Abstract
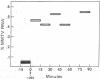
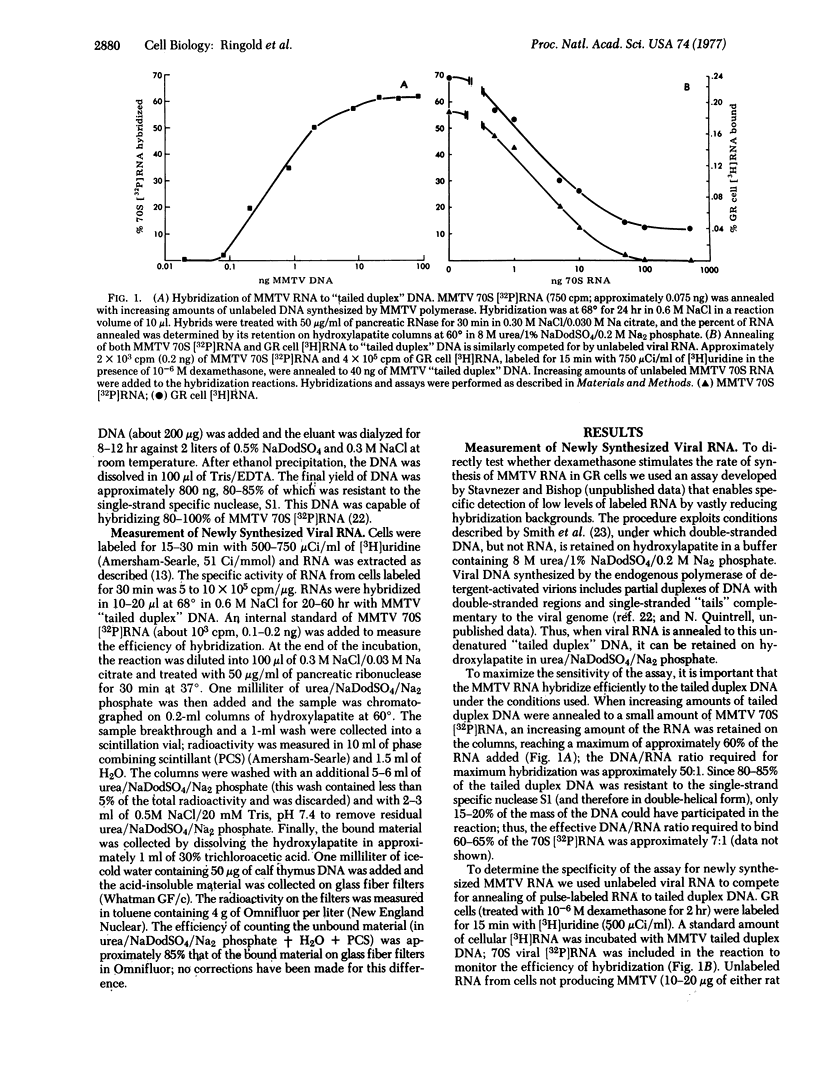
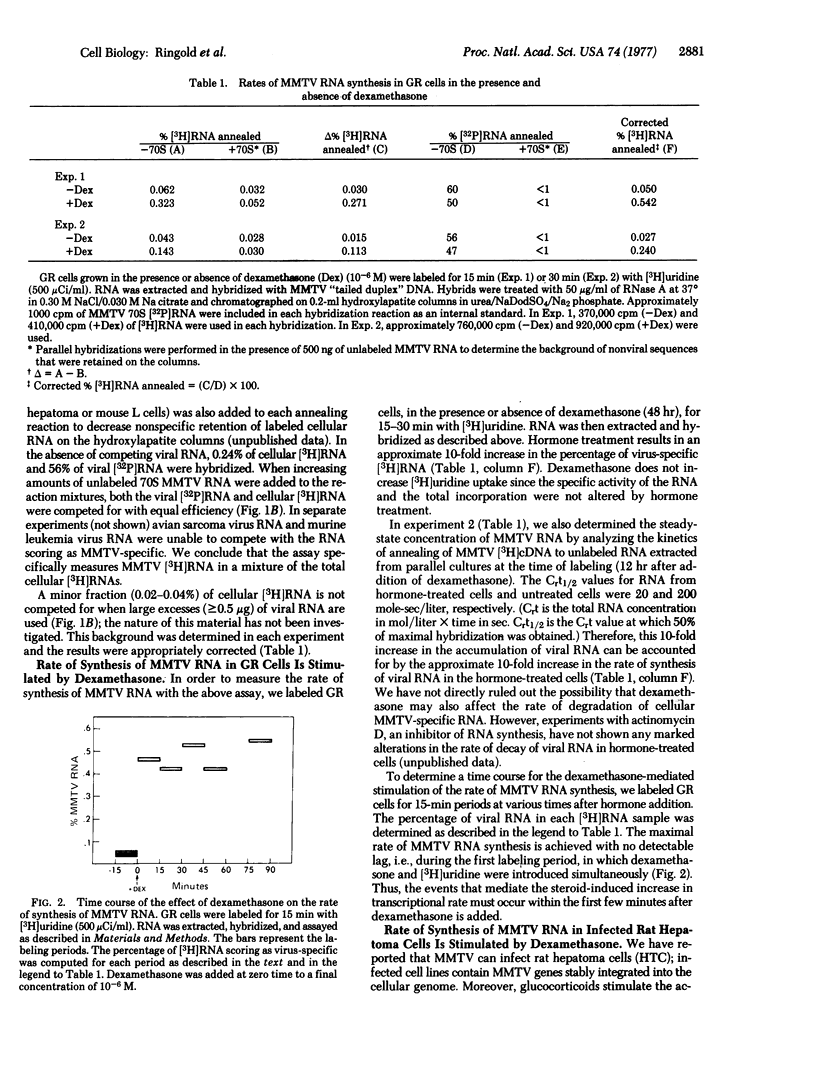
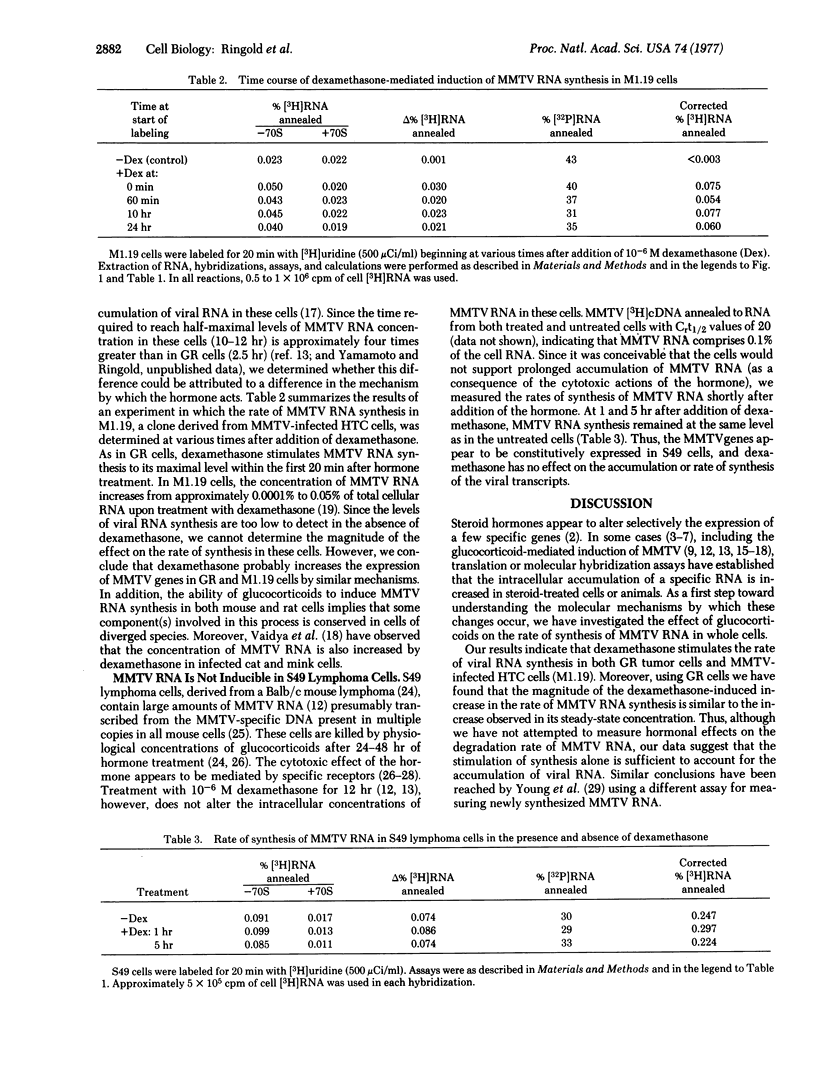
Glucocorticoid hormones specifically increase the intracellular concentration of mouse mammary tumor virus (MMTV) RNA in a cultured cell line from a GR mouse mammary carcinoma (GR) and in an MMTV-infected rat hepatoma cell line (M1.19). In contrast, these steroids have no effect on the concentration of MMTV RNA in a lymphoma line, S49, from a Balb/c mouse. Using a molecular hybridization procedure to detect newly synthesized RNA, we have directly measured the effect of dexamethasone, a synthetic glucocorticoid, on the rate of MMTV RNA synthesis. In GR cells the hormone causes a 10-fold increase in the rate of synthesis of viral RNA without appreciably affecting the overall rate of cellular RNA synthesis. The transition from the basal to the maximally stimulated rate of MMTV RNA synthesis occurs within the earliest labeling period, 0-15 min after addition of the hormone. Thus, it appears that glucocorticoids regulate MMTV genes principally by this rapid and specific alteration of their rate of transcription. Similar results are obtained in M1.19 rat hepatoma cells. In contrast, dexamethasone does not affect the rate of viral RNA synthesis in S49 lymphoma cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Dickson C., Haslam S., Nandi S. Conditions for optimal MTV synthesis in vitro and the effect of steroid hormones on virus production. Virology. 1974 Nov;62(1):242–252. doi: 10.1016/0042-6822(74)90319-5. [DOI] [PubMed] [Google Scholar]
