Abstract
A single multiplex PCR assay targeting seven virulence factors and the wzi gene specific for the K1 and K2 capsular serotypes of Klebsiella pneumoniae was developed and tested on 65 clinical isolates, which included 45 isolates responsible for community-acquired severe human infections. The assay is useful for the surveillance of emerging highly virulent strains.
INTRODUCTION
Klebsiella pneumoniae is associated with both community-acquired and nosocomial infections. It is responsible for a wide spectrum of infections, including urinary tract infections, pneumonia, bacteremia, meningitis, wound infections, and purulent abscesses, at various sites. A new virulent hypermucoviscous variant of K. pneumoniae has emerged. It initially manifested as the cause of community-acquired primary liver abscesses, sometimes complicated by metastatic meningitis and/or endophthalmitis, and it has been described by numerous investigators in Asian countries (1). Sporadic cases of severe infections have been reported throughout the world and are now increasingly recognized in Western countries (2–7). The reasons for this epidemiological change and global differences remain unexplained. Invasive K. pneumoniae isolates causing these infections exhibit a hypermucoviscous phenotype more often than is commonly observed and are frequently found to belong to serotype K1 or K2 (8). Shon, Bajwa, and Russo (1) recently defined this new type of variant as hypervirulent K. pneumoniae (hvKP) and reviewed the clinical data and bacteriological features associated with these isolates; briefly, these features include the ability to cause severe community-acquired infections (e.g., liver abscesses, pneumonia, and meningitis) in young healthy hosts, the ability to cause metastatic infections (e.g., endophthalmitis), and a hypermucoviscous aspect of colonies on agar plates that can be semiquantitatively appreciated by a positive string test. The hypervirulent serotype K1 clone belongs to sequence type 23 (ST23), whose presence has been demonstrated on three continents (7).
Two genes were initially associated with invasive infections, i.e., the mucoviscosity-associated gene A (magA) and the regulator of mucoid phenotype A (rmpA) (9, 10). It is now well established that magA is located within the gene cluster specifying K. pneumoniae capsular serotype K1 and encodes a particular capsular polymerase, WzyKpK1 (11). The rmpA gene is a plasmid-borne regulator of extracellular polysaccharide synthesis and is associated with the hypermucoviscous phenotype (10, 12). Yu et al. (10) demonstrated that rmpA-carrying strains were associated with the hypermucoviscous phenotype and with the clinical syndrome caused by invasive strains.
Molecular epidemiology has shown the hypervirulent strains to possess a combination of iron acquisition systems, i.e., enterobactin (Ent), the prototypical catecholate siderophore; aerobactin, a hydroxamate siderophore whose receptor is encoded by iutA; yersiniabactin (YbtS), a phenolate-type siderophore that is structurally distinct from Ent (13), and Kfu, which mediates uptake of ferric iron and is more prevalent in hypervirulent strains (14, 15). The allS gene (associated with allantoin metabolism) is strongly correlated with K. pneumoniae isolates from liver abscesses (16). Other genes that are involved in K. pneumoniae virulence include fimbrial and nonfimbrial adhesion genes, such as ycfM, KPN, and mrkD. mrkD is believed to function as the type 3 fimbrial adhesin and to mediate binding to the extracellular matrix (17).
Previous multiplex PCRs have targeted a few virulence genes and six capsular serotypes (7) or targeted virulence plasmid pLVPK-derived loci, i.e., iutA, rmpA, and two resistance genes (18). Recently, a multiplex PCR assay was designed to identify three groups of hvKP clonal groups of capsular serotype K2 (27). The purpose of the present study was to design a reliable PCR assay for the rapid detection of the most frequently encountered virulence genes and serotypes that are associated with hvKP.
A multiplex PCR was designed using the FastPCR software. The following genes were targeted: magA (K1 serotype), rmpA, entB, ybtS, kfu, iutA, mrkD, allS, and the K2 capsular serotype-specifying wzi gene (19) (Table 1); the primers targeting the capsular serotype K1- and K2-specifying genes were previously described (9, 20). As entB is widely spread among K. pneumoniae strains (G. Arlet and D. Decré, personal communication) (21), we included primers for entB in the design of the multiplex PCR as a positive control.
TABLE 1.
