Abstract
Human-to-human-transmitted Corynebacterium diphtheriae was historically the main pathogen causing diphtheria and has therefore been studied extensively in the past. More recently, diphtheria caused by toxigenic Corynebacterium ulcerans is an emerging disease in several industrial countries, including the United Kingdom, the United States, France, and Germany. However, toxigenic C. ulcerans has so far been almost neglected in the development of epidemiologic tools. One of the most important tools in modern epidemiology to understand transmission pathways is sequence typing of pathogens. Here, we provide a protocol for multilocus sequence typing (MLST) to type C. ulcerans strains rapidly and relatively cost-effectively. Applying MLST to C. ulcerans for the first time, we show that related sequence types (STs) might be associated with the presence of the diphtheria toxin gene, which encodes diphtheria toxin (DT), the most important diphtheria-causing virulence factor. Interestingly, we found only two very closely related STs in the isolates derived from six dogs. Additionally, our data show that all STs derived from animals which were at least twice present in our analysis were found in humans as well. This finding is congruent with zoonotic transmission of C. ulcerans.
INTRODUCTION
Diphtheria and diphtheria-like diseases are severe infectious diseases which can be caused by three species of the genus Corynebacterium (1). Diphtheria is caused by the local and systemic action of diphtheria toxin (DT), which is one of the most potent toxins produced by bacteria (2). DT enters the eukaryotic cell by endocytosis and carries out its catalytic function in the cytoplasm. DT ribosylates the translation factor EF-2 and leads to translational shutdown and thereby cell death (2). The diphtheria disease-causing cluster of Corynebacterium is formed by three species: C. diphtheriae, C. ulcerans, and C. pseudotuberculosis (1). It has been shown for all three species that a nontoxigenic Corynebacterium strain can be transformed by integration of a toxigenic phage into the bacterial chromosome (3). In the past, the main pathogen for diphtheria, C. diphtheriae, was extensively characterized. C. diphtheriae is nearly exclusively transmitted from human to human, making it necessary to develop epidemiologic tools to understand and to combat outbreaks. These efforts resulted in the development of a multilocus sequence typing (MLST) system (4), which enables fast and economical epidemiologic studies and outbreak analysis of C. diphtheriae strains. MLST is a very advantageous technique, since it offers high resolution and can be performed very rapidly. MLST is technically relatively undemanding in comparison to other techniques, such as pulsed-field gel electrophoresis or the former gold standard ribotyping. Additionally, MLST is sequence/allele based, and therefore, the resulting data can be shared conveniently with other scientists as the data can be organized, maintained, and searched in public databases, making MLST a perfect tool of choice for fast and accurate typing of transmission pathways.
Although an MLST scheme was published for C. diphtheriae (4), no such protocol has been published for C. ulcerans, leaving a gap in the epidemiological toolbox. However, in the last several decades C. ulcerans was recognized as an emerging pathogen causing diphtheria-like disease. This tendency further increased within the last several years and in many industrialized countries, including the United Kingdom (5), France (6), the United States of America (7), and Germany (8); the infections caused by toxigenic C. ulcerans even outnumbered diphtheria cases caused by C. diphtheriae. In marked contrast to C. diphtheriae, which to date has been almost exclusively isolated from humans, C. ulcerans is often found in domestic animals. In addition, C. ulcerans has so far not been described to be transmitted from human to human. Therefore, it is thought that the transmission pathways might be different for the two species. Among the animals described to be colonized with C. ulcerans are cats, dogs, and pigs (9–13), as well as nondomestic animals, such as cynomolgus macaques (14), ferrets (15), and game animals (16). Although C. ulcerans is considered a zoonotic pathogen, molecular indication for zoonotic transmission has been achieved in only four instances, two of them involving dogs (12, 17), one involving a cat (9), and one involving a pig (13).
Considering the fact that toxigenic C. ulcerans is gaining greater importance as a diphtheria-causing pathogen, we aimed to establish an MLST scheme for C. ulcerans to enhance the epidemiological research and outbreak analysis of C. ulcerans.
MATERIALS AND METHODS
Bacterial strain collection.
Forty-four isolates originating from human patients (n = 31) or from animals (n = 13) from the C. ulcerans collection of the National Consiliary Laboratory on diphtheria (NCLoD), as well as published whole-genome sequences, were analyzed. The strains were derived from patient isolates which were sent to the NCLoD from clinical microbiology laboratories for further differentiation and testing. Species were determined using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry and/or biochemical testing (API Corynebacterium). Additionally, the rpoB gene was partially sequenced and the isolates were tested for toxigenicity by tox-PCR as described previously (18).
DNA preparation.
