAAGC-3′), in which the 3′ end was blocked with a phosphate group to prevent involvement of the probe in the PCR extension reaction. The MbmTq probe was fluorescently labeled at the 5′ end with 6-carboxyfluorescein phosphoramidite (FAM) as a reporter dye and at the 3′ end with 5-carboxytetramethylrhodamine (TAMRA) as a quencher. The theoretical specificities of the primers and the probe were determined by comparison with those in the GenBank database using the Basic Local Alignment Search Tool (BLAST).
Plasmid preparation for standard curve.
Using the primers Mbm1 and Mbm2, the 166-bp amplification product from B. melitensis 16M was cloned into pJET1.2 and transferred into Escherichia coli DH-5α using the Clone JET PCR cloning kit (Thermo Scientific). The identity of the inserted bacterial DNA was confirmed by sequencing. A recombinant plasmid was purified using the AccuPrep Nano-Plus plasmid mini extraction kit (Bioneer) and was linearized by digestion with HindIII (Thermo Scientific). The DNA concentration was determined by measuring the absorbance at 260 nm in a NanoDrop 2000 spectrophotometer, and the corresponding copy number was calculated accordingly. Data from the standard dilution series, using linearized plasmid, were used to generate the standard curve (the cycle threshold [CT] values from the standard reactions plotted against standard quantities).
Real-time PCR assay conditions.
Real-time PCR amplifications were conducted in a total reaction volume of 20 μl, and comprised 10 μl of Premix Ex Taq (Perfect Real Time) reagents (TaKaRa), 100 nM of each primer, 250 nM of the MbmTq probe, and 100 ng of the template DNA from the patients and the controls. PCRs were carried out with an ABI 7500 apparatus (Applied Biosystems) according to the following profile: initial template denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s and 60°C for 34 s. Data analysis was performed by using 7500 system SDS software (Applied Biosystems). The second derivative maximum algorithm was used to calculate the CT value.
All runs included negative and positive controls. The positive control contained 5.5 × 104 copies of plasmid per reaction. All samples were tested in duplicate. A result was considered negative when no amplification occurred or when the CT value was >37 cycles; results were considered positive when both replicates were positive. The bacterial DNA load per milliliter of serum was calculated from the standard curve.
Statistical analysis.
The Student t test and the χ2 analysis were used to compare continuous variables and categorical variables, respectively. A P value of <0.05 was considered statistically significant.
RESULTS
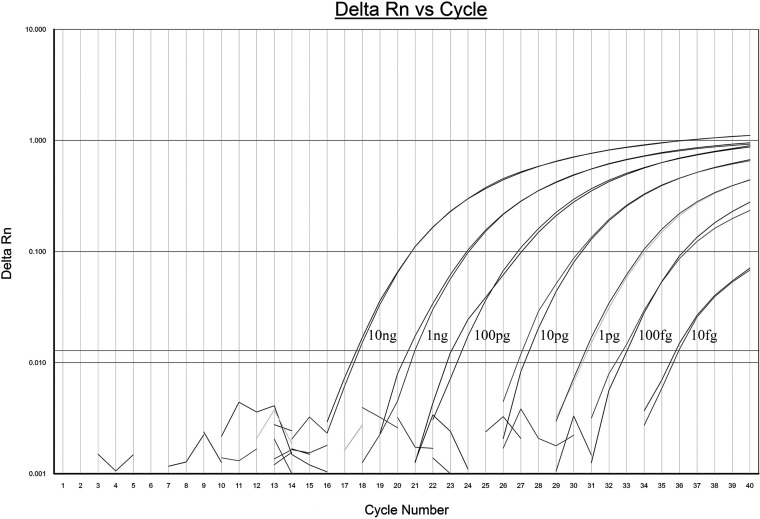
The analytical sensitivity of the real-time PCR assay was performed by amplifying 10-fold serially diluted B. melitensis 16M and B. abortus S19 genomic DNA samples (from 10 ng to 1 fg). The detection limit of the assay was 10 fg (equivalent to 2 genome copies), and we used the linear regression equation CT = −3.17 log (copy number) + 37.7; the correlation coefficient (R2) was 0.99, and the PCR efficiency (E) was 2.0 (Fig. 1).