Primers used in this study
| Primer name | DNA sequence (5′ to 3′) | Target gene product/function | EMBL accession no. | Amplicon size (bp) | Primer concn (pmol/μl) | Reference or source |
|---|---|---|---|---|---|---|
| ybtS_for | GACGGAAACAGCACGGTAAA | Siderophore | AB298504 | 242 | 0.4 | This study |
| ybtS_rev | GAGCATAATAAGGCGAAAGA | |||||
| mrkD_for | AAGCTATCGCTGTACTTCCGGCA | Adhesin type 3 fimbriae | EU682505 | 340 | 0.1 | This study |
| mrkD_rev | GGCGTTGGCGCTCAGATAGG | |||||
| entB_for | GTCAACTGGGCCTTTGAGCCGTC | Siderophore | CP000647 | 400 | 0.1 | This study |
| entB_rev | TATGGGCGTAAACGCCGGTGAT | |||||
| rmpA_for | CATAAGAGTATTGGTTGACAG | Regulator of mucoid phenotype A | X17518 | 461 | 0.2 | This study |
| rmpA_rev | CTTGCATGAGCCATCTTTCA | |||||
| K2_for | CAACCATGGTGGTCGATTAG | Capsular serotype K2 and hypermucoviscosity phenotype | EF221827 | 531 | 0.4 | 20 |
| K2_rev | TGGTAGCCATATCCCTTTGG | |||||
| kfu_for | GGCCTTTGTCCAGAGCTACG | Iron transport and phosphotransferase function | AB115591 | 638 | 0.075 | This study |
| kfu_rev | GGGTCTGGCGCAGAGTATGC | |||||
| allS_for | CATTACGCACCTTTGTCAGC | Allantoin metabolism | AB115590 | 764 | 0.1 | This study |
| allS_rev | GAATGTGTCGGCGATCAGCTT | |||||
| iutA_for | GGGAAAGGCTTCTCTGCCAT | Siderophore | AY378100 | 920 | 0.1 | This study |
| iutA_rev | TTATTCGCCACCACGCTCTT | |||||
| magA_for | GGTGCTCTTTACATCATTGC | Capsular serotype K1 and hypermucoviscosity phenotype | AY762939 | 1,283 | 0.3 | 9 |
| magA_rev | GCAATGGCCATTTGCGTTAG |
Crude DNA was prepared by lysis at 100°C for 10 min of 2 colonies in 500 μl of sterile distilled water, followed by centrifugation. The supernatant was used in the PCRs.
Multiplex PCR was carried out in a 25-μl volume using the Qiagen multiplex PCR kit (Qiagen, Courtaboeuf, France), according to the manufacturer's instructions. The final reaction mixture contained a 1× PCR mixture (which consists of preoptimized concentrations of hot start DNA polymerase, MgCl2, dinucleoside triphosphate [dNTP], and PCR buffer), various primer concentrations (Table 1), and 1 μl of the crude DNA extract. The PCR conditions were as follows: initial activation at 95°C for 15 min, followed by 30 cycles at 94°C for 30 s, 60°C for 90 s, and 72°C for 60 s, and a final extension at 72°C for 10 min. The amplicons were separated at 100 V for 2 h in a 2% (wt/vol) agarose gel containing ethidium bromide. The functionality and specificity of all primer pairs were tested in single reactions before their combined use in the multiplex PCR assay. In order to confirm primer specificity, bidirectional sequencing of the PCR amplicons was performed, and the sequences determined were compared with those in GenBank. Multilocus sequence typing (MLST) analysis was performed using the international K. pneumoniae MLST typing scheme (http://bigsdb.web.pasteur.fr/), as previously described (5, 22).
In the optimization assays, we used strains recently reported to cause severe and fatal infections (2). The nine primer pairs used in this study did not interfere with each other, and the PCR conditions allowed a highly reproducible synthesis of amplicons of the predicted size with the DNA of all isolates (Fig. 1). The intrarun reproducibility was examined by testing two hvKP strains of our collection (K. pneumoniae strains SA1 and SA2; see Table S1 in the supplemental material) 10 times (data not shown). Strain SA2 was added as an interrun control in each PCR (Fig. 1).
FIG 1.
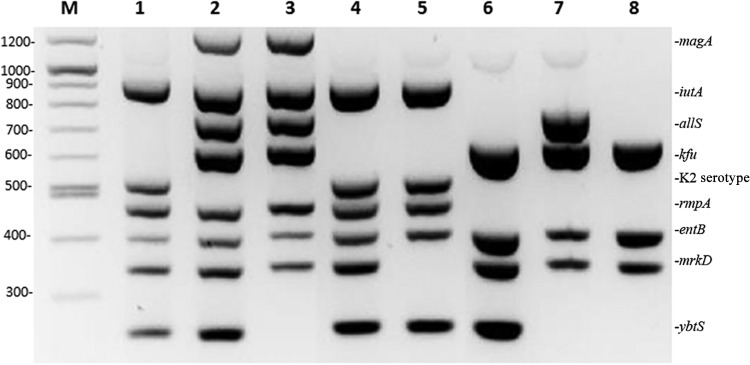
Virulence gene amplification assays for K. pneumoniae. The PCR products were separated on a 2% agarose gel. M, molecular size marker (in bp, measurements on left). Lane 1, SA1; lane 2, SA2; lane 3, SA55; lane 4, SA18; lane 5, H9; lane 6, SA4; lane 7, T3; lane 8, SA11.