C. ulcerans isolates were grown on plates overnight at 37°C. One colony was picked, and DNA was prepared using a Biosprint device (Qiagen) according to the manufacturer's instructions. DNA was quantified using a NanoDrop photometer (Thermo Scientific).
Locus amplification and sequencing.
Each PCR was carried out in a 50-μl total volume using HotStarTaq Master Mix (Qiagen). Sequences of the primers and the expected amplicon sizes are given in Table 1. Primers for atpA, dnaA, fusA, odhA, and rpoB are identical with ones from reference 4. The primer used for dnaK and leuA was adapted to C. ulcerans according to the genome of C. ulcerans 809 (19). Locus amplification for MLST analysis was performed according to the published scheme for C. diphtheriae with minor modifications (4). DNA was amplified in a thermal cycler (Eppendorf) with the following conditions for atpK, dnaE, dnaK, fusA, odhA, and rpoB: 95°C for 15 min and 35 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 2 min followed by 72°C for 5 min. leuA was amplified using a touchdown PCR: after 95°C for 15 min, 10 cycles of 94°C for 1 min, 60°C to 50°C (minus 1°C per cycle) for 1 min, and 72°C for 2 min. These 10 cycles were followed by 25 cycles with 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min with a final extension at 72°C for 5 min.
TABLE 1.
Oligonucleotide sequences of the primers used for amplification and sequencing for MLST of C. ulcerans with the calculated amplicon size
| Primer name | Sequence (5′–3′) | Amplicon/MLST fragment size (bp) | Reference |
|---|---|---|---|
| atpA_fwd | GCGATTGCGAACTACACC | 1,029/378 | 4 |
| atpA_rev | CTCGAGGAATACCTRACC | 4 | |
| dnaE_fwd | TGATTATGGCCAGCGTKC | 581/354 | 4 |
| dnaE_rev | ACCCATGGCYTTACGGAA | 4 | |
| fusA_fwd | TACCGCGAGAAGCTCGTT | 683/360 | 4 |
| fusA_rev | GAAGGTTGGGTCCTCTTC | 4 | |
| odhA_fwd | CGGCAAGGAAASCATGAC | 505/381 | 4 |
| odha_rev | GTTGTCGCCTAACATCTG | 4 | |
| rpoB_fwd | AAGCGCAAGATCCAGGAC | 845/342 | 4 |
| rpoB_rev | TCGAACTCGTCGTCATCC | 4 | |
| Culc_dnaK_fwd | ACTTGGGTGGCGGAACCT | 687/345 | This study |
| Culc_dnaK_rev | TGGTAAAGGTCTCAGAA | This study | |
| Culc_leuA_fwd | CGTTCACTTCTACAATTC | 864/384 | This study |
| Culc_leuA_rev | GCCGTGGTCAGTTTTCAT | This study |
The size of amplicons was estimated by agarose gel electrophoresis. The PCR products were sent to Source BioScience (Berlin, Germany) for purification and sequencing. Each product was sequenced using the forward and reverse primers, which was also used for the PCR amplification.
Allele numbers were assigned to each unique allele for a given locus. For each isolate, the allelic profile was generated by combining the allele numbers for each locus in the order atpA, dnaE, dnaK, fusA, leuA, odhA, and rpoB. The sequenced loci are homologous to the loci used for C. diphtheriae (4), giving the possibility of comparing the alleles between the two species.
MLST analysis.
Allele and sequence type number were assigned and used for goeBURST analysis using PHYLOViZ (20). For phylogenetic analysis, the MLST sequences were concatenated and aligned using Clustal W2 (21). Neighbor-joining (NJ) trees and bootstrapping were calculated using MEGA 6.0 (22).
RESULTS
Allelic variation.
In order to establish an MLST procedure for C. ulcerans which is applicable for epidemiological research, outbreak analysis, and identification of ST, we chose the orthologous genes used previously for the C. diphtheriae MLST (4), to ensure—if needed—cross-species comparability. The primers used for atpA, dnaA, fusA, odhA, and rpoB are identical with the ones used for C. diphtheriae. The primers used for dnaK and leuA were adapted to C. ulcerans according to the genome of C. ulcerans 809 (19) and are given in Table 1. We were able to amplify all MLST loci, atpA (1,029 bp), dnaA (581 bp), fusA (683 bp), odhA (505 bp), rpoB (845 bp), dnaK (687 bp), and leuA (864 bp), using two different PCR cycling conditions. The MLST sequences obtained for all isolates and isolate information can be found in Table S1 in the supplemental material and are deposited at http://pubmlst.org/cdiphtheriae (23).
