Abstract
We developed a new transport medium (GESA—Helicobacter pylori transport medium [publication no. WO/2014/019696, patent pending no. PCT/EP2013/002292; Liofilchem s.r.l., Roseto degli Abruzzi, Teramo, Italy]) for recovery of Helicobacter pylori from gastric biopsy samples. GESA transport medium, in a semisolid state, provides the optimal conditions for maintaining the viability of the microorganism over time. The efficacy of the transport medium was assessed through in vitro and ex vivo experiments. We were able to recover different suspensions of H. pylori ATCC 43629 and H. pylori 13 A in GESA transport medium stored at 4°C for up to 10 days. In particular, with a starting inoculum of ∼105 CFU, after 7 days of storage, 150 ± 25 CFU and 40 ± 7 CFU of the reference and clinical strains were detected, respectively. H. pylori colonies were isolated from gastric specimens taken from both the antrum and the fundus in 68 (90.66%) of 75 urea breath test (UBT)-positive patients. Moreover, GESA transport medium allowed the recovery and isolation of H. pylori colonies from additional biopsy samples from 13 of the 75 detected subjects at up to 10 days of biopsy sample storage at 4°C. Finally, GESA transport medium preserved its characteristics when stored at 4°C for 1 year from its preparation, thus allowing good recovery of H. pylori. GESA transport medium can be considered a standardized transport medium with high performance that optimizes the recovery rate of H. pylori grown by culture.
INTRODUCTION
Helicobacter pylori, the principal species of the genus Helicobacter, colonizes the human stomach early in the life of the host and tends to persist over time (1). The microorganism is also responsible for a variety of gastroduodenal pathologies, and it was also described as a risk factor for gastric carcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (2, 3).
Culture of H. pylori from gastric biopsy specimens represents a more accurate method for diagnosis in patients with suspected infection, with 100% specificity and a level of sensitivity which depends on the performing conditions (4–6). Currently recommended for populations with a high prevalence of drug resistance, culture allows isolation of the microorganism for testing susceptibility to antimicrobials before starting appropriate treatment (7, 8). Moreover, the isolation of the microorganism allows analysis of the genotypic status of the isolates, offering important information for bacterial characterization and, consequently, for therapy evaluation (9, 10).
Culture of H. pylori, however, can prove difficult because of the fastidious nature of the microorganism and its capability to become unculturable (11–14). Multiple gastric biopsy specimens and suitable transport media represent important tools for a high diagnostic yield (15, 16).
Therefore, to make H. pylori culture from gastric biopsy specimens possible in an endoscopy unit, rapid and efficient transportation to microbiological laboratories is essential, taking into account that the survival rate of H. pylori can be affected by both the time lapse between sampling and culture and the transport temperature (17).
Previous studies on the optimal transport conditions for H. pylori were conducted to minimize the loss of bacterial cultivability, increasing the recovery of the colonies (17–21).
In this report, we propose a new semisolid transport medium for cultural recovery and isolation of H. pylori for subsequent drug susceptibility testing from biopsy specimens, adding useful hints to enhance the recovery rate and to preserve the viability of the strains during long-term transportation.
MATERIALS AND METHODS
Composition of GESA transport medium.
GESA transport medium (Liofilchem s.r.l., Roseto degli Abruzzi, Teramo, Italy) for storage and transport of gastric specimens for the detection of H. pylori is based on the combination of soft Granulated agar (Liofilchem) (7 g/liter) with 10 g/liter of enzymatic digest of casein (Liofilchem), 9.5 g/liter of enzymatic digest of animal tissue (Liofilchem), 2 g/liter of yeast extract (Liofilchem), 1 g/liter of glucose (Liofilchem), and 5.5 g/liter of sodium chloride (Liofilchem) at pH 7.0 ± 0.2 in distilled water. After sterilization, 10 ml of enrichment products is added to the mixture (Table 1).
TABLE 1.