FIG 1.
Real-time PCR amplification curve of B. melitensis strain 16M genomic DNA. A 10-fold dilution series from 10 ng to 10 fg was used as a template.
Amplification of a 5-fold serial dilution of the linearized recombinant plasmid, harboring a 166-bp fragment from B. melitensis 16M, exhibited a linear correlation in the range of 101 to 105 copies per reaction mixture, with an R2 value of 0.99 and a PCR efficiency of 2. The linear regression equation we used was CT = −3.16 log (copy number) + 37.2.
Positive results were obtained from all Brucella genome samples, and none of the 14 non-Brucella species (Gram negative or Gram positive) tested was detectable by the Brucella-specific TaqMan real-time PCR assay developed in this study (data not shown).
As shown in Table 1, Brucella was detected in the blood cultures of 37 individuals who did not receive any antibiotic treatment before specimen collection. All of them were also positive based on the standard tube agglutination (STA) test, for which the titers ranged between 1:160 and 1:1280. In the negative-control group, 5 out of 25 individuals had an agglutination titer of ≤1:80.
TABLE 1.
Microbiological findings for patients with brucellosis, who were considered positive controls
| Patient | Gender | Blood culture result | Reciprocal STA titer | Initial serum DNA load (copies/ml) | History of brucellosis |
|---|---|---|---|---|---|
| 1 | Female | Positive | 160 | 11,420 | No |
| 2 | Male | Positive | 160 | 7,097 | No |
| 3 | Male | Positive | 1280 | 8,543 | Yes |
| 4 | Male | Positive | 160 | 53,295 | No |
| 5 | Female | Positive | 160 | 4,750 | No |
| 6 | Female | Positive | 640 | 25,475 | No |
| 7 | Female | Positive | 320 | 49,978 | No |
| 8 | Female | Positive | 320 | 5,000 | No |
| 9 | Female | Positive | 320 | 6,139 | No |
| 10 | Female | Positive | 320 | 4,634 | No |
| 11 | Male | Positive | 640 | 10,407 | Yes |
| 12 | Female | Positive | 320 | 13,420 | No |
| 13 | Male | Positive | 160 | 8,097 | Yes |
| 14 | Female | Positive | 160 | 8,510 | No |
| 15 | Female | Positive | 640 | 53,295 | No |
| 16 | Male | Positive | 640 | 4,765 | No |
| 17 | Male | Positive | 640 | 29,475 | No |
| 18 | Male | Positive | 640 | 50,212 | No |
| 19 | Female | Positive | 160 | 5,134 | No |
| 20 | Male | Positive | 320 | 11,420 | No |
| 21 | Female | Positive | 160 | 7,097 | No |
| 22 | Female | Positive | 160 | 8,543 | No |
| 23 | Female | Positive | 160 | 53,100 | Yes |
| 24 | Male | Positive | 320 | 4,780 | No |
| 25 | Male | Positive | 160 | 25,475 | No |
| 26 | Male | Positive | 320 | 50,000 | Yes |
| 27 | Male | Positive | 320 | 5,000 | No |
| 28 | Male | Positive | 1280 | 6,334 | Yes |
| 29 | Male | Positive | 640 | 6,079 | No |
| 30 | Male | Positive | 640 | 10,450 | No |
| 31 | Female | Positive | 320 | 13,427 | No |
| 32 | Female | Positive | 160 | 8,097 | No |
| 33 | Female | Positive | 320 | 8,576 | No |
| 34 | Male | Positive | 640 | 53,295 | No |
| 35 | Male | Positive | 160 | 4,715 | Yes |
| 36 | Male | Positive | 320 | 29,475 | No |
| 37 | Male | Positive | 160 | 50,000 | No |
All serum samples obtained from the 37 patients were positive for Brucella DNA, but none of the negative controls showed any signs of the Brucella genome; therefore, the specificity of the proposed assay proved to be 100%. In the positive-control group, the mean Brucella DNA load (± standard deviation [SD]) was 1.9 (±1.8) × 104 copies/ml (range, 4,634 to 5,3295 copies/ml). Patients who were undergoing treatment had a mean DNA load of 132 ± 68 copies/ml (range, 0 to 204 copies/ml). Patients at the end of treatment still showed a Brucella DNA load, with a mean of 27 ± 9 copies/ml (range, 14 to 34 copies/ml). The results for samples obtained 4 weeks after the end of the treatment consisted of 21 negative results, while others showed a mean DNA load of 35 ± 20 copies/ml (range, 8 to 59 copies/ml). The mean bacterial DNA load in relapsed patients was 2 (±1.8) × 104 copies/ml (range, 5,006 to 49,986 copies/ml) at the initial diagnosis. Statistically, results for those individuals with a history of the disease were not significantly different from those of first-time patients (P ≥ 0.05).