- Feigelson P., Beato M., Colman P., Kalimi M., Killewich L. A., Schutz G. Studies on the hepatic glucocorticoid receptor and on the hormonal modulation of specific mRNA levels during enzyme induction. Recent Prog Horm Res. 1975;31:213–242. doi: 10.1016/b978-0-12-571131-9.50010-4. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Plowman J. K., Kelley S. P., Arthur L. O., Hillman E. A. Enhanced production of mouse mammary tumor virus in dexamethasone-treated, 5-iododeoxyuridine-stimulated mammary tumor cell cultures. J Natl Cancer Inst. 1974 Jun;52(6):1881–1886. doi: 10.1093/jnci/52.6.1881. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- McGrath C. M. Replication of mammary tumor virus in tumor cell cultures: dependence on hormone-induced cellular organization. J Natl Cancer Inst. 1971 Aug;47(2):455–467. [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Ransom J. C., Young H. A., Scolnick E. M. Mammary tumor virus induction by glucocorticoids. Characterization of specific transcriptional regulation. J Biol Chem. 1975 May 10;250(9):3330–3336. [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Varmus H. E., Bishop J. M., Nicholson M. O., McAllister R. M. Homologies among the nucleotide sequences of the genomes of C-type viruses. Virology. 1974 Apr;58(2):568–575. doi: 10.1016/0042-6822(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Blair P. B., Bishop J. M., Varmus H. E. Nucleotide sequence homologies among mouse mammary tumor viruses. Virology. 1976 Apr;70(2):550–553. doi: 10.1016/0042-6822(76)90297-x. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Young H. A., Parks W. P. Biochemical and physiological mechanisms in glucocorticoid hormone induction of mouse mammary tumor virus. Virology. 1976 Jan;69(1):148–156. doi: 10.1016/0042-6822(76)90202-6. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Isolation of lymphoma cell variants resistant to killing by glucocorticoids. Cell. 1974 Aug;2(4):213–220. doi: 10.1016/0092-8674(74)90013-0. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Mechanisms of steroid resistance. Cell. 1974 Aug;2(4):221–227. doi: 10.1016/0092-8674(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Hough B. R., Chamberlin M. E., Davidson E. H. Repetitive and non-repetitive sequence in sea urchin heterogeneous nuclear RNA. J Mol Biol. 1974 May 5;85(1):103–126. doi: 10.1016/0022-2836(74)90132-6. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Heubel G., Lasfargues J. C., Moore D. H. Murine mammary tumor virus: characterization of infection of nonmurine cells. J Virol. 1976 Jun;18(3):911–917. doi: 10.1128/jvi.18.3.911-917.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Stampfer M. R., Tomkins G. M. Receptors from glucocorticoid-sensitive lymphoma cells and two clases of insensitive clones: physical and DNA-binding properties. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3901–3905. doi: 10.1073/pnas.71.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. J., Cardiff R. D., Ashley R. L. Long-term primary culture of mouse mammary tumor cells: production of virus. J Natl Cancer Inst. 1975 May;54(5):1215–1221. doi: 10.1093/jnci/54.5.1215. [DOI] [PubMed] [Google Scholar]