Composition of GESA transport medium
| Ingredient(s) (unit) or parameter | Value(s) |
|---|---|
| Enrichment products (ml)a | 10 |
| Granulated agar (g) | 7 |
| Enzymatic digest of casein (g) | 10 |
| Enzymatic digest of animal tissue (g) | 9.5 |
| Yeast extract (g) | 2 |
| Glucose (g) | 1 |
| Sodium chloride (g) | 5.5 |
| Distilled H2O (ml) | 961.2 |
| pH | 7.0 ± 0.2 |
Vitamin B12, 0.01 g/liter; l-glutamine, 10.0 g/liter; adenine, 1.0 g/liter; guanine hydrochloride, 0.03 g/liter; p-aminobenzoic acid, 0.013 g/liter; nicotinamide adenine dinucleotide, 0.25 g/liter; thiamine pyrophosphate, 0.1 g/liter; ferric nitrate, 0.02 g/liter; thiamine hydrochloride, 0.003 g/liter; l-cysteine hydrochloride, 25.9 g/liter; l-cystine, 1.1 g/liter; dextrose, 100.0 g/liter.
The efficiency of the semisolid transport medium in viable bacterial preservation was tested through the use of inocula of both different bacterial suspensions and biopsy specimens.
The performance of the transport medium, stored at 4°C, was evaluated until 12 months after its preparation.
H. pylori suspensions and viability assay.
For quantitative assessment of recovery of bacteria from GESA transport medium, serial dilutions of the reference strain H. pylori ATCC 43629 (ATCC LGC Standards S.r.l., Milan, Italy) and the clinical strain H. pylori 13 A were used. For the experiments, the strains were grown in chocolate agar plus 1% IsoVitaleX (AC; Becton Dickinson Italia, Milan, Italy) at 37°C for 5 days under microaerobic conditions consisting of 85% N2, 5% O2, and 10% CO2 (Rivoira, Milan, Italy). From each strain, a bacterial suspension in brucella broth (BB; Biolife, Milan, Italy) was prepared to achieve an optical density at 600 nm (OD600) of ∼0.2, corresponding to approximately 3.5 × 107 CFU/ml, and serial 10-fold dilutions were prepared at up to 10−5 times the original broth culture concentration. Ten-microliter volumes of each dilution were inoculated in duplicate on 150 μl of GESA transport medium and stored at 4°C until the 10-day time point. In addition, 10 μl of the first dilution was inoculated on GESA transport medium under the same conditions for the detection of the morphology and viability of the bacteria.
For the bacterial recovery assessments, at time zero and after 1, 2, 3, 4, 5, 7, and 10 days, the semisolid cylinders of GESA transport medium containing the bacterial suspensions were collected, spread on AC, and incubated for 3 to 5 days under microaerobic conditions. At each time point and for each broth culture dilution, the number of CFU was counted. Three experiments were performed in duplicate, and the CFU detected were indicated as the mean of the numbers of colonies counted independently by two microbiologists.
Similarly, for the viability test, Live/Dead Backlight bacterial viability staining (Molecular Probes Inc./Invitrogen, Italy) was performed directly on the inoculated semisolid cylinders of GESA transport medium; the morphology and viability of bacteria were visualized under a fluorescent Leika 4000 DM microscope (Germany). As for the bacterial shape, the number of spiral/bacillary (B) and coccoid (C) forms was determined by counting 10 randomly chosen fields of view. Two microbiologists carried out counts independently. The detected U-shaped bacteria were considered coccoid cells.
Biopsy specimens.
For the evaluation of the reliability of GESA transport medium in recovering H. pylori by gastric biopsies, 75 patients with a positive result from a 13C urea breath test (UBT), performed with citric acid and 75 mg of 13C urea, were included in this study. Gastric biopsy specimens were collected from endoscopy units in the Abruzzo area, in Italy. Two biopsy specimens both from the gastric antrum and from the fundus were taken from each subject for a total of 150 analyzed specimens, and from 13 of them, 6 additional biopsy samples were collected. Patients gave their informed consent to the study.
After endoscopy, biopsy specimens from the antrum and fundus were immediately put in GESA transport medium and processed within 2 h (day 1). The additional samples were stored in GESA transport medium at 4°C and randomly processed for up to 10 days.
Biopsy specimens were trimmed with a razor, homogenized, and cultured on chocolate agar plus 1% IsoVitaleX (CA; Becton Dickinson & Co., Cockeysville, MD) and Campylobacter selective medium (CP; Oxoid). Plates were incubated under microaerobic conditions at 37°C for 3 to 5 days.
H. pylori colonies were identified on the basis of their colony morphology, Gram staining, and positive reaction with urease, catalase, and oxidase.
Statistical analysis.
The statistical significance of the differences between the results determined for the experimental groups was evaluated using the Student t test. Probability levels of <0.05 were considered statistically significant.