The results of real-time PCR and culture for patients suspected of having brucellosis are presented in Table 2. Among sera from patients with suspected brucellosis, 18 (72%) were positive by real-time PCR, with a mean bacterial DNA load of 2.1 × 104 copies/ml (range, 64 to 68,078 copies/ml). The blood culture was positive for only 10 out of 18 patients with suspected brucellosis; it was positive in all samples with a CT of ≤35, but the culture was negative in some samples with a CT of >35 but ≤37. Only four out of eight patients with a history of brucellosis tested positive with our real-time PCR assay, and their infections were also detected by blood culture (Table 2).
TABLE 2.
Results of serology, culture, and real-time PCR
| Real-time PCR CT (no. of samples)a | Serology (STA) |
History of brucellosis (n) | Positive blood culture (n) | |
|---|---|---|---|---|
| Titer | n | |||
| ≤35 (7) | 160 | 1 | 1 | 1 |
| 320 | 2 | 1 | 2 | |
| 640 | 4 | 4 | ||
| >35 to ≤37 (11) | 160 | 4 | ||
| 320 | 4 | 1 | 2 | |
| 640 | 3 | 1 | 1 | |
| >37 or undetermined (7) | 160 | 3 | 3 | |
| ≤80 | 4 | 1 | ||
A CT of ≤37 indicates the presence of Brucella, and a CT of >37 indicates the absence of Brucella.
DISCUSSION
The clinical and laboratory diagnosis, treatment, and follow-up of human brucellosis have remained medical challenges; the tendency of brucellosis to persist and relapse has complicated the problem. Furthermore, long-term persistence of Brucella DNA along with nonspecific symptoms has hindered the diagnosis of the disease by classical techniques (18). The inefficiency of conventional diagnostic methods, particularly serology (20), has coerced laboratories and physicians into applying real-time PCR as a more sensitive and specific tool. Real-time PCR reduces the time required to accomplish the detection and also increases the specificity and sensitivity, and it has the ability to quantify the bacterial DNA load (5, 21, 22). Quantification of the microbial load is valuable for the diagnosis and control of numerous infectious diseases, such as hepatitis and HIV infection. Due to the intracellular nature of Brucella infection and its potential to persist in host organs, bacterial DNA loads may serve as an indirect index of pathogen presence, which can be used for diagnosis and also for evaluation of treatment efficiency (5).
In this work, we developed a quantitative TaqMan real-time PCR assay and evaluated its diagnostic efficiency for the detection and quantification of Brucella DNA in human serum samples. Based on samples from 37 patients who tested positive by blood culture and from 25 healthy control samples, the sensitivity of real-time PCR proved to be 100%; such sensitivity was also reported by Navarro et al. (25).
Although most diagnostic studies of human brucellosis have been undertaken with whole-blood samples, serum is the preferred clinical specimen due to its simplicity of use and reduced inhibition effects from hemoglobin and human DNA (12, 13, 24). We included a complete set of control groups comprising healthy donors and patients with confirmed brucellosis in our study. These controls led to a more accurate interpretation of results for suspected samples. Among patients suspected of having brucellosis, 72% showed positive results based on our real-time PCR procedure. We were unable to distinguish between relapse and reinfection using our method; however, patients who had a history of brucellosis dating back to 3 years tested positive.