All alleles obtained had similar sequence lengths, and the minimal identity of the different alleles within C. ulcerans varied within the data set presented here from 95% to 99%: atpA, 99%; dnaE, 96%; dnaK, 98%; fusA, 97%; leuA, 97%; odhA, 95%; and rpoB, 98%. The identity values obtained for the different loci in C. ulcerans in the presented study are very similar to the ones obtained for C. diphtheriae (90 to 95%) in the initial published study by Bolt et al. (4). In total, 12 STs were assigned to 33 isolates. We obtained 4 atpA, 6 dnaE, 5 dnaK, 3 fusA, 7 leuA, 4 odhA, and 4 rpoB alleles. Additionally, we extracted the sequences of the MLST loci from the published C. ulcerans genomic sequences of isolates 809 (NC_017317.1) (19), BR-AD22 (NC_015683) (19), 0102 (NC_018101.1) (24), and FRC58 (NZ_AYTI00000000) (25) as well as from 7 isolates sequenced in one of the projects at the NCLoD (D. M. Meinel, G. Margos, R. Konrad, S. Krebs, H. Blum, and A. Sing, unpublished data). Allele comparison led to the identification of one additional allele for dnaE and leuA as well as two for odhA, resulting in three new STs.
We assessed whether C. ulcerans and C. diphtheriae share common alleles due to their close relationship. As expected, the loci are very similar between the two species; nevertheless, we did not detect any shared allele within the first presented MLST data set for C. ulcerans. We detected identities of approximately 86 to 89% for atpA, dnaK, fusA, and rpoB. For odhA, we detected 80 to 82% identity, and for the leuA alleles, we detected a minimum of 78%, illustrating the separation of the two species and the idea that the species can be easily discriminated from each other by the alleles obtained with MLST.
goeBurst analysis.
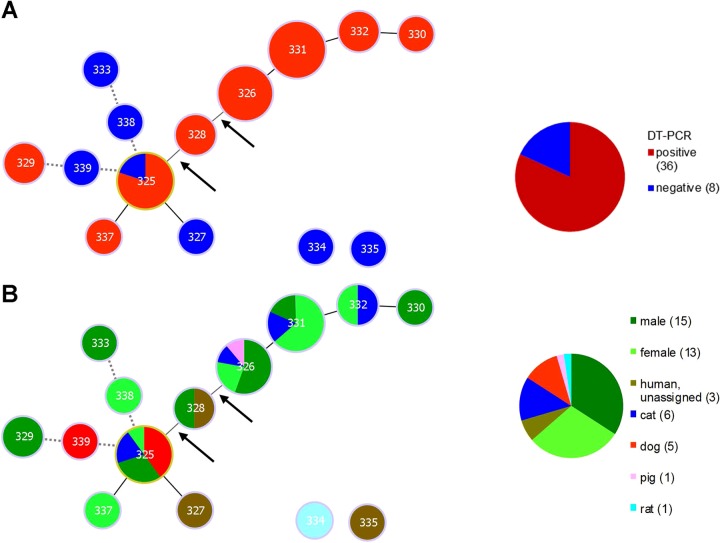
For a more detailed analysis of the population structure, we used PHYLOViZ to generate goeBurst diagrams of the typed isolates. First, we analyzed whether certain STs carry the DT gene more often. Interestingly, approximately 81% of the isolates analyzed carried a tox gene. We found very distinct STs in the different non-tox-bearing isolates (Fig. 1A). In contrast, the toxigenic isolates seem to form a lineage which carries the tox gene more often, suggesting that there might be an ST which is more susceptible to horizontal gene transfer by toxigenic transformation or that the DT genes are passed on over several generations in that lineage. Notably, this effect is more visible if not only single-locus variants are allowed in each lineage but also double-locus variants are considered: ST 325 and 326 get connected remotely via ST 328 (connections of double-locus variants are marked with an arrow in Fig. 1A). If triple-locus variants are allowed, STs 325, 339, and 329 and STs 325, 338, and 333 will be connected (dotted lines in Fig. 1A). STs 334 and 335 appeared as singletons showing no connection to other STs.
FIG 1.
goeBURST diagram for the MLST data set of 44 C. ulcerans isolates. (A) An eBURST diagram was calculated using PHYLOViZ with the goeBURST algorithm. STs were grouped according to their allelic profiles. Solid lines indicate single-locus variants, except for the two lines marked with arrows, which indicate double-locus variants. The dotted lines reflect triple-locus variants and therefore more distantly related isolates. Each circle represents one ST, and the size represents the number of isolates in each ST. Exact numbers of isolates per ST are given in Table 2. Isolates which tested positive for the tox gene by PCR are colored red; negative isolates are colored blue. In the right panel, a pie chart depicts the fractions of tox-positive and -negative strains in the analysis. (B) eBURST diagram tree as in panel A. The isolates are color coded according to their host, as given in the key at right. The pie chart depicts the isolates from each host as fractions of the total number of isolates.