RESULTS
Recovery of H. pylori from suspensions.
Table 2 displays the recovery rate of H. pylori suspensions after storage in GESA transport medium, over time. When 10 μl of the first microbial dilution was inoculated into GESA transport medium at 4°C, it was possible to recover H. pylori colonies at up to 10 days of storage. At this inoculum concentration and after 7 days, means of 150 ± 25 H. pylori ATCC 43629 colonies and 40 ± 7 H. pylori 13 A colonies were counted on AC medium, respectively. However, a significant reduction in the bacterial recovery rate was observed in the reference strain after 4 days of incubation (from 490 ± 70 to 88 ± 15, P < 0.05) and already after 1 day in the clinical isolate (from 106 ± 15 to 52 ± 20, P < 0.05) with a dilution of 10−2.
TABLE 2.
Number of CFU per plate obtained from Helicobacter pyloria
| Dilution of starting bacterial suspensions (10 μl) (OD600, ca. 0.2) | No. of CFU per plate at the indicated no. of days of storage at 4°C |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
1 |
2 |
3 |
4 |
5 |
7 |
10 |
|||||||||
| R | C | R | C | R | C | R | C | R | C | R | C | R | C | R | C | |
| 10 | >103 | >103 | >103 | >103 | >103 | >103 | >103 | >103 | >103 | >103 | >103 | 324 ± 35 | 150 ± 25 | 40 ± 7 | 4 ± 3 | 3 |
| 10−1 | >103 | >103 | >103 | 705 ± 90 | >103 | 208 ± 30 | >103 | 155 ± 25 | 321 ± 35 | 98 ± 20 | 375 ± 40 | 8 ± 3 | 3 | 0 | 2 | 1 |
| 10−2 | 490 ± 70 | 106 ± 15 | 450 ± 60 | 52 ± 20 | 471 ± 70 | 44 ± 8 | 420 ± 45 | 6 ± 2 | 88 ± 15 | 1 | 39 ± 8 | 0 | 0 | 0 | 0 | 0 |
| 10−3 | 170 ± 30 | 12 ± 6 | 165 ± 30 | 8 ± 4 | 158 ± 25 | 3 | 33 ± 8 | 0 | 7 ± 1 | 0 | 5 ± 3 | 0 | 0 | 0 | 0 | 0 |
| 10−4 | 9 ± 2 | 2 | 32 ± 7 | 2 | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
R, reference strain H. pylori ATCC 43629; C, clinical strain H. pylori 13 A. Data represent the results determined with suspensions after storage at 4°C in GESA transport medium over time. The number of CFU per plate are mean values of the results of three experiments performed in duplicate ± standard deviations (SD).
In all cases, efficacy of recovery of microorganisms was obtained even at a very low inoculum concentration; with a dilution of 10−4 of the starting bacterial suspension, H. pylori ATCC 43629 was detected until the 4-day time point.
No significant differences in terms of colonies recovered were detected when GESA transport medium was stored at 4°C for 12 months before use.
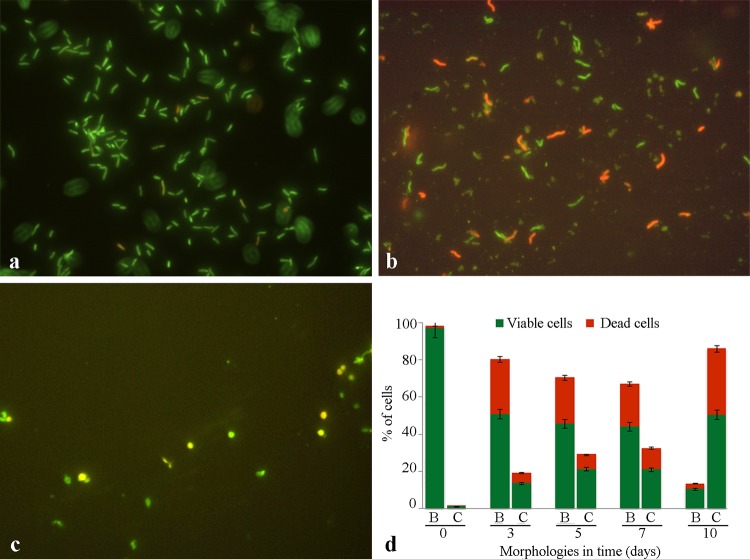
The morphology and viability of H. pylori suspensions inoculated in GESA transport medium detected over time are shown in Fig. 1. The typical green spiral morphology was prevalent until the 7-day time point (Fig. 1a and b). Green viable cells were detectable after 10 days of storage also but were in their unculturable coccoid shape (Fig. 1c). Histograms in Fig. 1d express the percentages of viable and dead cells in both spiral/bacillary (B) and coccoid (C) morphologies. The recorded morphologies were similar for the reference and the clinical H. pylori strains.