Our results obtained from patients under treatment clearly showed the significant decrease in bacterial DNA quantity. This is consistent with previous reports, which stated that during treatment of brucellosis, a significant decrease in bacterial DNA load occurs (25). The significance of the persistent bacterial DNA for a long period after antibiotic treatment is not clear. The detection of Brucella DNA in treated individuals coupled with clinical symptoms may support the occurrence of chronic disease (18). Detection of DNA with no clinical symptoms does not imply the presence of brucellosis, since many individuals who had recently undergone treatment may still have had positive real-time PCR results (25). Our data showed a significant decrease in the DNA load during treatment; only one patient had a negative real-time PCR result within this period.
Many studies have analyzed the Brucella DNA load in different stages of the disease. Of the suspected patients, 94.4% can be categorized as having active brucellosis according to a report by Queipo-Ortuño et al., in which 5 × 103 copies/ml was defined as the best cutoff value for bacterial DNA load in serum samples (12). Nevertheless, the mentioned cutoff point covered approximately 86% of patients in the positive-control group who had active brucellosis. Detection of the wide range of bacterial DNA load (from 0 to 68,078 copies/ml) in different stages of brucellosis (in different patients) showed that our system can be applied for diagnosis and that it is also useful in treatment follow-up surveys.
The precise determination of a genome copy number by real-time PCR necessitates shared targets with equal copy numbers among species and strains. IS711 has been targeted in several studies (4, 26–30). It is noteworthy that different copy numbers of IS711 in various species and biovars affect the accuracy of the bacterial load estimation by real-time PCR assays that target this fragment, particularly in culture-negative cases. Bounaadja et al. reported that the CT differences between IS711 and bcsp31 (ΔCT IS/bcsp) were 0.18 to 2.35 and 0.41 to 2.50 for the same B. abortus and B. melitensis DNA loads, respectively (31). Hence, the bcsp31 with one copy in brucellae can serve as an exact indicator for the detection of Brucella spp.
The real-time PCR signal from supercoiled DNA is less than that for linear conformations (32); therefore, in contrast to published work by Navarro et al. (25), we used a linear plasmid for the preparation of a standard curve. The detection limit of the bcsp31 real-time PCR in this work was 10 fg, which noticeably improved on results reported by Debeaumont et al. (11). Al Dahouk et al. (30) evaluated previously published real-time PCR assays targeting bcsp31 with IS711 and showed that the limit of detection varied widely among the assays (16 to 1,600 fg), concluding that some assays should not be applied to clinical samples (30). However, our results show that bcsp31-based assessments provide valued sensitivity.
Clinical management of human brucellosis remains problematic. It is difficult to determine an accurate cutoff point for serological methods, such as the STA test or the enzyme-linked immunosorbent assay (ELISA). Moreover, there is not a clear relationship between the antibody titer and the bacterial load in brucellosis patients. Of course, these differences seriously affect the diagnosis of new cases and follow-up of patients during the treatment. Although it cannot differentiate between relapses and new infections, our real-time PCR can be applied to more precisely detect brucellosis among patients with suspicious serological results or clinical symptoms. Additionally, this method is a useful monitoring tool for the evaluation of treatment efficacy and is a more reliable indicator of the patients' health status, helping clinicians make better and more informed diagnoses.