In the next step, we analyzed whether certain ST lineages are species specific. Since C. ulcerans is suspected to be transmitted zoonotically, we assessed whether all STs are present in both animals and humans (Fig. 1B). We found that, indeed for all STs for which more than two isolates were available, at least one human isolate was present, supporting the concept of zoonotic transmission for C. ulcerans. Furthermore, we found several STs in the isolates originating from cats, indicating no visible ST specificity. Interestingly, we found 4 of 5 isolates originating from dogs to have the same ST. The remaining fifth isolate (ST 339) showed two allelic changes compared to the 4 isolates with ST 325. ST 339 MLST sequences were extracted from a published whole-genome sequence of an isolate which originated from Brazil and might therefore be expected to be more different from the other isolates, derived from Germany.
Phylogenetic analysis.
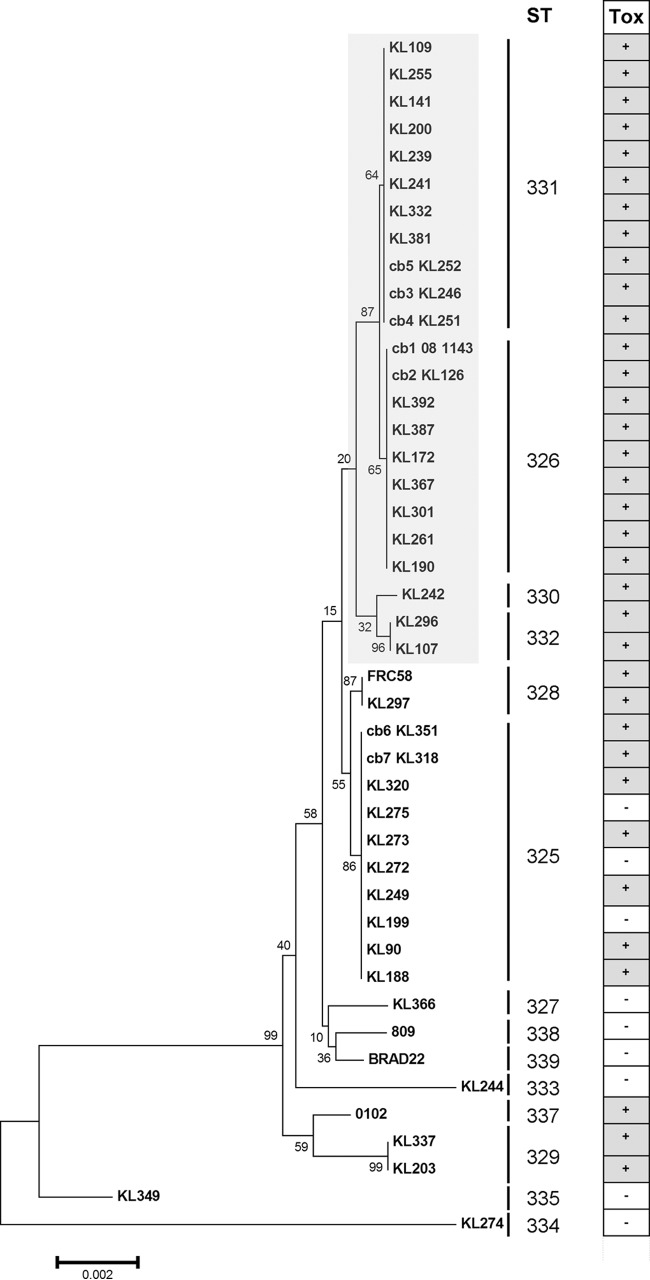
We also used the obtained sequence data to generate a phylogenetic tree. Concatenated sequences of all seven loci were used to calculate a neighbor-joining (NJ) tree. As already obvious from the goeBURST analysis, KL349 and KL274, which correspond to STs 335 and 334, respectively, are most distant from the other isolates (Fig. 2). The other isolates are very similar to each other (see Table S1 in the supplemental material), and we did not observe any strong lineages. As expected, all STs form exclusive clusters in the phylogenetic trees.
FIG 2.
Neighbor-joining tree based on concatenated MLST sequencing data for 44 C. ulcerans isolates. The NJ tree was calculated using MEGA 6.0 (22). The strain identifiers are given at the ends of the branches. The numbers give the values for the bootstrapping test of the tree with 100 repetitions. The sequence type is given under “ST,” and the results of the tox-PCR are given in the rightmost panel. The lineage of STs 326, 330, 331, and 332 is highlighted in light gray. All isolates in this cluster were tox positive.