The multiplex PCR was applied to 65 K. pneumoniae isolates, which included 45 clinical isolates collected from several French hospitals between 2004 and 2014 and that were identified as hvKP, i.e., (i) were responsible for severe invasive community-acquired infections, including pyogenic liver abscesses, pneumonia, meningitis, and/or bacteremia, and (ii) tested positive on a string test, with the exception of one isolate recovered from a liver abscess (K. pneumoniae SA67) (Table 2; see also Table S1 in the supplemental material). The results were compared with those obtained for 20 non-hvKP strains, including one multidrug-resistant strain responsible for a fatal nosocomial infection (K. pneumoniae KpS13) (23).
TABLE 2.
Results of multiplex PCR targeting virulence genes and K1/K2 capsular serotypes in 65 strains of K. pneumoniae according to their sequence type
| Capsular serotype (no.) | MLST (no.)a | No. of hvKP strainsb | No. of strains with virulence gene: |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ybtS | mrkD | entB | rmpA | kfu | allS | iutA | |||
| K2 (36) K1 (15) | ST23 (15) | 15 | 14 | 15 | 15 | 15 | 15 | 15 | 15 |
| ST13 (1) | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | |
| ST14 (2) | 0 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | |
| ST25 (2) | 1 | 1 | 2 | 2 | 1 | 0 | 1 | 1 | |
| ST65 (4) | 4 | 1 | 4 | 4 | 4 | 0 | 0 | 4 | |
| ST86 (12) | 12 | 9 | 12 | 12 | 12 | 0 | 0 | 12 | |
| ST133 (1) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| ST244 (1) | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | |
| ST375 (3) | 3 | 2 | 2 | 3 | 3 | 1 | 0 | 3 | |
| ST380 (9) | 9 | 9 | 9 | 9 | 9 | 8 | 0 | 9 | |
| ST556 (1) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Non-K1/K2 (14) | ST12 (1) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| ST15 (1) | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | |
| ST35 (1) | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | |
| ST36 (1) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| ST37 (2) | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | |
| ST45 (1) | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| ST60 (1) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| ST101 (2) | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | |
| ST416 (1) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| ST443 (1) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| ST528 (1) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| ST1604 (1) | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | |
MLST, multilocus sequence typing.
hvKP, hypervirulent K. pneumoniae.
Fifteen isolates were of capsular serotype K1 and belonged to ST23, with most of them (11/15) recovered from primary liver abscesses and 4 from pulmonary samples. The rmpA, kfu, mrkD, and iutA genes were detected in all 15 isolates. Only one was negative for ybtS. The gene cluster involved in allantoin metabolism, including allS, was present in all serotype K1 isolates, in agreement with previous reports (5, 16, 24). Only one non-K1/K2 isolate (from ST60) was recovered from a pyogenic liver abscess, a prevalence much lower than the 30% of the non-K1/K2 strains causing liver abscesses, as reported previously (24). A high genotypic diversity was found among the 36 isolates of capsular serotype K2, which were distributed among 10 STs, i.e., ST86 (n = 12), ST380 (n = 9), ST375 (n = 3), ST65 (n = 4), ST25 (n = 2), ST14 (n = 2), ST13 (n = 1), ST133 (n = 1), ST244 (n = 1), and ST556 (n = 1). Almost all isolates (64/65) were found to possess mrkD, a gene detected in 35/36 K2 isolates. In contrast, the presence of other virulence genes differed considerably among the STs. The allS gene was present in only two non-K1 isolates (from ST25 and a ST1604); rmpA and the aerobactin receptor-encoding gene iutA were detected in all isolates belonging to ST86, ST380, ST375, and ST65 but never in isolates belonging to ST13, ST14, ST133, ST244, and ST556. The kfu gene of the iron acquisition system was never detected in ST86 isolates and was detected in 8/9 ST380 isolates. The yersiniabactin-encoding gene ybtS was present in all nine ST380 isolates and was predominant in ST86 (9/12) and ST23 (14/15) isolates. The results of the multiplex PCR assay are highly concordant with the distribution of virulence factors reported in a recent analysis of the genomic content of K. pneumoniae clonal groups (28).