Footnotes
Published ahead of print 1 October 2014
REFERENCES
- 1. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. 2005. N. Engl. J. Med. 352:2325–2336. 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 2. Young EJ. 1995. An overview of human brucellosis. Clin. Infect. Dis. 21:283–289, quiz 290. 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]
- 3. Ratushna VG, Sturgill DM, Ramamoorthy S, Reichow SA, He Y, Lathigra R, Sriranganathan N, Halling SM, Boyle SM, Gibas CJ. 2006. Molecular targets for rapid identification of Brucella spp. BMC Microbiol. 6:13. 10.1186/1471-2180-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hinić V, Brodard I, Thomann A, Holub M, Miserez R, Abril C. 2009. IS711-based real-time PCR assay as a tool for detection of Brucella spp. in wild boars and comparison with bacterial isolation and serology. BMC Vet. Res. 5:22. 10.1186/1746-6148-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vrioni G, Pappas G, Priavali E, Gartzonika C, Levidiotou S. 2008. An eternal microbe: Brucella DNA load persists for years after clinical cure. Clin. Infect. Dis. 46:e131–136. 10.1086/588482. [DOI] [PubMed] [Google Scholar]
- 6. Queipo-Ortuño MI, Colmenero JD, Baeza G, Morata P. 2005. Comparison between lightcycler real-time polymerase chain reaction (PCR) assay with serum and PCR-enzyme-linked immunosorbent assay with whole blood samples for the diagnosis of human brucellosis. Clin. Infect. Dis. 40:260–264. 10.1086/426818. [DOI] [PubMed] [Google Scholar]
- 7. Mukherjee F, Jain J, Patel V, Nair M. 2007. Multiple genus-specific markers in PCR assays improve the specificity and sensitivity of diagnosis of brucellosis in field animals. J. Med. Microbiol. 56:1309–1316. 10.1099/jmm.0.47160-0. [DOI] [PubMed] [Google Scholar]
- 8. Ariza J, Pellicer T, Pallares R, Foz A, Gudiol F. 1992. Specific antibody profile in human brucellosis. Clin. Infect. Dis. 14:131–140. 10.1093/clinids/14.1.131. [DOI] [PubMed] [Google Scholar]
- 9. Young EJ. 1991. Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev. Infect. Dis. 13:359–372. 10.1093/clinids/13.3.359. [DOI] [PubMed] [Google Scholar]
- 10. Kattar MM, Zalloua PA, Araj GF, Samaha-Kfoury J, Shbaklo H, Kanj SS, Khalife S, Deeb M. 2007. Development and evaluation of real-time polymerase chain reaction assays on whole blood and paraffin-embedded tissues for rapid diagnosis of human brucellosis. Diagn. Microbiol. Infect. Dis. 59:23–32. 10.1016/j.diagmicrobio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11. Debeaumont C, Falconnet PA, Maurin M. 2005. Real-time PCR for detection of Brucella spp. DNA in human serum samples. Eur. J. Clin. Microbiol. Infect. Dis. 24:842–845. 10.1007/s10096-005-0064-0. [DOI] [PubMed] [Google Scholar]
- 12. Queipo-Ortuño MI, Colmenero JD, Bravo MJ, García-OrdoñezMÁ Morata P. 2008. Usefulness of a quantitative real-time PCR assay using serum samples to discriminate between inactive, serologically positive and active human brucellosis. Clin. Microbiol. Infect. 14:1128–1134. 10.1111/j.1469-0691.2008.02095.x. [DOI] [PubMed] [Google Scholar]
- 13. Zerva L, Bourantas K, Mitka S, Kansouzidou A, Legakis NJ. 2001. Serum is the preferred clinical specimen for diagnosis of human brucellosis by PCR. J. Clin. Microbiol. 39:1661–1664. 10.1128/JCM.39.4.1661-1664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ulu-Kilic A, Metan G, Alp E. 2013. Clinical presentations and diagnosis of brucellosis. Recent Pat. Antiinfect. Drug Discov. 8:34–41. 10.2174/1574891X11308010007. [DOI] [PubMed] [Google Scholar]
- 15. Mermut G, Ozgenc O, Avci M, Olut AI, Oktem E, Genc VE, Ari A, Coskuner SA. 2012. Clinical, diagnostic and therapeutic approaches to complications of brucellosis: an experience of 12 years. Med. Princ. Pract. 21:46–50. 10.1159/000331588. [DOI] [PubMed] [Google Scholar]
- 16. Sareyyüpoǧlu B, Cantekin Müştak Z, Kaan H. 2010. Investigation of Brucella antibodies in bovine sera by rose bengal plate test (RBPT), serum agglutination test (SAT), microagglutination test (MAT) and 2-mercaptoethanol-microagglutination (2-ME-MAT) test. Ankara Üniv Vet. Fak Derg. 57:157–160. [Google Scholar]