Interestingly, one of the two lineages seen in the goeBURST analysis, consisting of STs 326, 331, 332, and 330, forms a highly homogenous branch in the phylogenetic analysis. Notably, all isolates in this cluster bear a tox gene. Furthermore, both isolates of ST 328, which is located close to this cluster in the goeBURST analysis and which represents a double-locus variant of the closest member of the cluster (Fig. 1), bear a tox gene. ST 325, a triple-locus variant of the closest member of the cluster, also has a high proportion of toxigenic isolates.
As already noted before upon inclusion of all available MLST sequence data published on PubMLST.org, C. diphtheriae and C. ulcerans form two highly separated branches in the phylogeny, because although the sequences for the single MLST loci are very similar in the two species, the sequences are still clearly distinguishable from each other. This is expected from two distinct species with low horizontal gene transfer affecting the analyzed loci (data not shown).
DISCUSSION
C. ulcerans is an emerging pathogen in regions which are highly vaccinated against diphtheria. Since human-to-human transmission has so far been reported only anecdotally, most countries have not included toxigenic C. ulcerans in their list of notifiable diseases. However, alarming reports on rising numbers of cases of diphtheria and diphtheria-like disease caused by toxigenic C. ulcerans from several highly industrialized countries, such as the United Kingdom, the United States, France, and Germany, indicate that the diphtheria cases caused by C. ulcerans outnumber those caused by C. diphtheriae. Therefore, it might be reasonable to keep toxigenic C. ulcerans under closer epidemiological surveillance to gain deeper insight into the public health relevance of this pathogen. To better understand transmission pathways and to learn whether special sequence types of C. ulcerans have a higher pathogenic potential, we established MLST for C. ulcerans as an epidemiological tool. MLST is a fast and relatively cost-effective technique. The sequence-based MLST data can be stored in public databases, are portable, and can be easily searched and shared between laboratories. Here, we present MLST data for 33 isolates obtained by classical PCR and sequencing and data for 11 isolates extracted from next-generation sequencing (NGS) data, also illustrating the compatibility of MLST with the newly emerging NGS technology.
Our data nicely illustrate the importance of MLST for C. ulcerans, by showing that there might be—with the caveat that we analyzed only 44 isolates—certain C. ulcerans STs which carry the tox gene more often. Whether this is due to a higher susceptibility to horizontal gene transfer for uptake of the tox gene in those ST clusters or whether it is due to the tox gene being passed on from a common ancestor needs to be further investigated. However, this points to the possibility that certain STs might be connected with higher virulence and with a more severe course of disease. Since C. ulcerans can cause diverse clinical pictures, such as skin ulcers and cutaneous and pharyngeal diphtheria, it is of great importance to investigate whether certain STs are associated with particular courses of disease. However, we did not detect a clear connection of STs with the severity of the course of disease. Most likely, a more comprehensive data set is needed for such conclusions, for which the foundations could be easily laid when more and more labs apply MLST to C. ulcerans and make their data available.
Additionally, we found that all STs which were present at least twice in our data set and originated from animals were also among the isolates from human patients. This provides an additional indication for zoonotic transmission of C. ulcerans. Hence, it underlines the importance of molecular typing of these pathogens to understand transmission pathways between humans and animals as well as human-to-human or animal-to-animal transmission. Our study also included a porcine strain (KL126) which shares the same ST as an isolate obtained from the farmer who owned the pig (cb1 08-1143), as well as isolates from a dog (KL315) and its owner (KL318) both with the same ST, providing molecular evidence for zoonotic transmission (Table 2). Since all four isolates are toxigenic, both cases of likely zoonotic transmission underline that the companion animals of a patient also should be probed and, when needed, treated to avoid possible infection cycles with toxigenic C. ulcerans.
TABLE 2.