Except for the presence of entB and mrkD, the 14 non-K1/K2 isolates displayed poor virulence gene profiles: none possessed iutA, while kfu and ybtS were observed in five and three isolates, respectively, and allS and rmpA were observed in one isolate each.
Most hvKP isolates belonged to capsular serotypes K1 or K2 and, remarkably, contained both rmpA and iutA. Moreover, they belonged to a limited set of STs (i.e., ST23, ST25, ST60, ST65, ST86, ST375, and ST380), of which only ST25 included both hvKP and non-hvKP isolates. Our observations confirm that ST23 (K1 serotype) and ST86 and ST65 (K2 serotype) represent important clones causing severe community-acquired infections in humans (2, 25). In this study, ST86 was associated with 12 cases of severe infection, including seven fatal cases. In a previous genetic study of K2 strains, only one ST86 strain (K. pneumoniae CIP52.204) was not part of the two major K2 clones (5). ST65 and ST25 belong to the same clonal complex. ST375 is a single-locus variant of ST65, and both were composed of hvKP isolates. ST380, which was associated with fatal infection in seven cases, might correspond to a recently emerging virulent clone (2, 26).
In conclusion, the multiplex PCR described in this study allows the rapid, reproducible, and sensitive detection of virulence genes carried by hvKP isolates. In addition, the method is less time-consuming than MLST determination and is suitable for screening virulent clones. This PCR will be useful for comparing the virulence profiles of large collections of K. pneumoniae organisms, including hvKP strains. It would be of particular interest to characterize particular groups of K. pneumoniae (e.g., hvKP strains and multiresistant strains.) according to the source of isolation or the presence or absence of virulence factors in order to assess their correlation with clinical and epidemiological data. This multiplex PCR is anticipated to be valuable in epidemiological surveys of invasive infections due to K. pneumoniae.
Supplementary Material
ACKNOWLEDGMENTS
We thank G. Barnaud, T. Billard, and G. Marcadé (Hôpital Louis Mourier), L. Drieux (Hôpital Charles Foix), V. Fihman (Hôpital Henri Mondor), C. Muller (Hôpital Bichat), I. Mendes (Charleville-Mézières), A. Merens (Hôpital Begin), S. Robert (Tours), and I. Podglajen (Hôpital Européen Georges Pompidou) for providing strains of K. pneumoniae responsible for severe infections.
This work was partially supported by a grant from the Assistance Publique-Hôpitaux de Paris, contract CRC 08007 (KPath).
This study involves no commercial relationship or conflict of interest.
Footnotes
Published ahead of print 1 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02316-14.
REFERENCES
- 1. Shon AS, Bajwa RPS, Russo TA. 2013. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 4:107–118. 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Decré D, Verdet C, Emirian A, Le Gourrierec T, Petit J-C, Offenstadt G, Maury E, Brisse S, Arlet G. 2011. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J. Clin. Microbiol. 49:3012–3014. 10.1128/JCM.00676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frazee BW, Hansen S, Lambert L. 2009. Invasive infection with hypermucoviscous Klebsiella pneumoniae: multiple cases presenting to a single emergency department in the United States. Ann. Emerg. Med. 53:639–642. 10.1016/j.annemergmed.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 4. McCabe R, Lambert L, Frazee B. 2010. Invasive Klebsiella pneumoniae infections, California, USA. Emerg. Infect. Dis. 16:1490–1491. 10.3201/eid1609.100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, Diancourt L, Grimont P. 2009. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One 4:e4982. 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nadasy KA, Domiati-Saad R, Tribble MA. 2007. Invasive Klebsiella pneumoniae syndrome in North America. Clin. Infect. Dis. 45:e25–e28. 10.1086/519424. [DOI] [PubMed] [Google Scholar]
- 7. Turton JF, Perry C, Elgohari S, Hampton CV. 2010. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J. Med. Microbiol. 59:541–547. 10.1099/jmm.0.015198-0. [DOI] [PubMed] [Google Scholar]
- 8. Lee HC, Chuang YC, Yu WL, Lee NY, Chang CM, Ko NY, Wang LR, Ko WC. 2006. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J. Intern. Med. 259:606–614. 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 9. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. 2004. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 199:697–705. 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, Fung CP, Chuang YC. 2006. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 42:1351–1358. 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 11. Yeh KM, Lin JC, Yin FY, Fung CP, Hung HC, Siu LK, Chang FY. 2010. Revisiting the importance of virulence determinant magA and its surrounding genes in Klebsiella pneumoniae causing pyogenic liver abscesses: exact role in serotype K1 capsule formation. J. Infect. Dis. 201:1259–1267. 10.1086/606010. [DOI] [PubMed] [Google Scholar]