- 17. Cetin ES, Kaya S, Demirci M, Aridogan BC. 2007. Comparison of the BACTEC blood culture system versus conventional methods for culture of normally sterile body fluids. Adv. Ther. 24:1271–1277. [DOI] [PubMed] [Google Scholar]
- 18. Castaño MJ, Solera J. 2009. Chronic brucellosis and persistence of Brucella melitensis DNA. J. Clin. Microbiol. 47:2084–2089. 10.1128/JCM.02159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20. Çiftçi C, Öztürk F, Öztekin A, Karaoǧlan H, Saba R, Gültekin M, Mamikoǧlu L. 2005. Comparison of the serological tests used for the laboratory diagnosis of brucellosis. Mikrobiyol. Bul. 39:291–299 [In Turkish.] [PubMed] [Google Scholar]
- 21. Redkar R, Rose S, Bricker B, DelVecchio V. 2001. Real-time detection of Brucella abortus, Brucella melitensis and Brucella suis. Mol. Cell. Probes 15:43–52. 10.1006/mcpr.2000.0338. [DOI] [PubMed] [Google Scholar]
- 22. Queipo-Ortuño MI, Colmenero JD, Reguera JM, Garcia-Ordonez MA, Pachon ME, Gonzalez M, Morata P. 2005. Rapid diagnosis of human brucellosis by SYBR green I-based real-time PCR assay and melting curve analysis in serum samples. Clin. Microbiol. Infect. 11:713–718. 10.1111/j.1469-0691.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24. Kim SG, Kim YH, Chae MJ, Wan Kim JW, Lee YJ. 2010. Real-time PCR assay based glyceraldehydes 3-phosphate gene for identification of Brucella sp. J. Anim. Vet. Adv. 9:2315–2320. 10.3923/javaa.2010.2315.2320. [DOI] [Google Scholar]
- 25. Navarro E, Segura JC, Castano MJ, Solera J. 2006. Use of real-time quantitative polymerase chain reaction to monitor the evolution of Brucella melitensis DNA load during therapy and post-therapy follow-up in patients with brucellosis. Clin. Infect. Dis. 42:1266–1273. 10.1086/503035. [DOI] [PubMed] [Google Scholar]
- 26. Zhang B, Wear DJ, Stojadinovic A, Izadjoo M. 2013. Sequential real-time PCR assays applied to identification of genomic signatures in formalin-fixed paraffin-embedded tissues: a case report about Brucella-induced osteomyelitis. Mil. Med. 178:88–94. [DOI] [PubMed] [Google Scholar]
- 27. Sanjuan-Jimenez R, Morata P, Bermudez P, Bravo MJ, Colmenero JD. 2013. Comparative clinical study of different multiplex real-time PCR strategies for the simultaneous differential diagnosis between extrapulmonary tuberculosis and focal complications of brucellosis. PLoS Negl. Trop. Dis. 7:e2593. 10.1371/journal.pntd.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanjuan-Jimenez R, Colmenero JD, Bermudez P, Alonso A, Morata P. 2013. Amplicon DNA melting analysis for the simultaneous detection of Brucella spp and Mycobacterium tuberculosis complex. Potential use in rapid differential diagnosis between extrapulmonary tuberculosis and focal complications of brucellosis. PLoS One 8:e58353. 10.1371/journal.pone.0058353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cerekci A, Kilic S, Bayraktar M, Uyanik MH, Yasar E, Esen B. 2011. Comparison of conventional methods and real-time multiplex polymerase chain reaction for identification and typing of Brucella isolates of human origin. Mikrobiyol. Bul. 45:392–400 [In Turkish.] [PubMed] [Google Scholar]
- 30. Al Dahouk S, Nockler K, Scholz HC, Pfeffer M, Neubauer H, Tomaso H. 2007. Evaluation of genus-specific and species-specific real-time PCR assays for the identification of Brucella spp. Clin. Chem. Lab Med. 45:1464–1470. 10.1515/CCLM.2007.305. [DOI] [PubMed] [Google Scholar]
- 31. Bounaadja L, Albert D, Chenais B, Henault S, Zygmunt MS, Poliak S, Garin-Bastuji B. 2009. Real-time PCR for identification of Brucella spp.: a comparative study of IS711, bcsp31 and per target genes. Vet. Microbiol. 137:156–164. 10.1016/j.vetmic.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 32. Chen J, Kadlubar FF, Chen JZ. 2007. DNA supercoiling suppresses real-time PCR: a new approach to the quantification of mitochondrial DNA damage and repair. Nucleic Acids Res. 35:1377–1388. 10.1093/nar/gkm010. [DOI] [PMC free article] [PubMed] [Google Scholar]