C. ulcerans isolates analyzed in this study
| ST | Isolate | Yr of isolation | Place of isolation | Patient gender or animal | Age of patient (yr) | Result of Elek test: tox | tox-PCR result | Symptoms/site of colonization | Note |
|---|---|---|---|---|---|---|---|---|---|
| 326 | KL392 | 2012 | Baden-Württemberg, Germany | Cat | NAc | Negative | Positive | Animal, no symptoms detected | From same household as KL387 |
| 326 | KL387 | 2012 | Baden-Württemberg, Germany | Male | 86 | Negative | Positive | Wound | From same household as KL392 |
| 326 | KL172 | 2009 | Baden-Württemberg, Germany | Male | 56 | Negative | Positive | Wound | |
| 367 | KL367 | 2012 | Baden-Württemberg, Germany | Male | 80 | Negative | Positive | Wound | |
| 326 | KL301 | 2011 | Bavaria, Germany | Male | 57 | Positive | Positive | Wound | |
| 326 | KL261 | 2011 | Bavaria, Germany | Male | 57 | Positive | Positive | Wound/ulcer | |
| 326 | KL190 | 2010 | Baden-Württemberg, Germany | Female | 61 | Positive | Positive | Wound | |
| 327 | KL366 | 2012 | Baden-Württemberg, Germany | Human | NA | NA | Negative | NA | |
| 325 | KL320 | 2012 | Hesse, Germany | Dog | NA | Negative | Positive | Animal, no symptoms detected | |
| 325 | KL275 | 2011 | Baden-Württemberg, Germany | Dog | NA | NA | Negative | Animal | |
| 325 | KL273 | 2011 | Baden-Württemberg, Germany | Cat | NA | Positive | Positive | Cat with symptoms | |
| 325 | KL272 | 2011 | Baden-Württemberg, Germany | Cat | NA | Negative | Positive | Cat with symptoms | |
| 325 | KL249 | 2010 | Berlin, Germany | Dog | NA | Negative | Positive | Animal, no symptoms detected | |
| 325 | KL199 | 2010 | North Rhine-Westphalia, Germany | Male | 41 | NA | Negative | Ethmoid bone | |
| 325 | KL90 | 2005 | Bavaria, Germany | Female | 35 | NA | Positive | Throat | |
| 325 | KL188 | 2010 | Bavaria, Germany | Male | 64 | Negative | Positive | Ulcer | |
| 328 | KL297 | 2011 | Baden-Württemberg, Germany | Male | 74 | Negative | Positive | Ulcer | |
| 329 | KL337 | 2012 | Bavaria, Germany | Male | 58 | Positive | Positive | NA | |
| 329 | KL203 | 2010 | Baden-Württemberg, Germany | Male | 58 | Positive | Positive | Wound | |
| 330 | KL242 | 2010 | Saxony, Germany | Male | 62 | Positive | Positive | Ulcer | |
| 331 | KL381 | 2012 | Baden-Württemberg, Germany | Male | 73 | Negative | Positive | Wound | |
| 331 | KL332 | 2012 | Bavaria, Germany | Male | 72 | Negative | Positive | Wound | |
| 331 | KL241 | 2010 | Saxony, Germany | Female | 63 | Positive | Positive | Ulcer | |
| 331 | KL239 | 2010 | NA | Female | 89 | Negative | Positive | Wound | |
| 331 | KL200 | 2010 | NA | Female | 87 | Positive | Positive | Wound | |
| 331 | KL141 | 2009 | NA | Female | 62 | Positive | Positive | Throat | |
| 331 | KL109 | 2007 | Thuringia, Germany | Female | NA | Positive | Positive | NA | |
| 331 | KL255 | 2011 | Saxony, Germany | Female | 85 | Negative | Positive | Ulcer | |
| 332 | KL296 | 2011 | Hamburg, Germany | Female | 66 | Negative | Positive | Wound | |
| 332 | KL107 | 2007 | Brandenburg, Germany | Cat | NA | Positive | Positive | Animal, no symptoms detected | |
| 333 | KL244 | 2010 | NA | Male | 58 | NA | Negative | Ulcer | |
| 334 | KL274 | 2011 | Baden-Württemberg, Germany | Rat | NA | NA | Negative | Animal with symptoms | |
| 335 | KL349 | 2012 | London, UK | Human | NA | Negative | Negative | Throat | |
| 337 | 0102a | NA | Japan | Female | 52 | Positive | Positive | Throat | Reference 24 |
| 338 | 809a | NA | Brazil | Female | 80 | NA | Negative | Lung | Reference 26 |
| 339 | BR-AD22a | NA | Brazil | Dog | 5 | NA | Negative | Animal with symptoms | Reference 26 |
| 326 | 081143a | 2007 | NA | Pig | NA | NA | Positive | Animal, no symptoms detected | From same household as KL126 |
| 326 | KL126a | 2007 | NA | Female | 56 | Positive | Positive | Throat | From same household as 081143 |
| 331 | KL246a | 2010 | Saxony, Germany | Female | 86 | Weakly positive | Positive | Throat | From same household as KL251 and KL252 |
| 331 | KL251a | 2010 | Saxony, Germany | Cat | NA | Negative | Positive | Animal, no symptoms detected | From same household as KL246 and KL252 |
| 331 | KL252a | 2010 | Saxony, Germany | Cat | NA | Negative | Positive | Animal, no symptoms detected | From same household as KL246 and KL251 |
| 325 | KL315a | 2012 | Baden-Württemberg, Germany | Male | 52 | Negative | Positive | Ulcer | From same household as KL318 |
| 325 | KL318a | 2012 | Baden-Württemberg, Germany | Dog | NA | Negative | Positive | Animal, no symptoms detected | From same household as KL315 |
| 328 | FRC58a | NA | France | Human | 86 | NA | Positiveb | Lung | Reference 25 |
The corresponding MLST sequences were extracted from NGS data.