- 12. Nassif X, Honoré N, Vasselon T, Cole ST, Sansonetti PJ. 1989. Positive control of colanic acid synthesis in Escherichia coli by rmpA and rmpB, two virulence-plasmid genes of Klebsiella pneumoniae. Mol. Microbiol. 3:1349–1359. 10.1111/j.1365-2958.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 13. Bachman MA, Oyler JE, Burns SH, Caza M, Lépine F, Dozois CM, Weiser JN. 2011. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect. Immun. 79:3309–3316. 10.1128/IAI.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma LC, Fang CT, Lee CZ, Shun CT, Wang JT. 2005. Genomic heterogeneity in Klebsiella pneumoniae strains is associated with primary pyogenic liver abscess and metastatic infection. J. Infect. Dis. 192:117–128. 10.1086/430619. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh PF, Lin TL, Lee CZ, Tsai SF, Wang JT. 2008. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J. Infect. Dis. 197:1717–1727. 10.1086/588383. [DOI] [PubMed] [Google Scholar]
- 16. Chou HC, Lee CZ, Ma LC, Fang CT, Chang SC, Wang JT. 2004. Isolation of a chromosomal region of Klebsiella pneumoniae associated with allantoin metabolism and liver infection. Infect. Immun. 72:3783–3792. 10.1128/IAI.72.7.3783-3792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jagnow J, Clegg S. 2003. Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology 149(Pt 9):2397–2405. 10.1099/mic.0.26434-0. [DOI] [PubMed] [Google Scholar]
- 18. Tang HL, Chiang MK, Liou WJ, Chen YT, Peng HL, Chiou CS, Liu KS, Lu MC, Tung KC, Lai YC. 2010. Correlation between Klebsiella pneumoniae carrying pLVPK-derived loci and abscess formation. Eur. J. Clin. Microbiol. Infect. Dis. 29:689–698. 10.1007/s10096-010-0915-1. [DOI] [PubMed] [Google Scholar]
- 19. Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, Decré D. 2013. wzi gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J. Clin. Microbiol. 51:4073–4078. 10.1128/JCM.01924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu WL, Fung CP, Ko WC, Cheng KC, Lee CC, Chuang YC. 2007. Polymerase chain reaction analysis for detecting capsule serotypes K1 and K2 of Klebsiella pneumoniae causing abscesses of the liver and other sites. J. Infect. Dis. 195:1235–1236; author reply 1236. 10.1086/512686. [DOI] [PubMed] [Google Scholar]
- 21. Podschun R, Fischer A, Ullmann U. 1992. Siderophore production of Klebsiella species isolated from different sources. Zentralblatt Bakteriol. 276:481–486. [DOI] [PubMed] [Google Scholar]
- 22. Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182. 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kassis-Chikhani N, Decré D, Gautier V, Burghoffer B, Saliba F, Mathieu D, Samuel D, Castaing D, Petit J-C, Dussaix E, Arlet G. 2006. First outbreak of multidrug-resistant Klebsiella pneumoniae carrying blaVIM-1 and blaSHV-5 in a French university hospital. J. Antimicrob. Chemother. 57:142–145. 10.1093/jac/dki389. [DOI] [PubMed] [Google Scholar]
- 24. Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. 2008. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn. Microbiol. Infect. Dis. 62:1–6. 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25. Lin JC, Koh TH, Lee N, Fung CP, Chang FY, Tsai YK, Ip M, Siu LK. 2014. Genotypes and virulence in serotype K2 Klebsiella pneumoniae from liver abscess and non-infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathog. 6:21. 10.1186/1757-4749-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bialek-Davenet S, Nicolas-Chanoine MH, Decré D, Brisse S. 2013. Microbiological and clinical characteristics of bacteraemia caused by the hypermucoviscosity phenotype of Klebsiella pneumoniae in Korea. Epidemiol. Infect. 141:188. 10.1017/S0950268812002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bialek-Davenet S, Criscuolo A, Ailloud F, Passet V, Nicolas-Chanoine M-H, Decre D, Brisse S. 26 September 2014. Development of a multiplex PCR assay for identification of Klebsiella pneumoniae hypervirulent clones of capsular serotype K2. J. Med. Microbiol. 10.1099/jmm.0.081448-0. [DOI] [PubMed] [Google Scholar]
- 28. Bialek-Davenet S, Criscuolo A, Ailloud F, Passet V, Jones L, Delannoy-Vieillard A-S, Garin B, Le Hello S, Arlet G, Nicolas-Chanoine M-H, Decré D, Brisse S. 2014. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg. Infect. Dis. 20:1812–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