FIG 1.
Morphology and viability of Helicobacter pylori stored in GESA transport medium over time. (a to c) Representative images of H. pylori ATCC 43629 after 1 (a), 7 (b), and 10 (c) days of inoculum Live/Dead staining. Magnification, ×1,000. (d) Percentages of spiral/bacillary (B) and coccoid (C) H. pylori cells and their viability, detected over time (in days). The values are the means ± standard deviations (SD) of the results obtained with both the reference and the clinical H. pylori strains.
Recovery of H. pylori from biopsy specimens.
Biopsy specimens that were UBT positive were included in the recovery assay. H. pylori was recorded from both the antral and fundus specimens from GESA transport medium with a recovery rate of 90.66% in 68 of the 75 patients examined. Bacterial colonies were isolated after 3 to 4 days of biopsy culture, and the absence of contaminant colonies facilitated the subsequent antimicrobial testing. Among the 7 patients in whom H. pylori was undetected both in antrum and fundus, the bacterial recovery was compromised because of bacterial overgrowth due to forceps contamination in 3 cases, whereas no bacterial growth was detected in plated media in the remaining 4 cases.
Therefore, GESA is a standardized transport medium that ensures greater stability over time, preserving its features after 1 year of storage at 4°C.
Table 3 shows data from the positive H. pylori samples from multiple biopsy specimens taken from gastric antrum and fundus of 13 patients and stored until the 10-day time point and randomly processed. In all detected biopsy specimens and at each time point of recovery, cultivable H. pylori cells were detected and isolated. In each analyzed time of recovery, H. pylori colonies were suitable for subsequent antimicrobial testing or collecting for long-term storage.
TABLE 3.
Helicobacter pylori isolation from biopsy samples stored at 4°C for up to 10 days in GESA transport medium and randomly processed
| Biopsy sample | Presence of H. pylori in sample from day: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Antrum | ||||||||||
| 18 A 2011 | + | NTa | + | NT | + | NT | NT | NT | + | NT |
| 19 A 2011 | + | + | NT | + | NT | NT | NT | NT | NT | + |
| 20 A 2011 | + | + | NT | NT | NT | NT | NT | + | NT | + |
| 21 A 2011 | + | NT | + | NT | NT | NT | NT | + | NT | + |
| 22 A 2011 | + | NT | NT | + | NT | + | NT | NT | NT | + |
| 23 A 2011 | + | NT | NT | NT | + | NT | + | + | NT | NT |
| 24 A 2011 | + | + | NT | NT | NT | + | NT | NT | NT | + |
| 25 A 2011 | + | NT | + | NT | NT | NT | NT | + | NT | + |
| 1 A 2012 | + | + | NT | NT | NT | + | NT | NT | NT | + |
| 2 A 2012 | + | + | NT | NT | NT | NT | + | NT | NT | + |
| 3 A 2012 | + | NT | NT | + | NT | + | NT | NT | NT | + |
| 4 A 2012 | + | NT | NT | + | NT | NT | + | NT | + | NT |
| 5 A 2012 | + | NT | + | NT | + | NT | NT | + | NT | NT |
| Fundus | ||||||||||
| 18 F 2011 | + | NT | + | NT | NT | NT | + | NT | + | NT |
| 19 F 2011 | + | + | NT | NT | NT | + | NT | NT | NT | + |
| 20 F 2011 | + | NT | NT | + | NT | NT | NT | + | NT | + |
| 21 F 2011 | + | NT | + | NT | + | NT | NT | + | NT | NT |
| 22 F 2011 | + | + | NT | + | NT | NT | NT | NT | NT | + |
| 23 F 2011 | + | NT | NT | NT | NT | NT | + | + | NT | + |
| 24 F 2011 | + | NT | NT | NT | + | + | NT | NT | NT | + |
| 25 F 2011 | + | NT | + | NT | NT | NT | NT | + | NT | + |
| 1 F 2012 | + | + | NT | NT | + | NT | NT | NT | NT | + |
| 2 F 2012 | + | + | NT | NT | NT | NT | + | NT | NT | + |
| 3 F 2012 | + | NT | + | + | NT | NT | NT | NT | NT | + |
| 4 F 2012 | + | NT | NT | + | NT | + | + | NT | NT | NT |
| 5 F 2012 | + | NT | + | NT | + | NT | NT | + | NT | NT |
NT, not tested.