It is unknown whether a tox-PCR was performed, but inspection of NGS resequencing data showed that a full tox gene was present.
NA, data not available.
Supplementary Material
ACKNOWLEDGMENTS
We thank Wolfgang Schmidt for the cultivation and microbiological characterization of the corynebacteria and Cecilia Hizo-Teufel for help with the DNA preparation. We thank Edgar Badell-Ocando and Keith Jolley for curation of the http://pubmlst.org database and integration of our MLST data into the Corynebacterium diphtheriae database.
The study was partly supported by the Bavarian State Ministry of Health and Care and by the German Federal Ministry of Health via the Robert Koch Institute and its National Reference Laboratories Network (FKZ 1369–359 and FKZ 415).
The authors have declared that no competing interests exist.
Footnotes
Published ahead of print 15 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02291-14.
REFERENCES
- 1. Pascual C, Lawson PA, Farrow JA, Gimenez MN, Collins MD. 1995. Phylogenetic analysis of the genus Corynebacterium based on 16S rRNA gene sequences. Int. J. Syst. Bacteriol. 45:724–728. 10.1099/00207713-45-4-724. [DOI] [PubMed] [Google Scholar]
- 2. Holmes RK. 2000. Biology and molecular epidemiology of diphtheria toxin and the tox gene. J. Infect. Dis. 181:S156–S167. 10.1086/315554. [DOI] [PubMed] [Google Scholar]
- 3. Brussow H, Canchaya C, Hardt WD. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560–602. 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolt F, Cassiday P, Tondella ML, Dezoysa A, Efstratiou A, Sing A, Zasada A, Bernard K, Guiso N, Badell E, Rosso ML, Baldwin A, Dowson C. 2010. Multilocus sequence typing identifies evidence for recombination and two distinct lineages of Corynebacterium diphtheriae. J. Clin. Microbiol. 48:4177–4185. 10.1128/JCM.00274-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagner KS, White JM, Crowcroft NS, De Martin S, Mann G, Efstratiou A. 2010. Diphtheria in the United Kingdom, 1986–2008: the increasing role of Corynebacterium ulcerans. Epidemiol. Infect. 138:1519–1530. 10.1017/S0950268810001895. [DOI] [PubMed] [Google Scholar]
- 6. Bonmarin I, Guiso N, Le Fleche-Mateos A, Patey O, Patrick AD, Levy-Bruhl D. 2009. Diphtheria: a zoonotic disease in France? Vaccine 27:4196–4200. 10.1016/j.vaccine.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 7. Tiwari TS, Golaz A, Yu DT, Ehresmann KR, Jones TF, Hill HE, Cassiday PK, Pawloski LC, Moran JS, Popovic T, Wharton M. 2008. Investigations of 2 cases of diphtheria-like illness due to toxigenic Corynebacterium ulcerans. Clin. Infect. Dis. 46:395–401. 10.1086/525262. [DOI] [PubMed] [Google Scholar]
- 8. Sing A. 2008. Zur Charakterisierung von C.-diphtheriae-verdächtigen Isolaten. Robert Koch Inst. Epidemiol. Bull. 3:23–25. [Google Scholar]
- 9. Berger A, Huber I, Merbecks SS, Ehrhard I, Konrad R, Hormansdorfer S, Hogardt M, Sing A. 2011. Toxigenic Corynebacterium ulcerans in woman and cat. Emerg. Infect. Dis. 17:1767–1769. 10.3201/eid1709.110391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corti MA, Bloemberg GV, Borelli S, Kutzner H, Eich G, Hoelzle L, Lautenschlager S. 2012. Rare human skin infection with Corynebacterium ulcerans: transmission by a domestic cat. Infection 40:575–578. 10.1007/s15010-012-0254-5. [DOI] [PubMed] [Google Scholar]
- 11. Katsukawa C, Kawahara R, Inoue K, Ishii A, Yamagishi H, Kida K, Nishino S, Nagahama S, Komiya T, Iwaki M, Takahashi M. 2009. Toxigenic Corynebacterium ulcerans isolated from the domestic dog for the first time in Japan. Jpn. J. Infect. Dis. 62:171–172. [PubMed] [Google Scholar]
- 12. Hogg RA, Wessels J, Hart J, Efstratiou A, De Zoysa A, Mann G, Allen T, Pritchard GC. 2009. Possible zoonotic transmission of toxigenic Corynebacterium ulcerans from companion animals in a human case of fatal diphtheria. Vet. Rec. 165:691–692. [PubMed] [Google Scholar]