DISCUSSION
Detection of H. pylori through cultural isolation represents a procedure that requires microbiological expertise, and it is significantly influenced by the conditions of transport of the biopsy specimen from the endoscopy unit to the laboratory. Moreover, bacterial isolation is expensive and requires invasive techniques with great variability in susceptibility and time of recovery of the bacteria (22).
Despite these disadvantages, detection of the colonies represents the only methodology that allows testing for antimicrobial susceptibility and molecular analysis (22). In the last 2 decades, increases in drug resistance, particularly in some areas of the world (7, 23), strongly suggest the use of methods that incorporate antibiotic testing before therapy for effective management of H. pylori infections.
In our Italian area, the increase in levels of resistance, in particular to clarithromycin and fluoroquinolones (24, 25) and especially in cases of relapse, needs great attention; culture and, consequently, susceptibility testing represent, undoubtedly, the most correct way to administer a successful therapy for eradication, preventing emergence of multiresistant H. pylori strains.
GESA transport medium enables transportation of the gastric biopsy specimen from endoscopy to the microbiology laboratory, guaranteeing the long-term viability and cultivability of the microorganism.
This report highlights the benefits of this new patented medium for high-performance recovery of H. pylori.
Several other transport media have been described, such as Stuart's media (18, 26–28), serum-free transport medium with cyanobacterial extract (MH-CE) (20), brain heart infusion broth plus vancomycin, amphotericin B, and nalidixic acid (BHI-VAN) (29), and a commercial medium, Portagerm pylori (18, 26). The composition of each of experimental medium was not critical for the recovery of cultivable H. pylori strains within 1 day of storage, with good performance in bacterial isolation seen for each of them. When immediate culture was not feasible, few media were useful in prolonging survival of culturable H. pylori from cell suspensions and biopsy specimens (18, 29) with suitable yields of H. pylori recovery over time.
The composition of the proposed new semisolid medium allows long-term H. pylori recovery from both suspensions and gastric biopsy specimens; with respect to other proposed transport media (5, 17, 30), GESA shows a quantifiable bacterial recovery rate until the 10-day time point.
By the use of H. pylori suspensions in experiments, the microscopy fluorescence observations underline the preservation, over time, of a consistent part of the bacterial population with the spiral/bacillary viable H. pylori morphology useful for bacterial growth on cultural media. Storage at 4°C in GESA transport medium does not modify the typical cell morphology until the 7-day time point, thus guaranteeing bacterial recovery and, consequently, suitability for drug susceptibility testing and biomolecular analysis of gene targets of virulence factors. The loss in bacterial recovery after 10 days, with detection of few H. pylori CFU, was confirmed by the fact that the cells, which showed a marked morphological change from spiral to coccoid, despite the prevalent viability, were unculturable. These results underline the good performances of GESA medium. In a previous study, Vega et al. (20) reported recovery of bacterial cells in 5 of 7 H. pylori strains in MH-CE for up to 5 days of storage at 4°C, whereas other authors (28) reported H. pylori survival and cultivability for up to 3 days when inocula were stored in Stuart medium.
Regarding H. pylori recovery from biopsy specimens, we collected H. pylori colonies until 10 days of storage at 4°C, and, interestingly, saw no differences in the levels of colony recovery over time, thus supporting the hypothesis of the high performance of GESA transport medium in maintaining storage of viable and cultivable cells from gastric specimens. This is of particular interest when it is necessary to prolong the biopsy specimen storage with a guarantee of satisfactory bacterial isolation. Other selective transport media showed lower rates of recovery of H. pylori, with values of 76% for 5 days in BHI-VAN (29), 77% for 3 days in Portagerm pylori (18), and 61% for 4 days in MH-CE (20).
The better GESA performance in the rate of recovery in bacterial colonies from biopsy specimens than in bacterial colonies from suspensions was also noticed for other transport media (18, 19). In particular, Heep et al. (18) suggested that gastric tissue exerts a protective effect during cold storage. We suppose that the protective effect deriving from the adhesion of the microorganism to the gastric epithelial tissue can help the H. pylori to avoid responding to the stress condition by entering the viable but nonculturable (VBNC) state and becoming coccoid (11–14). Moreover, the formulation of GESA transport medium and the absence of antibiotics could favor the fast recovery of H. pylori colonies. In fact, we obtained bacterial isolation in a time period never exceeding 4 days in the absence of contaminant, allowing rapid bacterial susceptibility testing and a fast response for patients. These results could be attributed both to the formulation of the medium and to the absence of antibiotics that, even among those ineffective against H. pylori, could be capable of stressing the microorganism and inducing, in a part of bacterial population, a loss of cultivability. The GESA formulation could also avoid bacterial overgrowth, providing easy recovery of H. pylori colonies from biopsy specimens with very low rates of contamination until the 10-day time point. Obviously, good practices with respect to biopsy specimen collection and the sterility of forceps are important factors for successful isolation.
GESA can be considered a standardized transport medium with high performance that optimizes the rate of recovery of H. pylori by culture.
ACKNOWLEDGMENTS
We thank Valentina Cataldi for useful contributions to this study.
Footnotes
Published ahead of print 15 October 2014
REFERENCES
- 1. Moyat M, Velin D. 2014. Immune responses to Helicobacter pylori infection. World J. Gastroenterol. 20:5583–5593. 10.3748/wjg.v20.i19.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suerbaum S, Michetti P. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175–1186. 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 3. van Amsterdam K, van Vliet AH, Kusters JG, van der Ende A. 2006. Of microbe and man: determinants of Helicobacter pylori-related diseases. FEMS Microbiol. Rev. 30:131–156. 10.1111/j.1574-6976.2005.00006.x. [DOI] [PubMed] [Google Scholar]
- 4. Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, Bosques-Padilla FJ. 2014. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J. Gastroenterol. 20:1438–1449. 10.3748/wjg.v20.i6.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirschl AM, Makristathis A. 2007. Methods to detect Helicobacter pylori: from culture to molecular biology. Helicobacter 12(Suppl 2):S6–S11. 10.1111/j.1523-5378.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 6. Ramis IB, de Moraes EP, Fernandes MS, Mendoza-Sassi R, Rodrigues O, Juliano CR, Scaini CJ, da Silva PE. 2012. Evaluation of diagnostic methods for the detection of Helicobacter pylori in gastric biopsy specimens of dyspeptic patients. Braz. J. Microbiol. 43:903–908. 10.1590/S1517-83822012000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y; Study Group participants. 2013. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 62:34–42. 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 8. Megraud F, Lehours P. 2007. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 20:280–322. 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mishra S. 2013. Is Helicobacter pylori good or bad? Eur. J. Clin. Microbiol. Infect. Dis. 32:301–304. 10.1007/s10096-012-1773-9. [DOI] [PubMed] [Google Scholar]
- 10. Dorer MS, Talarico S, Salama NR. 2009. Helicobacter pylori's unconventional role in health and disease. PLoS Pathog. 5:e1000544. 10.1371/journal.ppat.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cellini L. 2014. Helicobacter pylori: a chameleon-like approach to life. World J. Gastroenterol. 20:5575–5582. 10.3748/wjg.v20.i19.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cellini L, Robuffo I, Spoto G, Di Campli E, Di Candia M, Donelli G. 2004. Population dynamics in ageing Helicobacter pylori. New Microbiol. 27:29–35. [PubMed] [Google Scholar]