- 13. Schuhegger R, Schoerner C, Dlugaiczyk J, Lichtenfeld I, Trouillier A, Zeller-Peronnet V, Busch U, Berger A, Kugler R, Hormansdorfer S, Sing A. 2009. Pigs as source for toxigenic Corynebacterium ulcerans. Emerg. Infect. Dis. 15:1314–1315. 10.3201/eid1508.081568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirai-Yuki A, Komiya T, Suzaki Y, Ami Y, Katsukawa C, Takahashi M, Yamamoto A, Yamada YK. 2013. Isolation and characterization of toxigenic Corynebacterium ulcerans from 2 closed colonies of cynomolgus macaques (Macaca fascicularis) in Japan. Comp. Med. 63:272–278. [PMC free article] [PubMed] [Google Scholar]
- 15. Marini RP, Cassiday PK, Venezia J, Shen Z, Buckley EM, Peters Y, Taylor N, Dewhirst FE, Tondella ML, Fox JG. 2014. Corynebacterium ulcerans in ferrets. Emerg. Infect. Dis 20:159–161. 10.3201/eid2001.130675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenberg T, Kutzer P, Peters M, Sing A, Contzen M, Rau J. 2014. Nontoxigenic tox-bearing Corynebacterium ulcerans infection among game animals, Germany. Emerg. Infect. Dis. 20:448–452. 10.3201/eid2003.130423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lartigue MF, Monnet X, Le Fleche A, Grimont PA, Benet JJ, Durrbach A, Fabre M, Nordmann P. 2005. Corynebacterium ulcerans in an immunocompromised patient with diphtheria and her dog. J. Clin. Microbiol. 43:999–1001. 10.1128/JCM.43.2.999-1001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuhegger R, Lindermayer M, Kugler R, Heesemann J, Busch U, Sing A. 2008. Detection of toxigenic Corynebacterium diphtheriae and Corynebacterium ulcerans strains by a novel real-time PCR. J. Clin. Microbiol. 46:2822–2823. 10.1128/JCM.01010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trost E, Al-Dilaimi A, Papavasiliou P, Schneider J, Viehoever P, Burkovski A, Soares SC, Almeida SS, Dorella FA, Miyoshi A, Azevedo V, Schneider MP, Silva A, Santos CS, Santos LS, Sabbadini P, Dias AA, Hirata R, Jr, Mattos-Guaraldi AL, Tauch A. 2011. Comparative analysis of two complete Corynebacterium ulcerans genomes and detection of candidate virulence factors. BMC Genomics 12:383. 10.1186/1471-2164-12-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Francisco AP, Vaz C, Monteiro PT, Melo-Cristino J, Ramirez M, Carrico JA. 2012. PHYLOViZ: phylogenetic inference and data visualization for sequence based typing methods. BMC Bioinformatics 13:87. 10.1186/1471-2105-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 22. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30:2725–2729. 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jolley KA, Chan MS, Maiden MC. 2004. mlstdbNet—distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics 5:86. 10.1186/1471-2105-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sekizuka T, Yamamoto A, Komiya T, Kenri T, Takeuchi F, Shibayama K, Takahashi M, Kuroda M, Iwaki M. 2012. Corynebacterium ulcerans 0102 carries the gene encoding diphtheria toxin on a prophage different from the C. diphtheriae NCTC 13129 prophage. BMC Microbiol. 12:72. 10.1186/1471-2180-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silva Ado S, Barauna RA, de Sa PC, das Gracas DA, Carneiro AR, Thouvenin M, Azevedo V, Badell E, Guiso N, da Silva AL, Ramos RT. 2014. Draft genome sequence of Corynebacterium ulcerans FRC58, isolated from the bronchitic aspiration of a patient in France. Genome Announc. 2:01132–13. 10.1128/genomeA.01132-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trost E, Gotker S, Schneider J, Schneiker-Bekel S, Szczepanowski R, Tilker A, Viehoever P, Arnold W, Bekel T, Blom J, Gartemann KH, Linke B, Goesmann A, Puhler A, Shukla SK, Tauch A. 2010. Complete genome sequence and lifestyle of black-pigmented Corynebacterium aurimucosum ATCC 700975 (formerly C. nigricans CN-1) isolated from a vaginal swab of a woman with spontaneous abortion. BMC Genomics 11:91. 10.1186/1471-2164-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.