- 13. Cellini L, Allocati N, Di Campli E, Dainelli B. 1994. Helicobacter pylori: a fickle germ. Microbiol. Immunol. 38:25–30. 10.1111/j.1348-0421.1994.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 14. Cellini L, Robuffo I, Di Campli E, Di Bartolomeo S, Taraborelli T, Dainelli B. 1998. Recovery of Helicobacter pylori ATCC43504 from a viable but not culturable state: regrowth or resuscitation? APMIS 106:571–579. 10.1111/j.1699-0463.1998.tb01386.x. [DOI] [PubMed] [Google Scholar]
- 15. Lee HC, Huang TC, Lin CL, Chen KY, Wang CK, Wu DC. 2013. Performance of routine Helicobacter pylori invasive tests in patients with dyspepsia. Gastroenterol. Res. Pract. 2013:184806. 10.1155/2013/184806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez-Perez GI. 2000. Accurate diagnosis of Helicobacter pylori. Culture, including transport. Gastroenterol. Clin. North Am. 29:879–884. 10.1016/S0889-8553(05)70155-2. [DOI] [PubMed] [Google Scholar]
- 17. Dierikx CM, Martodihardjo J, Kuipers EJ, Hensgens CM, Kusters JG, Suzuki H, de Groot N, van Vliet AH. 2007. Serum- and animal tissue-free medium for transport and growth of Helicobacter pylori. FEMS Immunol. Med. Microbiol. 50:239–243. 10.1111/j.1574-695X.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 18. Heep M, Scheibl K, Degrell A, Lehn N. 1999. Transport and storage of fresh and frozen gastric biopsy specimens for optimal recovery of Helicobacter pylori. J. Clin. Microbiol. 37:3764–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Windsor HM, Abioye-Kuteyi EA, Marshall BJ. 2005. Methodology and transport medium for collection of Helicobacter pylori on a string test in remote locations. Helicobacter 10:630–634. 10.1111/j.1523-5378.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 20. Vega AE, Silva HJ, Cortiñas TI. 2012. Evaluation of a serum-free transport medium supplemented with cyanobacterial extract, for the optimal survival of Helicobacter pylori from biopsy samples and strains. Eur. J. Clin. Microbiol. Infect. Dis. 31:135–139. 10.1007/s10096-011-1285-z. [DOI] [PubMed] [Google Scholar]
- 21. Yuen B, Zbinden R, Fried M, Bauerfeind P, Bernardi M. 2005. Cultural recovery and determination of antimicrobial susceptibility in Helicobacter pylori by using commercial transport and isolation media. Infection 33:77–81. 10.1007/s15010-005-4071-y. [DOI] [PubMed] [Google Scholar]
- 22. Ndip RN, MacKay WG, Farthing MJ, Weaver LT. 2003. Culturing Helicobacter pylori from clinical specimens: review of microbiologic methods. J. Pediatr. Gastroenterol. Nutr 36:616–622. 10.1097/00005176-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 23. Smith SM, Haider RB, O'Connor H, McNamara D, O'Morain C. 2014. Practical treatment of Helicobacter pylori: a balanced view in changing times. Eur. J. Gastroenterol. Hepatol. 26:819–825. 10.1097/MEG.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 24. Ciccaglione AF, Cellini L, Grossi L, Marzio L. 2012. Quadruple therapy with moxifloxacin and bismuth for first-line treatment of Helicobacter pylori. World J. Gastroenterol. 18:4386–4390. 10.3748/wjg.v18.i32.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toracchio S, Cellini L, Di Campli E, Cappello G, Malatesta MG, Ferri A, Ciccaglione AF, Grossi L, Marzio L. 2000. Role of antimicrobial susceptibility testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 14:1639–1643. 10.1046/j.1365-2036.2000.00870.x. [DOI] [PubMed] [Google Scholar]
- 26. Grove DI, McLeay RA, Byron KE, Koutsouridis G. 2001. Isolation of Helicobacter pylori after transport from a regional laboratory of gastric biopsy specimens in saline, Portagerm pylori or cultured on chocolate agar. Pathology 33:362–364. [PubMed] [Google Scholar]
- 27. Roosendaal R, Kuipers EJ, Peña AS, de Graaff J. 1995. Recovery of Helicobacter pylori from gastric biopsy specimens is not dependent on the transport medium used. J. Clin. Microbiol. 33:2798–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soltesz V, Zeeberg B, Wadström T. 1992. Optimal survival of Helicobacter pylori under various transport conditions. J. Clin. Microbiol. 30:1453–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siu LK, Leung WK, Cheng AF, Sung JY, Ling TK, Ling JM, Ng EK, Lau JY, Chung SC. 1998. Evaluation of a selective transport medium for gastric biopsy specimens to be cultured for Helicobacter pylori. J. Clin. Microbiol. 36:3048–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rizvi F, Hannan A. 2000. Evaluation of different transport and enrichment media for the isolation of Helicobacter pylori. JAMC 12:31–33 http://ayubmed.edu.pk/JAMC/PAST/12-3/Farhat.pdf. [Google Scholar]